

6 EASY ways to model OSMOSIS

Teaching osmosis?
Osmosis is one of my favorite topics to teach during the cells unit because it’s so easily visualized. There are quite a few ways to easily see osmosis in action! Here’s a round-up of six labs and the pros and cons of each:
1. EGG OSMOSIS
The “naked egg” lab is very popular in biology. Students really get a kick out of seeing the eggshell dissolve! In this lab, students dissolve the shell of an egg by soaking it in vinegar (this takes about 3 days, so its best to set up on a Friday). Once the shell is gone, students carefully transfer their naked eggs to hypotonic distilled water, and a hypertonic solution like corn syrup or molasses. Students compare the mass of the egg before and after soaking and figure out which way the water moved while attempting to reach equilibrium.

PROS: Students love this lab! Also, eggs are round like red blood cells, so they can visualize what happens to cells in different hypertonic or hypotonic solutions.
CONS: It can get expensive to purchase enough eggs for all of your classes. Also, expect some to break along the way, so soak some extras just in case. Overall, it feels a little wasteful of perfectly good eggs. Maybe do this one as a demonstration, and then choose one of the options below for students to do in groups.
2. PURPLE ONION SKIN
Of all the osmosis labs, this one might be my favorite because I’m partial to getting out the microscopes. In this lab, students get a small piece of onion skin and make a wet mount slide using fresh water. They will see nice rectangular purple onion cells. Next, they will swap out the fresh water for salt water, and watch the cytoplasm in each cell shrivel up. If your students already know how to use microscopes, this one is a hit! Here is a full blog post with more details and pictures.
Note: I used to use elodea for this lab, but it became difficult to find at pet stores since it is invasive in many states. Purple onion is easy and cheaper! You can also find a lab write-up here .

PROS: Inexpensive, and fool proof. It is impossible to mess up! Unlike other options, this lab uses real plant cells. Also, it is the only version that doesn’t require soaking things overnight so you can get it done in one class period.
CONS: Your classroom will smell like onion for a day.
3. DIALYSIS TUBING
This version is a little fancier, and is a great option if you have honors or AP students. In this lab, students will get dialysis tubing and fill them with varying concentrations of sugar water solution. They will measure the initial mass of the tubes, and then soak the dialysis tubing overnight in distilled water. The following day they will measure the new mass, and see how water moved across the dialysis tubing membrane. You can find a free version of this lab from Amy Brown on TpT.
PROS: Students collect quantitative data, and it feels more “scientific” than other options.
CONS: Dialysis tubing is expensive, and if students don’t tie the string tight enough they can leak.

4. GUMMY BEARS
I am the first to admit gummy bears are not my favorite option, but I know many teachers who love doing it this way. In this version, students soak gummy bears in tap water, distilled water, and salt water overnight. They measure the change in the size of the gummy bear using rulers.
PROS: A large bag of gummy bears is only a few dollars, and students always love working with candy.

CONS: I don’t love this lab because depending on the brand you buy, the gummy bears can begin to dissolve and fall apart. Also, since they are an irregular shape it is difficult to calculate the change in volume.
5. WATER BEADS
An alternative to gummy bears is water beads, or Orbeez. You set it up the same way by soaking them in fresh water and salt water. If your kid already has some at home, use them instead of gummy bears. I like them better for a few reasons:
PROS: Water beads won’t fall apart like gummy bears, even after soaking multiple days. Since they are round, you can have students measure the diameter, and calculate the volume of the sphere. Also, you can dry them out afterward, place them in a Tupperware, and re-use them the following year.

CONS: Orbeez are smaller than gummy bears, so if your students struggle taking small, accurate measurements that might be a point of struggle. (Note: Beware of cheaper off-brands you can find on Amazon, because they WILL fall part unlike Orbeez).
6. BABY CARROTS
Last but not least are baby carrots! In this lab, each lab group gets 2 baby carrots. Just like other labs, they measure the mass before and after soaking them in fresh and salt water. Students will also notice that the baby carrot soaked in salt water becomes flimsy and bendable overnight. If you have already covered organelles, this can lead to a discussion about vacuoles and how plant cells become limp and flimsy when they lose water. You can find a lab write-up on my website !

PROS: Inexpensive materials, and uses real plant cells.
CONS: You won’t see the size of the carrot change, only the flexible vs stiff texture.
Alright, which is your favorite? Choose one (or two) and have a blast!

- Read more about: Cells

Hi, I'm Becca!
Search the site, browse by category.
- A list of ALL blog posts
- Back to School
- Biochemistry
- Body Systems
- Classification
- Classroom Decor
- Classroom Management
- Distance Learning
- End of the School Year
- Experiments
- Field Trips
- For NEW Teachers
- Formative Assessment
- Media in the Classroom
- Microscopes
- Photosynthesis & Respiration
- Plate Tectonics
- Sustainability
- Teacher Tips
- Weather and Climate
Get Freebies!
You might also like....

Read Aloud Closure Assignments

Biology Picture Book Recommendations

Earth and Space Science Picture Book Recommendations

Want a fun way to practice science vocabulary? Try out seek and finds!

Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |
- Science & Math
- Sociology & Philosophy
- Law & Politics
Selective Permeability of Dialysis Tubing Lab: Explained
- Selective Permeability of Dialysis Tubing…
This dialysis tube experiment experiment was conducted to investigate the selective permeability of dialysis tubing. The permeability of the tubing to glucose, starch, and iodine (potassium iodide) was tested. The dialysis tubing was clipped to form a bag so that glucose and starch were fed into the bag through the other end, and was also clipped to avoid the seeping of the solution.
Water with several drops of iodine added to it until it was visibly yellow-amber was added to a 400ml beaker. The bag was then placed in the beaker, which was stirred with a magnetic stirrer. It was left there for 30 minutes. It was seen that the color of the solution in the bag changed to blue-black color, which showed that iodine was able to pass through the membrane into the bag.
The solution in the beaker became pale yellow-amber, showing that starch didn’t pass through the membrane into the beaker. To confirm the presence of glucose in the beaker and the bag, a Benedict test was performed on the solutions including tap water (control) too.
The beaker solution turned light brown after Benedict’s solution was added to it and suspended in a water bath for 10 minutes. The dialysis bag solution also changed to brown color, while tap water remained blue. This experiment showed that dialysis tubing is selective in its permeability to molecules. It was permeable to glucose and iodine but not starch.
INTRODUCTION:
PURPOSE: The purpose of the dialysis bag experiment was to test the permeability of dialysis tubing to glucose, starch, and iodine.
Living cells need to obtain nutrients from their environment and get rid of waste materials to their surroundings. This exchange of materials between the cell and its surroundings is crucial to its existence. Cells have membranes composed of a phospholipid bilayer embedded with proteins.
This cell membrane can distinguish between different substances, slowing or hindering the movement of other substances and allowing others to pass through readily. This property of the cell is known as selective permeability (Ramlingam, 2008).
Selective permeability is a property of a cell membrane that allows it to control which molecules can pass (moving into and out of the cell) through the pores of the membrane. Selectively permeable membranes only allow small molecules such as glucose and amino acids to readily pass through and inhibit larger molecules like protein and starch from passing through it.
The dialysis tubing is a semi-permeable membrane tubing used in separation techniques and demonstration of diffusion, osmosis, and movement of molecules across a restrictive membrane (Todd, 2012). It separates dissolved substances of different molecular sizes in a solution, and some of the substances may readily pass through the pores of the membrane while others are excluded. The dialysis tubing is made up of cellulose fibers shaped in a flat tube.
In this dialysis tubing lab experiment, the selective permeability of dialysis tubing to glucose, starch, and iodine (potassium iodide) will be tested. This experiment consists of two tests: the test for starch and the test for reducing sugar. When iodine (potassium iodide) is added to a solution in which starch is present, the solution turns blue-black or purple; otherwise, it remains yellow-amber.
When Benedict’s reagent is added to a solution in which reducing sugar is present and it is heated in a water bath, the solution turns green, yellow, orange, red, and then brick red or brown (with a high concentration of sugar present). Otherwise, the solution remains blue.
Will glucose, starch, and iodine (potassium iodide) readily pass through the pores of the dialysis tubing?
HYPOTHESIS:
Glucose, starch, and iodine (potassium iodide) will readily pass through the membrane of the dialysis tubing.
Dialysis Lab Report Prediction:
The solution in the bag and the beaker will both turn blue-black due to the presence of iodine and starch; the presence of glucose in the bag and beaker will be investigated using Benedict’s test.
- Dialysis Tubing
- Test Tubes rack
- Benedict’s reagent
- Iodine (Potassium Iodide)
EXPERIMENT PROCEDURE:
1) 250 ml of tap water was added to a beaker. Several droppers of Iodine (Potassium Iodide) solution was added to the water until it was visibly yellow-amber in color. The color was then recorded.
2) The dialysis tubing was soaked in water for a few minutes until it began to open. One end of the bag was folded and clipped in order to secure it so that no solution seeped through.
3) The other end of the tubing was opened so that it forms a bag and 4ml of glucose and 3ml of starch was fed into it. The bag was also closed and its content was mixed. The color of the solution was then recorded.
4) The outside of the bag was rinsed in tap water.
5) The magnetic stirrer and then the bag was placed in the beaker. The other end of the bag was made to hang over the edge of the beaker.
6) The bag was left in the beaker for about 30 minutes, as the beaker was being stirred.
7) After 30 minutes, the bag was carefully removed and made to stand in a dry beaker. The final color of the solutions was recorded.
8) Benedict test was performed to test for the presence of reducing sugar in the solution in the bag, beaker and tap water (serves as control).
- a) 3 test tubes were labelled control, bag and beaker.
- b) 2 ml of water was added to the control test tube. 2 ml of the bag solution was added to the bag test tube and 2 ml of the beaker solution was added to the beaker test tube.
- c) 2 ml of Benedict’s reagent was added to each test tube and was suspended in a boiling water bath for 10 minutes. The color change was recorded.
| Solution Source | Original Contents | Original Color | Final Color | Color after Benedict’s test |
| Bag | Starch and Glucose | Colorless | Blue-black | Brown |
| Beaker | Water and Iodine | Yellow-amber | Pale yellow-amber | Brown |
| Control | Water | Colorless | Blue | Blue |
The solution in the bag turned blue-black in color owing to the movement of molecules of iodine from the beaker to the bag which contains starch. The solution in the beaker turned brown after Benedict’s test, indicating the presence of glucose in the beaker. This means that the dialysis tubing was permeable to both glucose and iodine but not starch. It is known that starch didn’t pass because the solution in the beaker, which contains iodine, didn’t turn blue-black in color but remained yellow-amber.
DISCUSSION:
- How can you explain your results?
From the results of the experiment represented in a tabular form above, the hypothesis suggested before carrying out the experiment turned out to be incorrect. The dialysis tubing was not permeable to all three solutions: glucose, starch, and iodine (potassium iodide). Rather, the tubing was permeable to glucose and iodine but not starch.
This could be known from the color change in the solutions in the beaker and the bag. The dialysis tubing was permeable to iodine, so the content of the bag turned blue-black in color, indicating the presence of starch. Glucose also readily passed through the pores of the membrane. After performing Benedict’s test on the solutions, the bag’s solution as well as the beaker’s solution turned brown in color. This shows the presence of reducing sugar in both solutions, meaning that glucose passed into the beaker from the bag.
- From your results, predict the size of iodine (potassium iodide) relative to starch.
From the results of this experiment, it is obvious that glucose and iodine (potassium iodide) have smaller molecular sizes than starch. Because starch had a larger molecular size, the dialysis tubing was not permeable to it (it didn’t allow it to readily pass through the pores of its membrane).
- What colors would you expect if the experiment started with glucose and iodine (potassium iodide) inside the bag and starch in the beaker? Explain.
- The solution in the bag will remain yellow-amber in color at the end of the experiment.
- The solution in the beaker will turn blue-black in color at the end of the experiment.
- After performing Benedict’s test, both solutions will turn brown in color.
The solution in the bag remained yellow-amber in color at the end of the experiment because the dialysis tubing is not permeable to starch, so starch didn’t pass through from the beaker into the bag. The solution in the beaker turned blue-black in color at the end of the experiment because iodine passed from the bag into the beaker through the membrane. After performing Benedict’s test on the bag and beaker solution, both solutions turned brown in color because the dialysis tubing was permeable to glucose, so glucose readily passed from the bag into the beaker through the membrane.
Precautions
- It was ensured that the right quantity of solutions was used in every part of the experiment.
- It was also ensured that the time required for the successful complement of the experiment was adhered to.
- It was ensured that all apparatus used were handled with caution.
- The dialysis tubing was clipped well on both ends to secure it so that no solution seeped through.
It was concluded that the dialysis tubing investigation doesn’t allow all kinds of substances to pass readily through the pores of its membrane. This means that it is selective in its permeability to substances. The dialysis tubing was permeable to glucose and iodine but not to starch. Starch was excluded because it has a larger molecular size than glucose and iodine.
Ramlingam, S. T. (2008). Modern Biology. Onitsha: African First Publishers.
Todd, I. S. (2012). Dialysis: History, Development and Promise. World Scientific Publishing Co Pte Ltd.
Related Posts
- Percent Composition Lab: Explained
- Lab Explained: Ohm's Law Lab
- Single Displacement Reactions Lab Explained
- The Energy of Phase Changes Lab Explained
- TLC of ASPIRIN: Lab Explained
18 Comments
Could oxygen pass through the dialysis tubing
so what was the chemical formula for this experiment?
After 24 hours of leaving the bag in the iodine solution; -The dialysis bag turned dark blue/purple, Explain. -The fructose test strip turned positive when dipped in the solution, Explain.
if the dialysis represent the membrane of a root air cell, and the sugar solution inside represent the cells cytoplasm, which is hypotonic, hypertonic or isotonic. is there any movement of iodine molecules?
What is the purpose of the Iodine Solution?
you added starch and glucose to dialysis tubing, a semipermeable membrane that mimics the plasma membrane of cells. The filled tubing which was placed in a beaker of water containing iodine. What is the purpose of the iodine?
Is the iodine entering the dialysis tube an example of diffusion or osmosis? or can osmosis only occur with water?
what was the purpose of placing the dialysis tubing containing starch solution into the beaker of distilled water
What were the limitations of your experiment ?
What about the NaCl? I did this lab but we had a question if NaCl moved out of the tube.
what did not diffuse through the membrane
Starch and Benedict’s solution.
Maybe the starch and its size.
How can you explain the change in weight of the cells?
osmosis of water
Include an analysis maybe? all around good job though!
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Post comment
Quick links
- Make a Gift
- Directories
Chemicals and Solutions
Saturated salt solution (about 1g NaCl/mL of water)
- About 6 inches of dialysis tubing
- 50 mL buret with tip removed
- Food coloring
- Dialysis tubing
- Tall beaker of water
- Celery, cucumber & white carnation
- Cut about 6 inches of dialysis tubing and soften it in water.
- Tie one end of the dialysis tubing in a double knot to make a leak proof bag.
- Secure the buret to a stand. Slide the open end of the dialysis bag around the 50 mL buret and pull the bag up so that the bag and tubing overlap for about one inch. Secure the bag around the buret using a rubber band.
- Use food coloring to color about 100 mL of saturated salt solution and then add the salt solution to the dialysis bag by pouring it through the top of the buret. The bag should be filled with salt solution.
- Immerse the bag in a beaker of saturated salt solution until classtime.
- To present the demo, remove the beaker of saturated salt solution and immerse the bag in deionized water. Water will pass through the membrane into the bag causing the liquid level in the buret to rise.
The dialysis tubing is a semipermeable membrane. Water molecules can pass through the membrane. The salt ions can not pass through the membrane. The net flow of solvent molecules through a semipermeable membrane from a pure solvent (in this cause deionized water) to a more concentrated solution is called osmosis.
- Newsletter
- News Feed

- Classroom Practicals
Osmosis and Diffusion

AUSTRALIAN CURRICULUM ALIGNMENT:
- Movement of materials across membranes occurs via diffusion, osmosis, active transport and/or endocytosis
BACKGROUND:
The cell membrane maintains the cell a separate entity; it holds the cell contents within, and acts as a barrier to the external environment. It is selectively permeable and has various mechanisms to allow for the exchange of gases and nutrients. These mechanisms allow for the intake of anything that is required and allows for the expulsion of waste and toxins. This membrane does not resemble a sheet or bag; rather, it is many molecules of Phospholipid Bilayers held together by the combined forces of attraction and repulsion. They are comprised of a Phosphate head; which is hydrophilic (water-loving), and a Lipid (fatty acid) tail which is hydrophobic (repelled by water). As the internal and external environments of a cell are aqueous, these molecules arrange themselves into two layers; one with the Phosphate heads oriented out into the external fluid, and the other with the heads oriented inwards into the internal fluid (the Cytoplasm). The Lipid tails are between the two layers of Phosphate heads; thereby, protected from the water, and the strength of this attraction/repulsion mechanism keeps the molecules together as though the membrane were a single entity.
In this practical, dialysis tubing is used as a surrogate cell membrane for a visual demonstration of osmosis and diffusion. A solution containing large molecules (Starch) and small molecules (Glucose) is placed inside the tubing; which is then placed in a solution containing iodine. Students are able to observe as the solution inside the tubing turns dark blue, while the surrounding solution it is submerged in does not. From this, students can use their prior knowledge of the Starch-Iodine complex to surmise that Iodine is able to pass through the membrane while starch is not. The Glucose-testing strips indicate that glucose has been able to pass out of the tubing and into the external fluid. Thus proving the tubing allows movement in both directions.
This inexpensive and simple experiment provides students with a clear visual result that effectively demonstrates how the size of a molecule can affect its ability to be transported into or out of a cell. It also illustrates the mechanics of diffusion and osmosis by which a cell will attempt to create homeostasis, or equilibrium between its inner and outer environments.
PREPARATION - BY LAB TECHNICIAN
- Cut the dialysis tubing into 15cm lengths and soak for 15 minutes in a beaker filled with room temperature distilled water. Prepare one length of tubing per student or group. However, it is best to prepare extra strips for students, as some strips may tear or leak through handling.
- To create the Starch solution, dissolve 2g of Starch in 100mL of boiling hot water (2% solution) on a hot plate until the Starch powder has been fully dissolved. Stir as required.
- To create the Glucose solution, dissolve 30g of Glucose in 100mL water (30% solution) and continue stirring until the glucose has been fully dissolved.
- Combine the Starch and Glucose solutions in a single beaker. Use a stirring rod to mix well.
METHOD - STUDENT ACTIVITY
Glucose/ Starch Solution
- Measure 5-10 mL of the Glucose/Starch mixture in a small beaker or test tube.
- To determine the initial glucose concentration within the Starch/ Glucose solution, you will first need to dilute a sample of the mixture in water. To do this, collect 1mL of your mixture using a transfer pipette and add to a test tube filled with 9mL of water. Mix using a clean stirring rod.
- Measure the diluted Starch/Glucose by placing a Glucose-testing strip in the solution, immediately removing it and waiting 60 seconds to observe any colour change. Using the colour guide on the testing strip container, determine the approximate Glucose levels, and record the results.
Iodine Solution
- Fill a large beaker with 100mL water, and add 1mL of Iodine/KI solution. The solution should appear a yellowish colour.
- Measure the Glucose levels of the Iodine solution with another strip; following the same procedure as before. Ensure you record the results.
Preparing the "cell" tubing
- Retrieve your soaked piece of dialysis tubing and tie a knot in one end as though you are tying a balloon.
- Using a transfer pipette, half-fill the tubing with your undiluted Starch/Glucose solution and tie the other end to create a “cell”.
- Submerge the “cell” tubing into the Iodine solution.
Observing changes in the “cell”
- After 15 minutes, observe any colour changes in the tubing and in the beaker solution.
- Measure the Glucose levels in the Iodine solution.
- Carefully open the tubing and pour the contents into a clean beaker.
- To dilute the tubing contents for Glucose testing, collect 1mL of the contents using a pipette and deposit into a test tube filled with 9mL of water.
- Measure the Glucose levels in the diluted contents using a Glucose testing strip following the same procedure as before.
- Record the results of the Glucose testing.
- Compare the changes in Glucose levels before and after the 15 minute interval.
OBSERVATION AND RESULTS
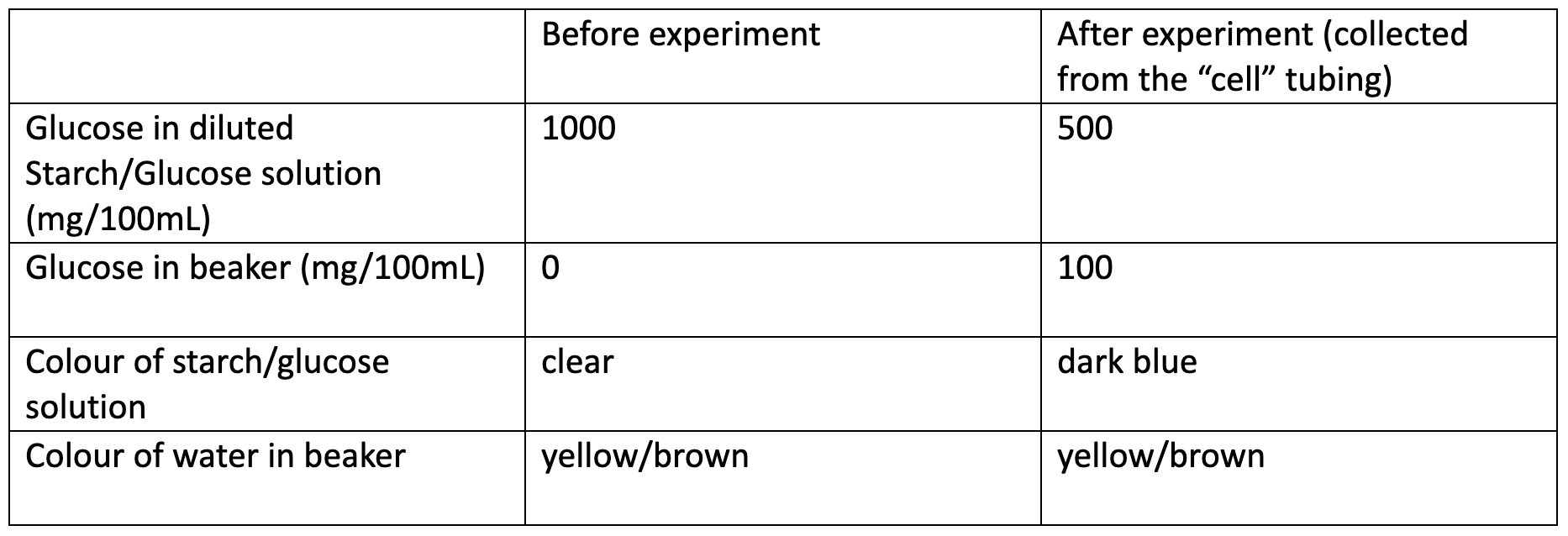
INVESTIGATION
- Provide students with the information that you prepared a 100mL solution of 2% starch and a 100mL solution of 30% Glucose. Based on this information, ask your students to calculate the concentration of each in the combined solution. Students should understand that double the volume without extra solute means half the concentration, so what was 2g of Starch in 100mL (2%) is now 2g of Starch in 200mL (1%); and what was 30g of Glucose in 100mL (30%) is now 30g of Starch in 200mL (15%).
- Ask students to identify what occurred the Starch, based on the fact that the blue colour is found inside the cell but not outside of it, students should be able to identify that the Starch has not been able to pass through the tubing, while the Iodine has. Students should understand that the Starch-Iodine complex has therefore been confined to the area where both Starch and Iodine are found, that is, the inside of the cell.
- Ask students to describe what is suggested by the Glucose results. The appearance of Glucose into the previously Glucose-free solution in the beaker should inform students that Glucose has been able to pass through the membrane.
- To provide students with a deeper understanding surrounding the molecular size of Glucose and Iodine, you may provide students with the information that our dialysis tubing typically allows passage to molecules of up to 12,000 to 14,000 daltons (g/mol). This should provide some guidance of the sizes that Starch molecules can reach. Remind students, however, that the shape of a molecule may affect the passage as a large linear molecule may be able to pass through more easily than a smaller but globular molecule.
TEACHER NOTES
The concentration of Glucose in this practical is quite high to enable shorter waiting times for students. This allows them to more readily measure the glucose which has diffused out of the “cell” using their test strips. However, this also means that the initial concentration is too high to show that the concentration inside the cell has decreased in line with the increase outside the cell. To manage this, students are asked to take a sample of the original combined Glucose/ Starch solution prior to being placed in the “cell” and also a sample of the now-blue solution inside the “cell” at the end of the prac. Both solutions are diluted by a factor of ten to bring the Glucose concentration into the range of the Uriscan strips.
EXTENSION EXERCISE
To observe the process of cell diffusion and osmosis over an extended period of time, make an extra “cell” and keep it in solution until the next class. By the beginning of next class, the Glucose inside and outside the cell should have somewhat equalised. This could be conducted as a class demonstration, or each student may make an extra cell. Once again, dilute both solutions by a factor of ten prior to measuring.
TEACHER TIPS:
Prepare extra dialysis strips for students, as some strips may tear or leak through handling as students attempt to tie them.
Time Requirements
- 45 mins
Material List
Dialysis tubing
- Starch
- Iodine/KI solution
- Glucose
- Glucose-testing strips
- Test tubes
- Test tube rack
- Beakers 500mL
- Beakers 100mL
- Transfer pipettes
- Stirring rod
Safety Requirements
- Wear appropriate personal protective equipment (PPE); particularly gloves and a lab coat as Iodine will stain clothing and skin on contact.
- Exercise caution when handling the chemicals used in this prac.
- Avoid any direct contact with the solution and wash hands thoroughly.
||Biology||Classroom Practicals||Cells||Year 11&12||
Buy The Kit
Related Resources
- Solution Sheets
- Benefits to Participating Communities
- Participating School Districts
- Evaluations and Results
- Recognition Accorded
- National Advisory Committee
- Establishing New Institutes
- Topical Index of Curriculum Units
- View Topical Index of Curriculum Units
- Search Curricular Resources
- View Volumes of Curriculum Units from National Seminars
- Find Curriculum Units Written in Seminars Led by Yale Faculty
- Find Curriculum Units Written by Teachers in National Seminars
- Browse Curriculum Units Developed in Teachers Institutes
- On Common Ground
- Reports and Evaluations
- Articles and Essays
- Documentation
- Video Programs
Have a suggestion to improve this page?
To leave a general comment about our Web site, please click here
Share this page with your network.
Teaching Osmosis and Diffusion through Kidney Dialysis
Introduction.
The idea of an artificial kidney, or any artificial organ for that matter, seems like such a complex idea only to be understood by those that have specialized in fields such as nephrology or biomedical engineering. The kidney is such a complex organ. How can its function be duplicated, let alone explained to medical laypeople? Amazingly, kidney dialysis, which for all practical purposes is an artificial kidney that functions in removing solutes and toxins from the blood, is actually not as complex as one might imagine. In fact, the mechanism by which dialysis works involves simple diffusion and osmosis, which is generally taught to children in the United States as early as middle school. The study of artificial organs can seem intimidating and beyond the comprehension of those with only a general knowledge of science. However, the study of artificial kidneys provides an amazing opportunity to display how even the most fundamental and seemingly simple concepts in science can be used to revolutionize modern medicine. Imagine a middle schooler's, or even a high schooler's excitement, when they realize they have the capability and background knowledge to create their own version of kidney dialysis.
The purpose of this unit is to teach students about the cellular processes of osmosis and diffusion. The concept of osmosis and diffusion is vital to understanding the nature of organisms and how they function, yet these have also proven to be among the most difficult concepts to get students to understand. In my experience, osmosis and diffusion are taught in isolation, generally using one cell and the surrounding environment as the ultimate reference point. It is hard for a student to understand the importance of equilibrium, when the process of diffusion is not linked to an idea bigger than a small two-dimensional drawing of a circle on paper to represent a cell. This is usually shown with an arrow pointing in the direction that water will flow in relation to the cell membrane.
The purpose behind this unit is to show how osmosis and diffusion are vital to the health of entire systems and organisms; it is not a rare occurrence that just happens in cells that have been isolated. In teaching kidney function, students will get to connect what is happening on the cellular level to what is happening within the entire organ system and then the entire human body. This will allow students to truly understand homeostasis and equilibrium as it relates to their own health and the state of their own bodies. In doing so, students will be able to see the importance of healthy living choices, especially as disease such as diabetes are on the rise.
The proposed unit will be designed for a middle school classroom, but can easily be adapted for high school. I am an 8 th grade science teacher in a high poverty, urban area. My students tend to test below grade level in reading, which can sometimes interfere with their success in science, so there will be a large emphasis on hands-on, inquiry-based learning to help engage students.
Background Information
The cell membrane.
The cell is the basic functional unit of all living things. If one is to understand what is happening within an organism, it is necessary to study what is happening to the individual cells within the organism 1 . This unit will cover the movement of materials—mainly water, salts and sugar—into and out of the cells of the kidney through passive transport. This will call for a thorough understanding of the cell membrane that surrounds all animal and plant cells.
The cell membrane, sometimes referred to as the plasma membrane, is a phospholipid bilayer that creates a definite boundary between the inside of the cell and the outside of the cell. The structure of cell membrane allows it to easily regulate the materials that enter and exit the cell 2 . The individual phospholipids that are arranged in the membrane are comprised of a head group, which is hydrophilic, and two fatty acid chains, which are hydrophobic 3 . This unique structure results in the bilayer that surrounds the cell, with the fatty acid chains or tails pointing inward (tail-to-tail) and the hydrophilic heads oriented outwards, so that the portion of the membrane that surrounds the inside of the cell (the cytoplasm) and the outside of the cell (the interstitial space) are hydrophilic. The inner portion of the cell membrane, where the fatty acid chains meet up from the inside and outside layers of the cell membrane, is a hydrophobic region. This arrangement is comparable to an oreo cookie, where in order for a material to pass through the whole cookie, it would have to pass through the chocolate cookie on one side (hydrophilic head), the cream-filled center (hydrophobic fatty acid tails), and once again the chocolate cookie on the other side. Materials that are unable to pass through the cream-filled center are unable to pass through the whole cookie.
The fact that the cell membrane is lined with both hydrophobic and hydrophilic layers makes it very difficult for most materials to pass in and out of the cell. This could be compared to a factory that continually makes products, yet is unable to ship any raw goods into the factory or ship out any of the final products. Obviously, no such factory could exist. Many materials that are necessary to cell functioning, such as salts, glucose, and amino acids, are not able to permeate the cell membrane because of the phospholipid bilayer. If the cell membrane were simply made of the phospholipid bilayer described above and nothing else, it would be a logical conclusion that anything made inside of the cell would not be able to leave the cell and anything that was found outside of the cell would not be able to enter, other than a few, very small, uncharged molecules. This is not the case, however, as it common knowledge that cells produce a variety of materials, such as hormones, and send them throughout the body; likewise, molecules, such as glucose and amino acids, enter the cell to be used for energy and to build proteins, respectively. How is this possible?
Transport of essential molecules is possible because embedded in the cell membrane are proteins (or clusters of proteins) that span the thickness of the membrane and act as transport channels for materials to move selectively in and out of the cell. These proteins come in contact with both the extracellular space and the intracellular space and, like the cell membrane itself, these proteins have areas that are hydrophobic and areas that are hydrophilic. The hydrophobic region of the protein is in the center of the cell membrane (i.e. the cream of the oreo cookie mentioned above), floating amongst the hydrophobic fatty acid chains, and the hydrophilic regions hang out slightly over the edges of the cell membrane surrounded by the hydrophilic region of the cell membrane. These proteins are capable of chemically recognizing certain materials and transporting them into or out of the cell 4 .
Because the cell membrane allows certain materials to pass through it, and keeps other materials out of it, it is referred to as a semi-permeable or selectively permeable membrane. Much like the security guard at a government center, movement into and out of the cell is highly regulated.
Diffusion and Osmosis
Water, carbon dioxide, and oxygen are a few substances that are able to pass through the cell membrane without any help from the proteins embedded in the membrane. These materials can simply pass through the membrane without the use of energy and they will do so in an attempt to reach an equal solute concentration inside and outside of the cell. This process is called diffusion, when substances move from a region of high concentration to a region of low concentration. The "goal" of diffusion is to reach a state of equilibrium. This does not mean that movement of molecules stops once equilibrium is reached. Equilibrium is a state of balance, where for every molecule of a substance that moves into a cell, another one moves out of the cell 5 . To visualize diffusion, imagine two rooms that are connected by one door. If the door connecting the two rooms is closed and everyone is crammed into only one of the rooms, as soon as the door opens, people will begin to walk into the other room to spread out. Most likely, they will not all move into the newly opened room. Instead, they will spread out until every area of the two rooms has roughly the same density (or concentration) of people. People will still be able to walk around and mingle, but they will likely readjust so that every part of the room is equally comfortable at all times. Diffusion is an easy concept to model. Simply place a drop of food coloring into a glass of water (being careful to make sure that the fluid in the glass is completely still). The food coloring will slowly spread out in the water until it is evenly mixed throughout 6 .
Diffusion In the Human Body
Within the body, diffusion is vital to the functioning of the cardiovascular system. When oxygen is inhaled into the lungs, it ends up at high concentration in the alveoli, the round sacs at the end of the bronchioles. Because blood that is flowing through your lungs is deoxygenated, there is a higher concentration of oxygen inside the alveoli than in the blood. In an attempt to reach equilibrium, the oxygen simply diffuses into the blood across the cells at the alveolar/capillary boundary. Similarly, the blood circulating through the capillaries in your lungs contains high amounts of carbon dioxide that has been picked up as a waste product from cellular respiration. The concentration of carbon dioxide in the blood is much higher than it is in the alveoli, and the carbon dioxide moves into the alveolar gas through simple diffusion; it is then exhaled out of the body 7 . In regards to cells, simple diffusion is only possible if the material is able to permeate the membrane.
The Diffusion of Water- Osmosis
Like carbon dioxide and oxygen, water is able to move across the cell membrane from areas of high concentration to low concentration. This movement is aided by the presence of small channels created by proteins, which are called aquaporins. When water diffuses across a membrane, it is referred to as osmosis 8 . Often, water will move across a membrane in order to balance the unequal concentrations of a solute, which is not able to move through the membrane itself. A solute is a material that is dissolved in a liquid. For example, in salt water, the solute is the salt.
Osmosis is most easily understood by imagining an experiment. Imagine a beaker that has been divided in half by a membrane that is permeable to water and impermeable to sugar, like most cell membranes in animals. Imagine that red sugar (for the purpose of this example, you will have to pretend that the sugar itself is actually red, so that as you add it to water, it turns light pink and the more you add the darker red the water gets) has been added to side A and very little sugar has been added to side B. Nature wants things at equilibrium, and there is not a state of equilibrium between the two sides of the beaker. At this point, side A should be dark red and side B would be a very light shade of pink. The simplest solution to the non-equilibrium problem would be for the sugar to move across the membrane until half of the sugar molecules are on side B and half are on side A. However, the membrane separating the two sides does not allow sugar to pass. An alternate solution to the non-equilibrium problem is possible. Water molecules can move from one side to the other to even out the concentration of sugar and water on both sides. If one side of the beaker (side A) is red and the other side (side B) is light pink, as it has very little sugar, then equilibrium could be achieved when both sides A and B are an equal shade of pink. This can happen when water passes through the membrane from side B into side A until the solute concentration of both sides was the same. (Notice that this would, of course, decrease the volume of side B and increase the volume of side A.) Once again, this does not mean that the water would simply stop moving once both sides turned pink. It means that water would be moving at an equal rate between sides A and B. (If this unit were designed for a high school class or even a high level middle school, it would be appropriate to discuss concentration and osmotic pressure in detail, but for the unit being proposed, the descriptors "higher concentration" and "lower concentration" will suffice. )
Osmosis—and the need to regulate solute concentrations in organisms, or maintain homeostasis—can be demonstrated by placing cells in solutions of varying solute concentrations. Cells already have a certain level of dissolved solutes within them. If a cell is surrounded by a solution with the same level of solute concentration as the cell itself, there will be no net movement of water into or out of the cell. This solution would be referred to as an isotonic solution, meaning the concentration of solutes inside the cell and outside of the cell were the same 9 .
If the same cell were surrounded by a solution that had more dissolved solutes than the cell itself, then water would leave the cell in order to reach equilibrium. Think back to the hypothetical red sugar example above. The end goal is pink, and water will flow from where the color is a darker red to where it is lighter or where there is no color at all. The darker red would be outside of the cell and the lighter pink would be inside of the cell. This would cause the volume of the cell to decrease as water left the cell. In this case, the solution surrounding the cell would be described as a hypertonic solution.
If the same cell were instead placed in distilled water or water with very few solute molecules, then water would enter the cell in an effort to reach equilibrium. This would cause the cell to expand in volume. If the concentration difference was large, so that a large volume of water had to move into the cell to equalize concentrations, this process could actually result in the cell bursting. In this case, the external solution would be described as a hypotonic solution. Because most cells have a relatively large number of solutes in their cytoplasm, maintaining an environment that is isotonic to the cell is imperative. For the paramecium, a single-celled protist usually found in freshwater, there is a constant struggle to remove water that flows into the cell in a futile effort to reach equilibrium. In order to prevent the paramecium from rupturing, the paramecium has a contractile vacuole which continually pumps the water out of organism 1 0 .
The Kidneys
Adult kidneys, of which there are two, are approximately 3 centimeters thick, 6 centimeters wide, and 12 centimeters long 1 1 . The kidneys lie in the back abdominal wall. Their major function is to maintain homeostasis within body and they accomplish this by filtering the blood 1 2 . The end process of kidney function is urine, which is produced to maintain the internal body environment through the regulation of certain solutes, such as potassium and sodium ions 1 3 .
The kidneys are arguably one of the most complex of all the organs of the human body. There is an overwhelming amount of information regarding the many different mechanisms of kidney function, including how it regulates solute concentration in urine, how it adjusts itself according to changes in the internal environment, and the physiology that explains how it all works. However, not all of this information is necessary for the proposed curriculum unit. In order to help focus on only what is necessary to teach osmosis and diffusion, only urine formation and the transport of salt, water and glucose will be discussed in detail.
The Nephron and Urine Production
The basic functional unit of the kidney is the nephron. The nephron is a tubular structure that is lined with one layer of epithelial cells. On average, each kidney is comprised of approximately one million nephrons. Blood first enters the kidneys by way of the renal artery. A large volume (1300 mL) of blood enters the kidney every minute. Upon entering the renal artery, blood then travels through the afferent arteriole, which leads into an individual nephron 1 4 .
The Glomerulus
The afferent arteriole directs the blood into a capillary structure called the glomerulus. The glomerulus functions as a high pressure filter. As blood passes through the glomerulus, proteins and blood cells are separated from the plasma. The filtrate, a protein-free plasma solution, ends up in the Bowman's capsule of the nephron. The Bowman's capsule is a membrane bound sac that surrounds the glomerulus. The blood cells and proteins that were not filtered into the Bowman's capsule flow out of the nephron through the efferent arteriole, which carries blood out of the nephron. At this point, the fluid that has been collected in the nephron is referred to as filtrate and is the precursor to what will eventually become urine 1 5 .
The glomerulus is essentially a capillary bed, but it has unique properties that make it possible for simple filtration to occur without the use of any energy (other than the energy that is consumed in the heart to create pressure in the blood). The first is that the pressure in the glomerular capillaries is much higher than an average capillary bed and the second is that the capillaries are much more permeable than most capillary beds 1 6 .
The Proximal Tubule
The filtrate that is produced in the glomerulus is protein-free plasma that contains glucose, amino acids, vitamins, minerals and any other solutes that are contained in the blood. If the nephron's structure allowed for only simple filtration, as occurs in the glomerulus, all of these important chemicals necessary in the body for healthy functioning would be excreted in the urine and would not be available for use. If these molecules were lost, they would have to be continually replaced in the diet. Fortunately, the nephron is able to recover most of these useful, filtered molecules. As the filtrate moves through the remainder of the nephron, these materials are reabsorbed into the body, since a healthy kidney does not produce urine that contains glucose or many of the other solutes found in the filtrate at this early stage of urine production.
The next region of the nephron is the proximal tubule, which receives about 120 mL of filtrate per minute. The proximal tubule is the region of the nephron responsible for the reabsorption of certain solutes, especially glucose. To understand how glucose is moved out of the fluid in the tubule, and eventually reabsorbed into the blood stream by way of the peritubular capillary bed that surrounds the nephrons, it is necessary to follow the movement of sodium ions that are also present at high concentration in the filtrate. There is a high concentration of sodium ions inside of the proximal tubule because of the initial filtration process. But sodium concentrations are kept relatively low in the epithelial cells that line the proximal tubules. Because of the concentration gradient, sodium ions will move from inside the tubule, through the cells and into the interstitial space. Of course, this requires a protein to transport them out of the cell, since ions cannot pass through the lipid bilayer. Sodium ions are transported into the cells and then pumped from the cells to the interstitial space outside of the cells. The peritubular capillary bed that surrounds the nephron has a low salt concentration as well, since salt was originally removed in the glomerulus. Therefore, the sodium ions diffuse into the capillary bed and are reabsorbed. As salt concentrations increase in the interstitial space outside of the proximal tubule, osmotic pressure increases and water moves outside of the proximal tubule as well. Ultimately, this water is also reabsorbed by the peritubular capillaries 1 7 .
Reabsorption of Glucose
Glucose, like most molecules, is not capable of simply diffusing across the cell membrane when there is a concentration gradient. In order for glucose to be reabsorbed by the body after being filtered out of the blood, glucose transport is actually coupled with the sodium ion transport, as described above. Transport proteins in the cell membrane are activated when both glucose and Na + are available. The transporters require both substances to be present and they operate without the use of any energy. As previously stated, Na + concentrations are kept low in the cells that line the proximal tubules. Therefore, sodium will continue to move down its gradient, so long as there is glucose available in order to activate the transport protein. In this mechanism, sodium is transported from the lumen of the proximal tubule into the cells that line the tubule. Once inside, the sodium and the glucose molecules are still at a higher concentration than they are in the surrounding interstitial space and the peritubular capillaries. There are specialized transport proteins on the side of the nephron cells closest to the peritubular capillaries that are capable of transporting glucose separately from hydrogen. Since glucose is moving down its concentration gradient, as is sodium, this movement of molecules is passive and requires no energy 1 8 .
Figure 2. A. Movement of solutes and water from the tubular fluid to the blood is regulated by tubular epithelial cells. B. Tubular epithelial cells transport sodium ions from the luminal fluid to the interstitium. C. Tubular cells have co-transporters that allow glucose to be reabsorbed together with sodium. Reproduced with permission from ref. 2 (Saltzman, 2009).
The Loop of Henle and Reabsorption of Water
At this point in the journey to create urine from blood filtrate, the glucose absorbed in the filtrate has been reabsorbed by the body, as has some salt and some water. However, it is in the body's best interest for the kidney to recover as much water as possible from the filtrate, creating the most concentrated urine. In order to get water to leave the filtrate through diffusion, the area surrounding the nephron must have a high salt concentration. A high salt concentration in the interstitial fluid outside of the nephron will provide a driving force for osmosis, allowing water to be recovered from the filtrate.
The reabsorption of water occurs in many places in the nephron, but especially in the collecting duct, which is the final segment of tubule in the nephron. To allow for the reabsorption of water, the nephron needs a mechanism for creating high solute concentrations (i.e. high osmotic forces) in the fluid outside the collecting duct. This is accomplished by the Loop of Henle. If you could imagine a tube shaped like a U, this is how the Loop of Henle looks. In the descending side of the loop, the cells are water permeable. In the ascending portion of the loop, the cells are not permeable to water. The cells in the ascending tubule also contain pumps that use energy to transport salt into the interstitial fluid surrounding the Loop and collecting duct. These pumps that remove salt from the ascending loop are important because they pump NaCl into the interstitial fluid that surrounds the Loop of Henle. This helps keep the concentration of salt higher in the interstitial fluid outside of the descending loop 1 9 . Because of the presence of high salt concentrations in the interstitial fluid, as the filtrate travels down the collecting duct—on the way out of the nephron—there is a strong driving force for water to diffuse through the cells that line the collecting duct. In these last stages of flow through the tubule, the filtrate can become extremely concentrated 2 0 .
As osmosis continues and more water moves into the interstitial fluid surrounding the loop, the filtrate becomes much more concentrated. This is the instance of osmosis that the curriculum unit will focus on, and rather than get into specifics of osmotic pressure and the role of the ascending loop, the "story" will be simplified and students will simply be informed that the body uses energy—within the Loop of Henle—to keep the area surrounding the Loop of Henle and collecting duct high in salt concentration, in order to encourage osmosis and the reabsorption of water by the body. Of course, this eliminates much of the more subtle detail involved in this process. If this were to be taught in a high school class, the role of the ascending loop would be appropriate to include and could also be used to further explain active transport, as opposed to the passive transport that happens more frequently in the kidney.
Tubular Secretions
The final stage of urine formation removes certain waste products from the blood that could be toxic if they were allowed to accumulate. Tubular secretions are how metabolic wastes, certain drugs, hydrogen and potassium ions and other materials end up in the urine 2 1 . The collection of these materials occurs in the distal tubule. The filtrate now flows into the collecting duct of the nephron. As the filtrate travels through the collecting duct, certain materials may once again be reabsorbed, depending on the current conditions of the body. The mechanism for this response to the internal environment is key to understanding how the kidney maintains homeostasis 2 2 . It is not important to this unit however, and will not be described in detail.
The Collecting Duct and Excretion of Urine
The collecting duct contains the filtrate, which is now referred to as urine. The process described above is occurring in every one of the one million nephrons in each kidney. Each collecting duct at the end of each nephron will funnel into a larger tubule. This happens repeatedly until all of the urine is funneled into the ureter 2 3 . The ureter is a tubular structure that carries the urine from the renal pelvis to the bladder. Once urine volume in the bladder reaches a specific volume, approximately 200- 400 mL, a receptor in the wall lining of the bladder is stimulated, creating the feeling that the bladder needs to be emptied. From the bladder, urine leaves the body through the urethra 2 4 .
Kidney Malfunction
It is the kidney that regulates the concentration of solutes in our blood and maintains the correct balance of sodium, water and other materials 2 5 . The kidney is a finely tuned organ that responds to our constantly changing internal conditions, adjusting and re-adjusting itself to maintain homeostasis. The filtration system of the kidneys maintains the necessary ion levels in the blood. This allows our muscles to function, which in turn keeps our heart functional and allows our diaphragm to contract in order for us to breathe. Additionally, it removes the waste products our body creates, especially urea, which could lead to death if allowed to accumulate. In the event of kidney malfunction, it is not possible to regulate our own blood composition by simply being selective with our diet. When the kidney malfunctions or shuts down completely, the rest of the body soon follows suit.
There are multiple causes of renal failure today, some genetic or rare, and seemingly unpreventable. Examples of these are shock due to fluid loss after an accident or the use of certain necessary medications 2 6 . Currently, however, more than two-thirds of all cases of kidney disease are diabetes and hypertension. Prolonged kidney disease can eventually lead to renal failure. When a person reaches end stage kidney failure, which is defined as the loss of at least 85% of kidney functionality, kidney dialysis is necessary 2 7 .
Hemodialysis
Once a person has reached the point that their kidneys are, for all practical purposes, non-functioning, it is necessary to filter the blood through artificial means to keep the person alive. A solution to renal failure is dialysis, in which an artificial apparatus filters the blood, removing the waste products such as urea. The first dialysis machine was constructed by Dutch physician, Dr. Willem Kolff, in 1941. His machine was fashioned from the cooling system of an old Ford, cellophane wrapped sausage skin, parts from an old downed German airplane and a porcelain bathtub 2 8 .
Today's kidney dialysis machines are obviously made a little differently than Dr. Kollf's, but the basic principle remains: remove a small amount of blood from the body at a time and filter out urea from the blood through simple diffusion. Today's dialysis, however, is able to remove more than just urea from the blood. Kidney dialysis is able to remove other waste products and balance essential ion concentrations 2 9 .
There are now two types of dialysis: hemodialysis and peritoneal dialysis. In hemodialysis, blood is removed from the body and filtered through an artificial membrane 3 0 . In peritoneal dialysis, the actual lining of the abdominal cavity is used as the filter and a solution is injected directly into the abdomen. As blood flows around the peritoneal cavity, diffusion of solutes from the blood occurs directly through the peritoneal membrane and then the fluid is removed later. For the purpose of this unit, only hemodialysis will be used as an example, to simplify the material.
The way that current hemodialysis works is to temporarily remove blood from the body, flowing the blood through a tube surrounded by a carefully selected permeable membrane which is surrounded by a fluid called the dialysate, and then return the filtered blood back into the body 3 1 . Dialysis works simply because molecules naturally want to be in a state of equilibrium and will diffuse through a membrane (assuming the pores are large enough) in order to reach this state. If a hypertonic solution is surrounded by a hypotonic solution, the solute particles will diffuse across the membrane. The dialysate is a solution that has been specially formulated to remove specific materials from the blood before sending the blood back into the body. For example, ideally, little to no urea should be present in the blood when it is sent back into the body. As the blood passes through the tube, which would have pores large enough for urea to pass through, the dialysate would contain no urea. This would cause urea to move through the membrane into the dialysate, thereby reducing the concentration of urea in the blood. Of course, this is a slight oversimplification and there are multiple factors that will determine the rate of diffusion during dialysis. The rate of diffusion, and therefore the success of the dialysis, is dependent of the concentration gradient between the blood and the dialysate, the material used for the membrane and the size and properties of the solute that is diffusing 3 2 .
Morbidity and Mortality of Kidney Dialysis
In the past twenty years there has been a dramatic increase in the prevalence of kidney disease. While kidney dialysis was a huge breakthrough in medicine, it is by no means a perfect solution for treating kidney failure. Morbidity and mortality rates are still high in patients receiving dialysis. Patients that receive dialysis have been shown to have a much shorter life expectancy than average 3 3 .
While all strategies that are being proposed are appropriate for a middle school classroom, they have all been chosen specifically to meet the needs of high risk students that traditionally perform below grade level. The strategies have been chosen to optimize engagement and lend themselves well to differentiation. The main strategies that will be used will be: demonstrations, modeling, hands-on lab activities and problem based learning.
Demonstrations
In the middle school classroom, it is not always possible or wise to let students do certain activities on their own. Demonstrations allow for a quick "gotcha" moment in the classroom without losing all the time with a full lab set-up. The questions that are asked by the teacher and the questions asked by the students determine the value of a demonstration.
Hands-On Labs
There is little question that hands-on learning is beneficial to students and their level of engagement. The hands-on activities being proposed will allow students to witness osmosis and diffusion and to experiment with ways to control it.

Writing Activity
Writing is usually a struggle for students, especially middle school students. In this unit, students will write a story book for small children using their notes and other background information. This will provide them with an opportunity to create their own differentiated product.
Demonstration Lab
Purpose: The purpose of this lab will be to introduce students to osmosis and diffusion using chicken eggs, since they are visible to the naked eye. This demonstration is "the hook" that can be used as a reference point throughout the rest of the unit.
Procedures (for the teacher):
1. Soak 2 chicken eggs in vinegar for 24-48 hours in order to dissolve the shell.
2. Have students measure the mass of each egg after the shell has been removed. (To save time, the teacher could measure the mass and give the data to the students.) Have students record the mass of each egg. Be sure to point out that the egg is a very large cell and it is surrounded by a cell membrane.
3. Create 2 different beakers with each of the following solutions: water and corn syrup and distilled water.
4. Measure the mass of each solution.
5. Place 1 egg into each beaker and let them sit overnight.
6. After at least 24 hours, remove the eggs from the solution.
7. Find the mass of the eggs and the remaining solution.
Questions for students:
1. What happened to the size of the egg when placed in the corn syrup?
2. What happened to the size of the egg when placed in the distilled water?
3. A hypertonic solution is one where there are more solutes compared to the inside of the cell. Which solution was hypertonic?
4. A hypotonic solution is one where there are less solutes compared to the inside of the cell. Which solution was hypotonic?
5. What could you do to make the cell (egg) get larger?
6. What could you do to make the cell (egg) get even smaller?
7. What do you think would happen if you placed the cell in really salty water?
In between the egg lab and The Potato Lab, students should have some form of instruction that covers the specifics of what is able to enter and exit the cell and what equilibrium is. Prior to beginning this lab, remind students that potatoes are living, so they are made out of cells. Students may also need to be reminded that cells contain a number of solutes.
Procedure for students:
1. Cut out 2 small potato cubes that are roughly the same size. (In my classroom, I would cut these myself).
2. Find the mass of each potato cube.
3. In a beaker, pour in 100 ml of water. Add salt in order to make a saturated solution. This will be beaker 1.
4. In another beaker, pour 100 ml of distilled water. This will be beaker 2.
5. Add one potato cube to each beaker.
6. Let the potatoes sit for at least 24 hours.
7. After 24 hours, measure the mass of the two potatoes.
Pre and Post Lab Questions:
1. In which beaker is the solution hypertonic?
2. In which beaker is the solution hypotonic?
3. What happened to the mass of each potato?
4. Explain why the mass of each potato changed using what you know about osmosis.
Writing Assignment- The Story of Pee
Students will receive notes on kidney function and how pee is made. These notes will be used by students in order to make a short book explaining how pee is made. They will trace their lunch, including the water they drink, from the time they eat the food to the digested food particles entering the bloodstream and ultimately the kidneys. This activity can easily be differentiated based on the level of the students. Some students may need additional diagrams and flow charts to help them follow all of the steps.
Dialysis Lab/ Guest Speaker
Prior to this lab, students should briefly be exposed to some of the causes of kidney failure and a short explanation of kidney dialysis. I will also get a guest speaker from the community to come talk to my students. The guest speaker will be someone from a dialysis lab. This will help provide students with some of the background information necessary for understanding the lab.
In the following lab, students will experiment with a simulation of kidney dialysis, using artificial blood and a dialysate. For the purposes of my classroom, we will only focus only the salt, proteins, red blood cells and urea in the blood 3 4 .
1. Students will make "artificial blood" by combining the following ingredients in a large test tube:
1. 2 mL of table salt
2. 2 mL of "protein" (baking soda)
3. Approximately 1 teaspoon of red glitter or sequins to represent red blood cells
4. 2 drops of yellow or orange food coloring to represent urea and other waste
5. Enough warm water to fill the test tube up
2. Place a stopper in the test tube and gently shake the "blood".
3. Obtain a piece of dialysis tubing. Wet one end and tie a knot.
4. Pour the "blood" into the dialysis tubing and tie another knot at the top of the tubing so that it is sealed. This represents the blood that would be removed from the body during dialysis.
5. Place the dialysis tubing into a cup of warm distilled water and let it sit for 10 minutes.
6. At the end of the 10 minutes, test the dialysate to see if it contains each solute. Read each question below for directions on how to determine if the solute left the blood.
a. If any of the urea or waste products left the blood, the dialysate would be orange/yellow. Did the urea leave the blood?
b. Dip the salt indicator strip into the dialysate. Did the salt leave the blood?
c. Dip the chromatography paper into the dialysate. If it turns blue, protein is present. If it remains white, protein is not present. Did the protein leave the blood? (The chromatography paper will not change colors. You could also call it "protein indicator paper".)
d. Observe the dialysate. Did any red blood cells move through the membrane?
Post Lab Questions:
1. Why do you think the protein did not move through the membrane but the salt did?
2. Do you think all of the salt left the blood? How do you know?
3. How would you change the dialysate if you wanted more salt to remain in the blood?
Through the activities proposed above, students should gain a thorough understanding of osmosis and diffusion and how imperative these processes are to the health and well- being of organisms. In addition to teaching students cellular processes, this unit touches on some of the health issues that Americans are facing today, namely diabetes. What I hope that my students will take from this unit is not only an understanding of osmosis and diffusion, but also an appreciation for what their bodies do for them every day. It is my hope that this will encourage students to take better care of themselves and think about the long term consequences of unhealthy living.
Implementing District Standards:
North Carolina Goal 6.01 Describe the cell theory. According to the Standard Course of Study in North Carolina, students are expected to understand the basics of the cell theory. Students should know that cells are the basic units of structure and function. This unit gives students a relevant example of how cell structures are related to their function. This unit also provides a model for how cells provide structure and carry on major functions to sustain life.
North Carolina Goal 6.02- Analyze structures, functions and processes within animal cells. Students are expected to understand the role of cell organelles and the processes which occur inside of every animal cell. This unit teaches students about the role of the cell membrane as well as the removal of waste products.
Bibliography:
Annotated Teacher Resources
Applegate, Edith. The Anatomy and Physiology Learning System . 1995. Reprint, Philadephia: Elsevier, Inc., 2006. This is a great book that reviews all the basics about the kidney.
Callaghan, C. A.. The renal system at a glance . 2nd ed. Malden, Mass.: Blackwell Pub., 2006. This book provides specific details about kidney functioning. It is much more in depth than necessary for middle school, but still a great source of detailed information.
Friedman, Eli A., and Mary C. Mallappallil. Present and future therapies for end-stage renal disease . New Jersey: World Scientific, 2010. This was a great up-to-date analysis of the state of kidney disease treatment. It provided insight and analysis of how kidney failure has been treated and brings up some of the current issues in medicine.
Hill, Lisa. Cells biology . Chandni Chowk, Delhi: Global Media, 2007. This book provides great explanations of osmosis and diffusion and is very easy to understand.
Kapit, Wynn, Robert I. Macey, and Esmail Meisami. The physiology coloring book . 2nd ed. San Francisco: Addison Wesley Longman, 2000. This is an awesome resource for the classroom. While it is very detailed, the basic material can easily be adapted for the classroom.
University of Rochester. "Kidney Dialysis." Life Sciences Learning Center. lifesciences.envmed.rochester.edu/curriculum/SEPAClass/3.TEACHERKidneyDialysis7-23-09.pdf (accessed August 17, 2011). This is a great unit that focuses on teaching about kidney dialysis in more detail.
"National Kidney Foundation." National Kidney Foundation. http://www.kidney.org/ (accessed August 10, 2011). This website provides lots of up-to-date data regarding kidney diseases. It would be a great resource for kids to use.
Rennke, Helmut G., Bradley M. Denker, and Burton David Rose. Renal pathophysiology: the essentials . 2nd ed. Philadelphia: Lippincott Williams & Wilkins, 2007. This book is written more for medical students, but it has some great sections about kidney dialysis.
Rizzo, Donald C.. Fundamentals of anatomy & physiology . 2nd ed. Clifton Park, NY: Thomson Delmar Learning, 2006. This is a tool for learning more background knowledge on the kidney and its structure and function.
Saltzman, W. Mark. Biomedical engineering: bridging medicine and technology . Cambridge: Cambridge University Press, 2009. This is a magnificent resource for up-to-date material on cell biology, kidney function and dialysis.
"Willem Kolff - Telegraph." Telegraph.co.uk - Telegraph online, Daily Telegraph and Sunday Telegraph - Telegraph. http://www.telegraph.co.uk/news/obituaries/4604625/Willem-Kolff.html (accessed July 10, 2011). This website provides some interesting biographical information about the first person to perform kidney dialysis
- 1 Donald Rizzo, Fundamentals of Anatomy and Physiology (Clifton Park: Thomson Delmar Learning, 2006), 42.
- 2 Mark Saltzman, Biomedical Engineering: Bridging Medicine and Technology (Cambridge: Cambride University Press, 2009), Chapter 5, pg 3.
- 3 Wynn Kapit, Robert I. Macey and Esmail Meisami, The Physiology Coloring Book (San Francisco: Addison Wesley Longman, 2000), 1.
- 4 Lisa Hill, Cells Biology (New Delhi: Global Media, 2007), 47.
- 7 Rizzo, Fundamentals , 30.
- 9 Hill, Cells , 48.
- 10 Ibid, 49
- 11 Edith Applegate, The Anatomy and Physiology Learning System (Philadelphia: Elsevier, Inc., 2006), 352.
- 12 Saltzman, Biomedical , Chapter 9, 6.
- 13 Kapit, Physiology , 58.
- 14 Ibid, 58
- 15 Ibid, 58
- 16 Ibid, 59
- 17 Ibid, 60
- 18 Ibid, 60
- 19 Applegate, Anatomy , 357.
- 20 Kapit, Physiology , 67.
- 21 Applegate, Anatomy , 358.
- 22 Rizzo, Fundamentals , 428.
- 23 Kapit, Physiology , 58.
- 24 Rizzo, Fundamentals , 428.
- 25 Saltzman, Biomedical, Chapter 9, 6.
- 26 Helmut G. Rennke, Bradley M. Denker and Burton David Rose, Renal Pathophysiology: The Essentials (Philadelphia: Lippincott Williams and Wilkins, 2007), 28.
- 27 "National Kidney Foundation", accessed August 10, 2011. http://www.kidney.org/
- 28 "Willem Kolff - Telegraph." Telegraph.co.uk, accessed July 10, 2011. http://www.telegraph.co.uk/news/obituaries/4604625/Willem-Kolff.html
- 29 Saltzman, Biomedical , Chapter 16, 9.
- 30 Rennke, Renal Pathophysiology , 45.
- 31 Saltzman, Biomedical , Chapter 16, 9.
- 32 Ibid, Chap 16, 11
- 33 Eli A. Friedman and Mary C. Mallappallil, Present and Future Therapies for End-Stage Renal Disease (New Jersey: World Scientific, 2010), 149.
- 34 "Kidney Dialysis", University of Rochester, accessed August 17, 2011, lifesciences.envmed.rochester.edu/curriculum/SEPAClass/3.TEACHERKidneyDialysis7-23-09.pdf
Comments (1)
Send us your comment

Search My Blog
Very Simple Diffusion and Osmosis Experiment

5 comments:
Good blog. Very easy to understand the point of each piece of text.

How do you make your starch solution and glucose solution?

Since no quantitative data is being collected, there is no need to make solutions of a specific concentration. I put some corn starch in a beaker of water and boil it until it clears up a bit. I put several teaspoons of Karo syrup in a beaker of water and stir until throughly mixed.
where can i find these dialysis tubing??
We order them with our lab supplies. You can also use a plastic sandwich bag.
- Faculty Resource Center
- Biochemistry
- Bioengineering
- Cancer Research
- Developmental Biology
- Engineering
- Environment
- Immunology and Infection
- Neuroscience
- JoVE Journal
- JoVE Encyclopedia of Experiments
- JoVE Chrome Extension
- Environmental Sciences
- Pharmacology
- JoVE Science Education
- JoVE Lab Manual
- JoVE Business
- Videos Mapped to your Course
- High Schools
- Videos Mapped to Your Course
Diffusion and Osmosis
Instructor prep, student protocol.
- Diffusion in Agar
- Movement of Molecules Across a Semi-Permeable Membrane
- NOTE: In this exercise, you will be given agar containing an indicator chemical called phenolphthalein. When phenolphthalein is exposed to the normal alkaline conditions in the agar, it will look pink. But when it is exposed to neutral or acidic conditions, it changes from pink to clear. You will make different size and shaped agar cubes as a model for cells to study the impact of cell size and shape on diffusion rate.
- To make the first set of cells, measure out and cut a small cube of agar where each side measures one centimeter.
- Next, measure and cut out a medium cell cube with sides of 2 cm and a large cell of 3 cm on each side.
- Knowing the length of the sides of your cube cells, calculate their surface area using this equation, where lower case a represents the length of the sides: Surface Area = 6a^2
- Record these values in the appropriate column in Table 1. Click Here to download Table 1
- Then, use the same length value and the equation below to calculate the volume of each cube and add these to the table: Volume = a^3 HYPOTHESES: The experimental hypothesis might be that the acid will diffuse completely to the center of the small cell faster than the medium and large cells. The null hypothesis could be that the acid will diffuse to the center of the small and two larger cubes at around the same time.
- Add 100 mL of 0.1 M HCl to each of the three 400 mL beakers to make the diffusion baths.
- Working in a team, have one experimenter ready with the timer and the second and third experimenters ready to drop each cube into one of the beakers.
- When the first experimenter says go, simultaneously drop all three cubes into their respective beakers and start the timer.
- Observe carefully until one of the cubes becomes completely clear or 10 min have passed.
- Stop the timer, remove the agar cubes from the beakers and place the cubes into a Petri dish.
- Make a note of which of the three cells became clear or had the smallest remaining pink area. Then, also note which cell had the most remaining pink agar.
- Next, in Table 1, calculate the surface area to volume ratio for each cell. Surface Area: Volume Ratio = (surface area)/volume
- As the cell size increases, note whether the surface area to volume ratio increases or decreases. Also consider whether this correlates with your observation of the depth of diffusion into the agar cells. If cells rely on diffusion to deliver essential nutrients and molecules to the whole cell, discuss with your group if it would be better to have a smaller or larger surface area to volume.
- Now, with the remaining agar, cut three rectangular shaped blocks of different sizes and record their length, width, and height. This will test what happens when the shapes of cells are different.
- Calculate the surface area of your rectangular cells using the formula below, where length is l, width is w, and height is h. Surface Area = 2lw + 2lh + 2wh
- Then, calculate the volume of your rectangles using this formula: Volume = l * w * h
- Repeat the experiment by dropping the new shapes into the hydrochloric acid solution for 10 min or until one cube becomes completely clear.
- Remove the cell shapes from the solutions and observe the depth that the hydrochloric acid diffused into each of these cells, and which shapes have the smallest and largest remaining pink areas not reached by the solute.
- Using the surface area and volume data you recorded for your rectangular shapes, calculate the surface area to volume ratio of these cells. Surface Area: Volume Ratio = (surface area)/volume
- Consider whether these values correlate to which cells had the most and least complete diffusion. Additionally, discuss with the group whether these rectangular cells displayed a similar or different pattern of diffusion to that observed with the cube shaped cells, and what this might mean.
- Before beginning the experiment, add 250 mL of distilled water to each of four 1 L beakers.
- Then, label the beakers from 1-4, and add 0.5 mL of iodine to the first beaker. HYPOTHESES: In this experiment, the experimental hypothesis is that some of the solutes will be able to pass through the dialysis tubing membrane and others will not. The null hypothesis is that there will be no difference in the ability to diffuse through the dialysis tubing membrane between the solutes.
- To prepare the dialysis tubing, remove the pieces one at a time from the distilled water bath and tie a tight knot at one end of each tube. These tubes, when filled, will act as model cells with the dialysis tubing acting like the semipermeable membrane.
- Add 10 mL of starch solution to the first tube and tie off the open end, making sure to leave space in case the tubing expands during the experiment.
- Then add 10 mL of the NaCl and dextrose solutions to the second and third pieces of tubing, respectively, and tie off both tubes, again, leaving space in case of expansion.
- After adding 10 mL of distilled water and tying off the fourth tube, weigh each of your model cells.
- Record the initial weight values in grams and the colors of the starting solution in each tube in the appropriate columns of Table 2. Click Here to download Table 2
- After quickly rinsing the outside with tap water, place each piece of tubing in its corresponding beaker for 1 h at room temperature. NOTE: For example, the starch solution tube should be placed into the beaker containing the iodine.
- At the end of the diffusion period, weigh the tubes again.
- Then, observe the tubes carefully, noting any color changes.
- Record all of these data in Table 2.
- Next, to perform a Benedict's Reagent test for simple sugars, make a water bath by adding 250 mL of water to a 600 mL beaker and placing it onto a hot plate.
- Set the plate to high, to boil the water.
- Label two new glass test tubes as H 2 O and dextrose, respectively.
- Use a graduated cylinder to transfer 1 mL of solution from the water and dextrose beakers into the corresponding test tubes.
- Then, add 2 mL of Benedict's Reagent to each tube.
- Once the water is boiling, place each test tube into the water bath for 3-5 minutes.
- After this time, note the color of the solution in each tube.
- Then use this key to assess whether the test is positive or negative and record these data in the appropriate column in Table 2. Click Here to download Figure 1
- First, look at the mass of your four dialysis tube cells at the beginning versus the end of the experiment. Calculate the change in mass for each of the four cells and plot it onto a bar chart.
- Note which cells demonstrated the most change, and whether any of the cells appeared visibly different in size.
- For the experiment with the starch and iodine indicator, note whether there was a color change in the fluid in the artificial cell. Also consider whether there was a color change in the water in the beaker, and what both of these observations say about the properties of the dialysis tubing membrane.
- Finally, in the Benedict's Reagent test for dextrose, note whether this simple sugar was able to pass through the semipermeable membrane of the “cell” into the water in the beaker. Discuss with the class which of the molecules you think could and could not pass through the semipermeable membrane.

Complete Guide to Dialysis Technician Training
Considering dialysis technician training? Gain clarity on the qualifications, educational programs, certification process, and hands-on experience you need in our actionable guide. Step into a crucial healthcare role and make a difference in the lives of those battling kidney failure, starting with the right training.
Get information on Hemodialysis Technician programs by entering your zip code and request enrollment information.
- Dialysis technicians are vital healthcare professionals who operate and maintain dialysis machines, support patients emotionally, and ensure the efficacy of treatments during dialysis procedures.
- To become a certified clinical hemodialysis technician, one must complete accredited training programs, pass certification exams, and gain hands-on experience through externships.
- Continual professional development is required for dialysis technicians, including regular recertification, completing continuing education hours, and actively participating in professional associations and networking.
The Importance of Dialysis Technicians
In the medical world, dialysis technicians often function as unsung heroes. They ensure the well-being of patients suffering from kidney failure by meticulously delivering dialysis treatments. They are trained to maintain and operate kidney dialysis machines, prepare the patient for the procedure, and monitor their health during the process.
But their role is not just technical. Dialysis technicians not only perform their duties but also provide emotional support patients need, addressing their concerns and making the dialysis experience as comfortable as possible. This aspect of their job is often as important as the technical side; after all, their patients are dealing with a potentially life-threatening condition.
The importance of dialysis technicians is recognized at a national level during the National Dialysis Technician Recognition Week. This event is a celebration of their significant contributions to patient health and the broader dialysis community.
Pathway to Becoming a Certified Clinical Hemodialysis Technician
The journey to becoming certified hemodialysis technicians demands commitment, determination, and a thirst for knowledge. The first stepping stone is enrolling in an accredited dialysis technician program. These programs are evaluated for education quality, ensuring that the curriculum meets industry standards and equips you with the necessary skills.
The hemodialysis technician training can be completed in as little as twelve months, preparing you for three industry-recognized certification exams. Read more about cost and duration of dialysis technician training . These exams test your competency and declare you fit to take on the responsibilities of a dialysis technician.
However, the journey extends beyond that. To become a well-rounded professional, you will need to gain hands-on experience . This is where an externship comes in. An externship allows you to apply your learned skills in real-world settings, giving you a taste of what it’s like to work in the field.
Dialysis Tech Training Components
The training process for dialysis technicians is multifaceted, encompassing several critical components. It includes learning renal physiology and medical terminology, understanding dialysis equipment and procedures, gaining practical experience through clinical hours, and procedure patient training.
We should explore these components in further detail.
Renal Physiology and Medical Terminology
Renal physiology is the cornerstone of dialysis technician training. It’s vital to have a clear understanding of kidney function and their role in maintaining the body’s homeostasis. This understanding helps dialysis technicians comprehend how kidney diseases, such as renal failure, affect the filtration of blood and the overall health of the patient.
Furthermore, troubleshooting problems with dialysis machines and providing emergency care when necessary, especially in cases of dialysis acute renal failure, demands a strong knowledge of renal physiology. The focus is not solely on the kidneys but the entire interconnected system.
Alongside renal physiology, medical terminology is another pillar of the training. Such knowledge equips dialysis technicians with the ability to interact effectively with the healthcare team and grasp the complexities of renal replacement therapies.
Dialysis Equipment and Procedures
A key part of dialysis technician training revolves around mastering the use of dialysis equipment, including those used for at home dialysis treatment. This involves understanding the setup and takedown of hemodialysis machines, as well as kidney dialysis machines preparation, integral to effective dialysis treatment.
Moreover, technicians are also skilled in troubleshooting machines and are prepared to provide emergency care if needed, forming a critical part of the dialysis safety protocol. They are responsible for disinfecting supplies, sanitizing equipment, and communicating any patient concerns to nurses, highlighting their importance in the broader care team, including dialysis teams administration.
In addition to the machinery, dialysis technicians must also be proficient with the extracorporeal blood circuit, which is central to the blood purification process during dialysis. They must understand the purpose and proper use of various components involved in the dialysis water treatment, such as:
- Water softeners
- Carbon tanks
- Reverse osmosis systems
- Ultraviolet light
Dialysis Tech Training: Practical Experience and Clinical Hours
While classroom learning is crucial, the application of these lessons in a real-world context presents a different challenge. That’s where clinical hours come in. They provide dialysis technicians with invaluable hands-on experience and the opportunity to test their skills in an actual clinical setting.
Completion of these clinical hours during the externship is crucial for mastering the skills necessary to be a successful hemodialysis technician. It’s this practical experience that truly prepares you for the responsibilities you will face in the field.
Ultimately, dialysis technician programs are designed with a blend of both classroom instruction and clinical practicums . This combination ensures that you are equipped with both the theoretical knowledge and the practical skills needed to kickstart your career in this rewarding field.
Continuing Education and Professional Development
Continuing education and professional development extend beyond buzzwords; they form key components of a dialysis technician’s career trajectory. As the field of dialysis care continues to evolve, so too must the knowledge and skills of those providing the care. Keeping up-to-date with latest industry practices, advances in technology, and research findings is paramount for delivering top-notch patient care and sustaining certification.
Recertification and Skill Enhancement
For a dialysis technician, professional development continues even after achieving initial certification. In fact, recertification is a crucial part of maintaining your standing as a competent professional. Dialysis technicians must:
- Obtain a formal dialysis technician certification from a nationally recognized organization
- Recertify every three to four years
- Complete at least 30 hours of continuing education
- Prove at least 3000 hours of work experience in the dialysis field within the last certification period
This process ensures that dialysis technicians stay up-to-date with the latest advancements in the field and continue to provide high-quality care to their patients, making dialysis technician jobs essential in the healthcare industry.
However, if you don’t have the required work experience but have a high school diploma and have worked as a dialysis technician within the past 18 months, you can still recertify by retaking the CCHT examination.
Professional associations like the National Association of Nephrology Technicians/Technologists (NANT) provide resources for recertification and continuing education opportunities. By attending events organized by NANT, you can earn up to 35 contact hours towards your recertification requirements.
Networking and Professional Associations
In addition to continuing education, networking and involvement in professional associations are also crucial for a dialysis technician’s career development. Here are some ways to enhance your professional network and stay updated on industry advancements:
- Attend conferences and events organized by nephrology organizations
- Join professional associations for dialysis technicians
- Participate in online forums and discussion groups
- Connect with colleagues and industry experts on social media platforms
- Volunteer for committees or leadership positions within professional associations
These activities not only help you expand your network but also provide opportunities to earn contact hours for recertification.
Such events provide platforms for you to engage with peers, exhibitors, and certification boards, fostering a collaborative community. The Networking lounges and Dialysis Solutions Center at NANT events, for instance, are designed to facilitate these interactions.
Moreover, professional associations grant access to a repertoire of industry journals like the American Journal of Kidney Disease, Kidney Medicine, and the Journal of Renal Nutrition. Such resources contribute to an all-inclusive knowledge base, bolstering the ongoing learning and professional advancement of dialysis technicians, especially when dealing with patients suffering from end stage renal disease.
Becoming a successful dialysis technician requires a comprehensive education, hands-on training , and a commitment to continuous learning and professional development. From understanding renal physiology and mastering dialysis equipment to gaining practical experience and engaging in professional networking, each step of the journey is crucial to developing a rewarding career in this life-saving field.
So, are you ready to embark on this fulfilling journey? Remember, as a dialysis technician, you’re not just earning a living; you’re making a difference in the lives of those who need it most. Read more about vocational training here .
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- v.8(10); 2022 Oct

Transformer maze for the evaluation of the learning and memory in rodents
Associated data.
Data will be made available on request.
Currently, different types of mazes are used to assess spatial learning and memory of rodents. The typical disadvantage is the inability to separate and exclude coincidences of the result of random choice with the correct one. The other problem is the impossibility of knowing whether the animal is guided by particular cues of the environment, or a map.
Our novel transformer maze can be used to test learning and memory of rodents and their navigation. It is a multiple T-maze with passages in the interior walls. Its modular design allows to quickly change routes. The task can include external signals; for example, the colors of the interior walls, or it can be used without any cues.
We compared Wistar and dopamine transporter heterozygous (DAT-HET) rats’ behavior in this novel paradigm using the black color of the wall as a cue. Entering a cul-de-sac compartment was considered an error. While Wistar rats learned the rule abruptly with the total number of errors rapidly decreasing, DAT-HET rats’ errors decreased gradually. We suppose that this reflects different strategies: insightful learning behavior is typical for Wistar rats, and trial-and-error learning is typical for DAT-HET rats.
Comparison with existing methods
The diversity of the chains of choices gives us confidence that trained animals do not make a choice randomly and are guided precisely by the cues. Moreover, we propose to use the same arena for a task with route-based navigation without any cues, and for a task with a visible and invisible feeder to study the path integration navigation within one box.
Conclusions
We suggest that the transformer maze could be a valuable tool for behavioral and pharmacological research to study learning, memory and navigation mechanisms.
Maze; Rat; Behavior.
1. Introduction
Mazes are commonly used to study animal behavior, such as spatial navigation and memory, and the brain mechanisms underlying such behavior. Some methods are more focused on allocentric navigation ( Morris, 1984 ) and some on egocentric navigation ( Vorhees and Williams, 2016 ). However, it is obvious that any navigation relies on the integration of both mechanisms. One of the most widely used methods for spatial memory testing is the Morris water maze, where the animal is trained to find a platform hidden underwater ( Morris, 1981 ). Rats simultaneously use several strategies to solve spatial navigation tasks, and find a visible or hidden platform in a swimming pool ( Whishaw and Mittleman, 1986 ). Another model is the Cincinnati water maze which focuses on remembering the path. The animal makes a chain of alternative choices leading to the platform. However, the maze layout is constant, which limits its use ( Vorhees and Williams, 2016 ). One limitation of water mazes is that not all rodents solve cognitive tasks well when under stressful conditions, and being in the water, they are stressed. The Barnes maze is similar to the Morris water maze, but less stressful for animals, as it’s an overland maze. In this maze, rats tend to try to get out of open space and bright light. This maze consists of an elevated circular platform with 18 holes along its edge. Rats search for an escape hole using distal visual cues to determine the spatial location of the escape hole ( Barnes, 1979 ). Another advantage of this labyrinth is that there is no need of food deprivation. Food reinforcement is used in other non-swimming mazes. The T-maze, the Y-maze and radial arm mazes allow measurement of decision making abilities and short-term memory ( Swonger and Rech, 1972 ; Olton and Samuelson, 1976 ; Zhang et al., 2018 ). One of the disadvantages of the Barnes maze and radial mazes isthe possibility for animals to use non-spatial strategies, like a serial strategy. The paired-associate learning was explicitly designed to investigate the recall of foodcache locations and sequential memory of two paired associations: flavors of food and their spatial locations ( Day et al., 2003 ; Tse et al., 2007 ).
We use a two-ring maze, which is a looped T-maze, to study neural mechanisms of decision-making ( Filatova et al., 2015 ). The disadvantage of a bilateral choice is the inability to distinguish random choices from a correct one, which affects the results of the experiment. It always remains a guess what strategy the animal uses to solve the problem. The Hebb-Williams maze provides different routes between fixed start and finish points, but the set of maze layouts is not equivalent in terms of route difficulty levels ( Hebb and Williams, 1946 ; Pritchett and Mulder, 2004 ).
Different navigational systems are mediated by different neural networks ( O'Keefe and Nadel, 1978 ; Buzsáki and Moser, 2013 ; Sherrill et al., 2013 ). One of the methodical goals in investigating navigation is to model tasks which require different navigation strategies ( Vorhees and Williams, 2016 ). In nature, all navigation systems overlap extensively. To navigate, animals simultaneously use external cues, like distal landmarks located outside, and internal determinants such feedback from limb movements, direction and turns, and also signposts.
The aim of the current study was to develop a maze which can be used to test spatial learning and memory of rodents and different types of navigation. Its modular design allows for creation of different routes, comparable in difficulty level. Moreover, the task can either include external cues or not. Thus, the maze can be used to assess both allocentric and egocentric navigation.
We decided to compare cognitive and motor performance in the new transformer maze using the dopamine dysfunction model rats. It has been shown that striatal dopamine (DA) dysfunction induces spatial information processing deficits ( De Leonibus et al., 2007 ), and neostriatal DA modulates, in both egocentric (route-based) and allocentric (spatial, map-based) learning ( Braun et al., 2012 ). Manipulation of the dopamine transporter (DAT) gene in animal models results in delayed clearance of DA and down-regulation of its receptors, leading to behavioral abnormalities. Genetically-modified rats and mice that lack DAT are hyperdopaminergic, and often are used as models for attention deficit hyperactivity disorder, obsessive-compulsive disorder, addiction and mania ( Cinque et al., 2018 ).
Also, we used DAT-heterozygous (DAT-HET) and Wistar rats to compare their learning patterns and navigation in the novel task, using the color of the interior walls as a cue in the new transformer maze.
2. Material and methods
2.1. apparatus, 2.1.1. design №1.
The maze is made of opaque white plexiglass. The first design of the rat maze is a square 42 cm × 42 cm arena with 30 cm high walls. The maze consists of 9 square compartments (3 × 3) divided by interior partitions. Each compartment is a 14 × 14 cm square, formed by removable barrier walls. With the barriers’ position changed, the maze is reconfigured for each trial. Removable barriers are fixed using grooves on the inner surface of the side walls of the maze, and on the 4 columns located in the corners of the central square compartment. Two different types of interior barriers are used to build the routes of the maze. There are solid white barriers and barriers with arch-shaped openings for animal passage that are white on one side and black on the other side, so that the black color is used as a cue, and there is only one black wall with a hole in each compartment. The route is arranged in such a way, that each maze compartment is either a cul-de-sac or a correct choice. The first compartment, where the rat is placed at the beginning, has two passages, one through the black barrier and one through the white barrier. Choosing the black passage leads to the next compartment with the next choice. Choosing the white passage leads to the cul-de-sac compartment which has only one black passage for return. Figure 1 shows one possible route.

Maze schematic. Example of one possible route. 1 – external walls, 2 – interior walls, 3 – feeder, 4 – arch passage. The gray arrow shows the correct path to the finish compartment with positive reinforcement. The feeder is mounted on the black side of the barrier wall 10 cm above the floor.
The modular design allows us to build 4 different routes in this maze. All these routes ( Figure 2 a–d) are of the same length, and consist of the same number of choice compartments (four), cul-de-sac compartments (four), and two turns per route.

Four different routes. Ch1 – first choice compartment, F – finish compartment with a feeder, C – cul-de-sac compartment, Ch – choice compartment. The white circle - passage through the white barriers, the black circle - passage through the black barriers. The grey line shows the correct route to the feeder.
The position of the first choice compartment and finish compartment relative to the external environment can be changed by rearranging the partitions or rotating the maze. Figure 3 shows the options for turning one of the routes ( Figure 3 a1) by 90 ( Figure 3 a2), 180 ( Figure 3 a3) and 270 ( Figure 3 a4) degrees.

Four options for one route (a1) after a 90 (a2), 180 (a3) or 270 (a4) degrees rotation. Ch1 – first choice compartment, F – finish compartment with a feeder, C – cul-de-sac compartment, Ch – choice compartment.
2.1.2. Design №2
The second sample of the rat maze is a square 56 cm × 56 cm arena separated into 16 square compartments (4 × 4) by interior partitions. Each compartment is also a 14 × 14 cm square.
White and black interior barriers are used in this maze, with the black color used as a cue. This design allows us to build many different routes of the same or different length, consisting of the same or different number of choice and cul-de-sac compartments. Moreover, some compartments may not have an entrance and may remain unused, or may have a through-passage without any choice. This design gives an opportunity to add a separate start chamber to our 3 × 3 maze ( Figure 4 ).

An example of a 3 × 3 maze with an additional start compartment built in a 4 × 4 maze. S-start compartment, Ch1 – first choice compartment, F – finish compartment with a feeder, C – cul-de-sac compartment, Ch – choice compartment. Crosses indicate inaccessible compartments.
In this case, the start chamber has only one black barrier with an opening (passage) to the first choice compartment. Using this kind of a start chamber helps to avoid the impulsive choice, which is possible in the first choice compartment in the 3 × 3 maze.
Any new longer routes can be used for testing after learning in a 3 × 3 maze. Figure 5 (a, b) shows two examples of routes with six choice compartments and three turns.

Examples of the test routes in a 4 × 4 maze, a-test1, b-test2. S – start compartment, Ch1 – first choice compartment, F – finish compartment with a feeder, C – cul-de-sac compartment, Ch – choice compartment. Crosses indicate inaccessible compartments.
For illustration, the video of the route assembling and test execution in the 4 × 4 maze is represented (Supplemental file video 1).
2.2. Optional equipment
The maze is equipped with a video camera located above. Recording and tracking analysis is carried out by a blinded observer. Tracking animal coordinates at each point of time allows analysis of the trajectory, timing and errors. During subsequent analysis, the video recording was viewed by the experimenter to eliminate tracking errors; episodes of rear and head entrances were also manually noted. Returns and visits to the cul-de-sac compartments were counted as errors.
2.3. Subjects
The subjects used in this study were 10 outbred Wistar rats and 10 DAT-HET 4-month-old male rats. Wistar rats were bred in house at the Sechenov Institute of Evolutionary Physiology and Biochemistry. DAT-HET rats were obtained from the Institute of Translational Biomedicine, St. Petersburg State University, St. Petersburg, Russia. The rats, initially weighing between 200-270 g (Wistar) and 250–320 g (DAT-HET) were group housed (5 rats per cage). Animals were maintained on a 12/12 h light/dark cycle at a constant temperature of 21 ± 1 °C. Behavioral testing was carried out during the light phase.
All experimental procedures were conducted in accordance with European, national, and institutional guidelines for animal care (EU Directive, 2010/63/EU), and were performed in conformity with the Ethics Committee for Animal Research of the Sechenov Institute of Evolutionary Physiology and Biochemistry, Saint-Petersburg, Russia.
2.4. Training procedure
Before the training, all animals were food-restricted and kept at 90% of their free-feeding body weight. During the whole duration of training, the rats’ weight was measured in order to monitor and control the level of food restriction.
One peeled sunflower seed per trial was used as a food reward.
At the beginning of training, an animal is placed in one compartment with solid walls without any exits, with a feeder attached to the black wall by double sided tape. The rat is taken out after emptying the feeder. When food reinforcement is replaced, the procedure is repeated. Usually less than 10 trials are needed to habituate rats to the feeder and type of reinforcement.
During the next experimental session, an animal is placed in the start compartment of the maze and the reinforcement is placed in the feeder in the finish compartment. After consumption of reinforcement, the animal is removed and placed into an individual cell for 2–5 min to wait for the next trial. If the rat does not move and does not leave one compartment within 10 min, it is removed and the trial is considered a failure. The test is also considered not complete if the rat moves in the maze for 10 min but does not consume food reinforcement. Then the route or position of the route is changed, and a rat is placed again in the start compartment. During subsequent experimental sessions, the rat is offered other maze configurations, trials are carried out after maze rotations. Thus, different tests are carried out every day, using different routes in different positions. Rotating the maze allows exclusion of distal environmental cues. The final goal is to get each rat acquainted with all 4 routes in 4 different positions. A total of 16 maze variants were used during training (all routes a, b, c, d ( Figure 2 ) with a start compartment ( Figure 4 ) and with 4 rotation options). Figure 6 shows the protocol of successive trials during the 5 learning sessions ( Figure 6 (a–e)) and one testing session ( Figure 6 f). Trials are presented so that during one experimental session there are at least two different routes from one starting position. After the learning sessions in all modifications of the 3 × 3 maze, rats undergo two final tests with unfamiliar routes in the 4 × 4 maze (test 1 and test 2). These routes are longer than the ones used for training, and include more turns ( Figure 5 a, b). Each rat is given one attempt at 2 test routes.

The protocol of successive trials, (a–e)-training sessions, f-final tests session. S-start compartment, Ch1-first choice compartment, F-finish compartment with a feeder, C-cul-de-sac compartment, Ch-choice compartment. Crosses indicate inaccessible compartments.
Between trials, the floor and walls are cleaned by 70% ethanol to minimize the possibility of any marks being used as reference points. It should be noted, that it is also possible to obtain the same route placement options in space without turning the maze by changing the start and finish compartments' placement and replacing barrier walls. It takes more time, but in this case, you can be sure that the rat did not use any external cues except the walls’ color.
2.5. Analysis
For the analysis, a custom-written “seeker” program (Shevelev_pro) was used. This software analyzes movement tracks and calculates the number of visits to the cul-de sac and choice compartments, the number of returns, time spent in each compartment, total distance traveled and time to reach the feeder. It is important to note, that visits and peeps into the compartments are distinguished. The rat is considered to have entered the next compartment, if two of the rat's limbs cross the border. Just the rat's head crossing the border is considered as peeking. Error-free execution does not involve returns and visits to the cul-de-sac compartments.
The track can be drawn manually while watching the video, or automatically if animal recognition is used. Behavioral acts such as standing upright, peeking, grooming and defecation are also manually marked. If the rat has not reached the feeder in 10 min, the test is considered a failure and excluded from analysis.
2.6. Statistical analysis
Analyses were performed using Statistica software, version 8.0, StatSoft, ink, Tulsa, USA. A Shapiro-Wilk test was used to assess the normality of data distribution. Because of the non-normal distribution of some parameters, the significant differences in parameters between groups of the Wistar and DAT-HET rats were identified using the nonparametric Mann-Whitney U test. To compare repeated measures of learning parameters in the same animals, nonparametric Wilcoxon Matched Pairs Test was used. The chi-square statistical procedure for a 2 × 2 research design was used to test for differences in the number of errors and correct executions in different groups. Differences were considered statistically significant at p ≤ 0.05. Data is presented as mean ± standard error of the mean (SEM).
5 training sessions and one test session were performed with Wistar and DAT-HET rats. The first training session consisted of 4 trials, the rest of the training sessions had three trials. The test session included two trials. Each time, the mazes were new or in a new position. Outcomes of each trial were combined for each animal. Examples of one rat's tracks in different mazes during the training are shown in Figure 7 .

The superposition of the rat tracks and maze schemes. Samples of the same rat in different routes at the beginning (a), middle (b) and the end of training (c). S – start compartment, F – finish compartment. Crosses indicate inaccessible compartments.
Out of a total of 180 trials of 10 Wistar rats, only two rats did not complete the task within 13 trials; in the DAT-HET group, only 5 trials were not completed by three rats. So, out of the total number of 360 trials, only 5% were not finished, which did not affect final learning outcomes, since there were no such cases in the final tests, during which all rats reached the finish and got food reinforcement. Analysis of the number of the error-free executions, when the animal did not visit cul-de-sac compartments and there were no returns, was carried out for each test session. Figure 8 shows the percentage of error-free executions. Significant differences were observed for the groups in the last training session (t5) (p = 0.0003, Chi-square test) and the final test session (t6) (p = 0.0003, Chi-square test).

Error-free task execution. Ordinate - percentage of the error-free executions to a total number of the trials. Abscissa – experimental sessions (training sessions – t1–t5, final tests – t6). Statistical significance ∗∗∗ – p < 0,0001 (Chi-square test).
Wistar rats have abruptly improved their accuracy levels, in contrast to DAT-HET rats, only about 10% of which trials performed the task accurately both during the training and during the final testing. More than 60% trials of Wistar rats had no errors during the final test. If we reduce the criteria so that one error (one visit to a cul-de-sac or one return) per trial is allowed, differences between the groups become significant only in the last training session ( Figure 9 ) (p = 0.019, Chi-square test). The percentage of error-free and error-one tests for Wistar rats reached 85% and 70% for DAT-HET rats.

The task executed without mistakes or with one mistake. Ordinate – trials with ≤1 error, % to the total number of the trials. Abscissa – experimental sessions (training sessions – t1–t5, final tests – t6). Statistical significance ∗ – p < 0.05 (Chi-square test).
The main characteristic of the learning curve is the change in the total number of errors. Figure 10 shows the average number of visits to cul-de-sac compartments for each experimental session for rats of both groups.

Number of visits to the cul-de-sac compartments. Ordinate – number of errors (mean ± SEM). Abscissa – experimental sessions (training sessions – t1–t5, final tests – t6). Statistical significance ∗∗ – p < 0.001 (differences between groups – Mann-Whitney U test and between different sessions – Wilcoxon test).
Wistar rats demonstrated a significant decrease in the number of errors during the 5th learning session, in contrast to DAT-HET rats (t4–t5, p = 0.008, Wilcoxon test ) . At the same time, there were significant differences between strains in their performance during the last training session and the final test (p = 0.002 and p = 0.002, Mann-Whitney U test).
The average velocity of rats’ movement was analyzed ( Figure 11 ). A significant increase in velocity was detected during the learning phase, and it happened earlier in the Wistar rats (t3–t4, p = 0.0002, t4–t5, p = 0.013, Wilcoxon test) and later in the DAT-HET rats (t4–t5, p = 0,004, Wilcoxon test). There was a trend towards higher velocity in Wistar rats in comparison with DAT-HET rats in the t5 (p = 0.057, Mann-Whitney U test).

Velocity analysis results. Ordinate – average velocity (mm/s) (mean ± SEM). Abscissa - experimental sessions (training sessions – t1–t5, final tests – t6). Statistical significance ∗ – p < 0.05; ∗∗ – p < 0.001; ∗∗∗ – p < 0,0001 (differences between different sessions -Wilcoxon test).
There are 4 types of compartments in the maze: start, finish, cul-de sac and choice compartments. An animal has to decide which passage to choose in the choice compartments. Analysis of the time spent in the choice compartments showed a sharp decrease of the decision-making time starting with the t4 learning session in Wistar rats (t3–t4, p = 0.001, Wilcoxon test) in contrast to the DAT-HET rats (t4, p = 0.014 and t5, p = 0.0005, Mann-Whitney U test) ( Figure 12 ). Only successful trials were included in this analysis. In the final test session, the decision-making time did not differ between the groups.

Decision-making time (s). Ordinate – average time, seconds (mean ± SEM). Abscissa – experimental sessions (training sessions – t1–t5, final tests – t6). Statistical significance ∗– p < 0.05; ∗∗ – p < 0.001; ∗∗∗ – p < 0,0001 (differences between groups -Mann-Whitney U test and between different sessions -Wilcoxon test).
Analysis of the number of rears ( Figure 13 ) showed its decrease by the end of the training in Wistar rats (t4–t5, p = 0.024, Wilcoxon test), in contrast to the DAT-HET rats, where significant differences between the groups were observed on day 5 of training and during final test session (t5, p = 0.005 and t6, p = 0.036, Mann-Whitney U test).

Rears. Ordinate – average number of rears per second (mean ± SEM). Abscissa – experimental session (training sessions – t1–t5, final test day – t6). Statistical significance ∗ – p < 0.05; ∗∗ – p < 0.001 (differences between groups -Mann-Whitney U test and between different sessions -Wilcoxon test).
The number of head entrances did not differ between groups during training sessions, however, Wistar rats peeked significantly more than DAT-HET rats during the final tests (t6, p = 0.0044, Mann-Whitney U test) ( Figure 14 ).

Head entrances. Ordinate – average number of head entrances per second (mean ± SEM). Abscissa – experimental sessions: training sessions – t1–t5, test day – t6. Statistical significance ∗∗ – p < 0.001 (differences between groups – Mann-Whitney U).
4. Discussion
4.1. training procedure and its advantages.
In this paper, we present a new technique as an alternative to the currently available mazes. The transformer maze is a compact, land-based and multifunctional device. It allows us to quickly change the route and orientation of the maze, to use different types of cues, or avoid them. When we use our cue (black side of the barrier with the arch) and present non-recurring routes, we make sure that the cue serves as the only reference point. Protocol presented in this paper provides fast and reliable learning. Animals can receive different tasks under identical conditions. In the current experiment, training consisted of 5 learning sessions and one test session. On average, total time spent by one animal in the maze during the whole experiment was about 40 min. Taking into account the time it takes to rebuild routes and cleaning, the needed experimental total time spent can be calculated.
Analysis of the time spent in the maze and total distance traveled allows us to assess memory and the level of general locomotor activity. Analysis of rears, head entrances, grooming, and defecation can indicate the level of anxiety and exploratory behavior ( Seibenhener and Wooten, 2015 ). Time spent in cul-de-sac compartments and choice compartments may indirectly indicate different search strategies.
4.2. Comparison Wistar and DAT-HET rats learning patterns
Comparison between Wistar and DAT-HET rats did not reveal differences at the initial stages of learning in the 3 × 3 maze, but showed significant differences at the late training stage and during final tests. DAT-HET rats’ inferior performance in new conditions may be associated with worse memory or lower cognitive flexibility. The final test does not just involve solving the same problem while taking a new route within the same space, but forces the animal to make more steps to make the choices. If there were 4 steps in the training mazes, in the test trials there were 6 steps. The essential characteristic of short routes in the 3 × 3 maze is that they always pass through the central compartment of the maze, and this passage is always straight and never includes a turn. Test routes in the 4 × 4 maze include longer sections with no turns. Reactions to the met with these new route features also may indicate flexibility.
Spatial navigation requires egocentric and allocentric strategies, meaning that the animal is guided simultaneously by the cues and features of the routes. These mechanisms are mediated by various neurochemical pathways ( Rubio et al., 2012 ; Gutierrez et al., 2019 ). Disturbances of DA metabolism in DAT-HET rats may lead to impaired functioning and integration of these different systems. Previously it has been shown, that methamphetamine impairs both egocentric and allocentric learning and memory ( Gutierrez et al., 2019 ).
A fundamental difference was found between Wistar and DAT-HET rats in the learning patterns and transformer maze test performances. While Wistar rats learned the rule quickly with the total number of errors rapidly decreasing, DAT-HET rats’ errors decreased gradually.
In the final tests, 65% of Wistar rats trials were error-free, with rats perfectly following visual cues. At the same time, only in Wistar rats was there an increase in the number of peeks in the final test. Apparently, they checked their options without fully going into the cul-de-sac compartments.
Increased velocity during the 4th training session and decreased time of decision making in the choice compartments precedes a sharp decrease in the number of errors observed in the 5th training session in Wistar rats. Also, during the 5th training session, Wistar rats showed a decrease in the number of rears, which may indirectly indicate a change in the strategy of their choice. Perhaps it is connected with the switch to the cue-based orientation. The absence of this shift in DAT-HET rats may indicate different learning mechanisms in these animals.
There are studies that were aimed at separating allocentric and egocentric navigation strategies. For example, a four-arm plus-shaped maze was used for this ( Ramos, 2017 ). To determine a predominant strategy, there 10 consecutive trials were carried out, in the probe test, and there was used a mean percentage of correct responses obtained by an allocentric or egocentric strategy. In this maze, it is difficult to distinguish between a random and a conscious choice. In the present transformer maze, the animal has got to make a sequence of choices, so the possibility of a random choice is minimized. When we observe animals making error-free successive choices without any mistakes, we can be sure that they have used the cue.
Two forms of learning have been described: trial and error strategy and “insightful learning”, which manifests as a sudden change in behavior ( Wolfensteller and Ruge, 2012 ; Neves Filho et al., 2015 ). In Wistar rats, the observed learning behavior is closer to insightful learning, leading to successful cue-based performance. They showed sudden changes of behavior parameters while learning, and error-free trials in the final tests can be taken as a criterion of the animal using cues. In contrast to that, DAT-HET rats continue to use trial and error strategy, and demonstrate gradually changing behavior parameters.
There are various models describing the role of DA in learning and memory. The prediction error model suggests that dopamine is important for matching expectation and reward, while the stimulus model suggests that dopamine promotes motivated behavior ( Saddoris et al., 2015 ). DA also plays a role in novelty and the significance of stimuli ( Kutlu et al., 2021 ).
Any of these roles of DA may be considered as a cause of DAT-HAT rats’ inferior performance and an absence of insightful learning.
Overall, the new transformer maze makes it possible to discover the differences in learning and performance patterns of rats.
4.3. Comparison of the transformer maze and its additional variations with other mazes
The transformer maze has several advantages in comparison with other mazes. Being a multiple T-maze, like the Cincinnati water maze, it can be quickly modified. Using visual cues like The Morris water maze, it does not require a large volume of warm water, which makes it possible to precisely evaluate the use of cues by animals. In addition, during the training, there is no need to stress the animals by making them swim, although food deprivation and weight monitoring are required. Rats explore the space and find a feeder without human intervention. Thus, the behavior execution is under less stressful conditions as compared to with water mazes.
The layout of the maze can be changed to contain any number of compartments, and it can be used to assess egocentric route-based navigation by placing same-colored barriers and setting up one route. In order to change the location of the route relative to the external environment, it can be turned at any angle. The principle of choosing between cul-de-sac and next choice compartments on the way to the feeder is preserved. Figure 15 shows an example of one route built this way.

An example of the route without any cues built in a 4 × 4 maze that allows us to assess egocentric navigation. F – finish compartment with a feeder, C – cul-de-sac compartments, Ch – choice compartment; crosses indicate inaccessible compartments.
In this maze, there are no proximal or distal landmarks that could help the animal to navigate. The rat has got to remember that it needs to go straight-straight-left-straight-left-straight-left. We only started to use this configuration in our experiments, but now we already have native recording of the tracks at the start of learning and at the finish. Figure 16 shows the samples of these tracks for one rat. It is obvious, that after a series of training trials when the animal wanders through the maze, the trained animal follows the route and uses_ route-based navigation without any landmarks.

The superposition of the rat tracks in the route-based navigation task in the maze configuration without any cues. Samples of the same rat in different turns of the route at the beginning (var1), middle (var2, var3, var4) and the end of training (var1). Var.2, 3, 4 are route turns of 90, 180 and 270°. S – start compartment, F – finish compartment. Crosses indicate inaccessible compartments.
We also suggest using the same arena to study path integration navigation ( Figure 17 ). To do this, the environment above the maze changes. Landmarks are added above the maze and learning find-the–feeder sessions occur in the empty arena without any barriers (similar to searching for the visible platform in the Morris water maze) ( Figure 17 a). Then the animal's ability to navigate is tested by placing white barriers with holes, so that the animal does not see the feeder, but still sees distal landmarks (similar to searching for the invisible platform in the Morris water maze) ( Figure 17 b). You can also change the starting point ( Figure 17 c). Figure 17 shows the samples of these tracks for one rat. It can be seen that the trained animal uses the vector, finding the shortest path.

The superposition of the rat tracks in the path integration navigation task in the maze configuration with cues above the maze, “a” – arena without any barriers, except start and finish compartment barriers, “b” and “c” – arena with white barriers with holes, without any cul-de-sac compartments with different place of the start point. S – start compartment, F – finish compartment. Crosses indicate inaccessible compartments.
We supposed, that when animals in the transformer maze choose the black passages, it can be estimated that they are moving by signposts. In this case, the animal must refuse to remember the routes or form a map, because it only interfere with the work. This allows us to study mechanisms of navigation without using spatial memory. The Barnes maze or radial arm mazes ( Barnes, 1979 ; Olton and Samuelson, 1976 ) allow us to study different aspects of spatial navigation; however, they can't provide a task where the animal, on its way to the goal point, should use only external cues, without any location-related skills.
Researchers use different mazes in one experiment to evaluate different cognitive aspects. But different motivations, different ways of moving, and different environments influence the result. ( Lewejohann et al., 2004 ). Comparing the behavior in three different tasks — moving by signposts, remembering the route, and the task using the arena with a visible and invisible feeder within one box—could be a precise instrument to study the brain processes.
5. Limitations
However, the design of this maze leads to certain limitations in research. The animal has to go through holes, so it's impossible to use any equipment connecting its skull to an external device, such as dialysis tubing or electrophysiological wires. To record brain processes, you can use only wireless methods. In addition, it is necessary to consider the animals' physiological ability to recognize the cues, so only relevant signals should be used.
6. Conclusion
We show that the new transformer maze can serve as a tool to evaluate the learning and decision-making patterns of rats. The comparison of Wistar and DAT-HET rats performance in this novel task revealed insightful learning typical for Wistar rats and trial and error-based learning in DAT-HET rats.
Declarations
Author contribution statement.
Elena Filatova: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Sechenov Institute of Evolutionary Physiology and Biochemistry of the Russian Academy of Sciences, the State assignment No. 075-0152-22-00.
Data availability statement
Declaration of interest’s statement.
The authors declare no conflict of interest.
Acknowledgements
I express my gratitude to the Institute of Translational Biomedicine, St. Petersburg State University, St. Petersburg, Russia that provided DAT-HET rats. I would like to express my appreciation to Shevelev Artem for excellent software development, patience and invaluable assistance. I am grateful to Galina Gromova for her help and support in the work. I am also immensely grateful to Dr. Maria Dorofeikova for the comments and editorial work on this manuscript.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2022.e11211 .
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Sample of the 4 × 4 maze assembling and test execution.
- Barnes C.A. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979; 93 (1):74–104. [ PubMed ] [ Google Scholar ]
- Braun A., Graham D.L., Schaefer T.L., Vorhees C.V., Williams M.T. Dorsal striatal dopamine depletion impairs both allocentric and egocentric navigation in rats. Neurobiol. Learn. Mem. 2012; 97 (4):402–408. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Buzsáki G., Moser E.I. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat. Neurosci. 2013; 16 (2):130–138. Epub 2013 Jan 28. PMID: 23354386; PMCID: PMC4079500. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cinque S., Zoratto F., Poleggi A., Leo D., Cerniglia L., Cimino S., Tambelli R., Alleva E., Gainetdinov R.R., Laviola G., Adriani W. Behavioral phenotyping of dopamine transporter knockout rats: compulsive traits, motor stereotypies, and Anhedonia. Front. Psychiatr. 2018:9–43. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Day M., Langston R., Morris R.G. Glutamate-receptor-mediated encoding and retrieval of paired-associate learning. Nature. 2003; 424 (6945):205–209. PMID: 12853960. [ PubMed ] [ Google Scholar ]
- Filatova E.V., Orlov A.A., Afanas'ev S.V. A two-ring maze for studies of the behavior of laboratory animals. Neurosci. Behav. Physiol. 2015; 45 (7):765–770. [ Google Scholar ]
- Gutierrez A., Regan S.L., Hoover C.S., Williams M.T., Vorhees C.V. Effects of intrastriatal dopamine D1 or D2 antagonists on methamphetamine-induced egocentric and allocentric learning and memory deficits in Sprague-Dawley rats. Psychopharmacology. 2019; 236 (7):2243–2258. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hebb D.O., Williams K.A. A method of rating animal intelligence. J. Gen. Psychol. 1946; 34 :59–65. [ PubMed ] [ Google Scholar ]
- Kutlu M.G., Zachry J.E., Melugin P.R., Cajigas S.A., Chevee M.F., Kelly S.J., Kutlu B., Tian L., Siciliano C.A., Calipari E.S. Dopamine release in the nucleus accumbens core signals perceived saliency. Curr. Biol. 2021; 31 (21):4748–4761. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- De Leonibus E., Pascucci T., Lopez S., Oliverio A., Amalric M., Mele A. Spatial deficits in a mouse model of Parkinson disease. Psychopharmacology. 2007; 194 (4):517–525. [ PubMed ] [ Google Scholar ]
- Lewejohann L., Skryabin B.V., Sachser N., Prehn C., Heiduschka P., Thanos S., Jordan U., Dell'Omo G., Vyssotski A.L., Pleskacheva M.G., Lipp H.P., Tiedge H., Brosius J., Prior H. Role of a neuronal small non-messenger RNA: behavioural alterations in BC1 RNA-deleted mice. Behav. Brain Res. 2004; 154 (1):273–289. PMID: 15302134. [ PubMed ] [ Google Scholar ]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods. 1984; 11 (1):47–60. [ PubMed ] [ Google Scholar ]
- Morris R.G.M. Spatial localization does not require the presence of local cues. Learn. Motiv. 1981; 12 (2):239–260. [ Google Scholar ]
- Neves Filho H.B., Dos Reis Stella L., Harder Ferro Dicezare R., Garcia-Mijares M. Insight in the white rat: spontaneous interconnection of two repertoires in Rattus norvegicus. Eur. J. Behav. Anal. 2015; 16 (2):8. [ Google Scholar ]
- O’Keefe J., Nadel L. Clarendon Press; Oxford, UK: 1978. The Hippocampus as a Cognitive Map. [ Google Scholar ]
- Olton D.S., Samuelson R.J. Remembrance of places passed: spatial memory in rats. J. Exp. Psychol. Anim. Behav. Process. 1976; 2 (2):97–116. [ Google Scholar ]
- Pritchett K., Mulder G. Hebb-Williams mazes. Contemp. Top. Lab. Anim. Sci. 2004; 43 (5):44–45. [ PubMed ] [ Google Scholar ]
- Ramos J.M.J. Perirhinal cortex involvement in allocentric spatial learning in the rat: evidence from doubly marked tasks. Hippocampus. 2017; 27 (5):507–517. [ PubMed ] [ Google Scholar ]
- Rubio S., Begega A., Méndez M., Méndez-López M., Arias J.L. Similarities and differences between the brain networks underlying allocentric and egocentric spatial learning in rat revealed by cytochrome oxidase histochemistry. Neuroscience. 2012; 223 :174–182. [ PubMed ] [ Google Scholar ]
- Saddoris M.P., Cacciapaglia F., Wightman R.M., Carelli R.M. Differential dopamine release dynamics in the nucleus accumbens core and shell reveal complementary signals for error prediction and incentive motivation. J. Neurosci. 2015; 35 (33):11572–11582. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Seibenhener M.L., Wooten M.C. Use of the Open Field Maze to measure locomotor and anxiety-like behavior in mice. JoVE. 2015; 96 [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sherrill K.R., Erdem U.M., Ross R.S., Brown T.I., Hasselmo M.E., Stern C.E. Hippocampus and retrosplenial cortex combine path integration signals for successful navigation. J. Neurosci. 2013; 33 (49):19304–19313. PMID: 24305826; PMCID: PMC3850045. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Swonger A.K., Rech R.H. Serotonergic and cholinergic involvement in habituation of activity and spontaneous alternation of rats in a Y maze. J. Comp. Physiol. Psychol. 1972; 81 (3):509–522. PMID: 4265289. [ PubMed ] [ Google Scholar ]
- Tse D., Langston R.F., Kakeyama M., Bethus I., Spooner P.A., Wood E.R., Witter M.P., Morris R.G. Schemas and memory consolidation. Science. 2007; 316 (5821):76–82. PMID: 17412951. [ PubMed ] [ Google Scholar ]
- Vorhees C.V., Williams M.T. Cincinnati water maze: a review of the development, methods, and evidence as a test of egocentric learning and memory. Neurotoxicol. Teratol. 2016; 57 :1–19. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Whishaw I.Q., Mittleman G. Visits to starts, routes, and places by rats (Rattus norvegicus) in swimming pool navigation tasks. J. Comp. Psychol. 1986; 100 (4):422–431. [ PubMed ] [ Google Scholar ]
- Wolfensteller U., Ruge H. Frontostriatal mechanisms in instruction-based learning as a hallmark of flexible goal-directed behavior. Front. Psychol. 2012; 3 :192. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhang Q., Kobayashi Y., Goto H., Itohara S. An automated T-maze based Apparatus and protocol for analyzing delay- and effort-based decision making in free. Moving rodents. JoVE. 2018; 138 [ PMC free article ] [ PubMed ] [ Google Scholar ]
About Kidney Disease
- Understanding Chronic Kidney Disease
- Kidney Disease Stages
- What to Expect with CKD
- Understanding Acute Kidney Injury
- How Kidneys Work
- Kidney Disease Management
- Managing Medications
- What Is a Nephrologist?
- Take a FREE CLASS on Kidney Disease
- Dialysis Basics
- Dialysis Access
- Find a Dialysis Center
- Home Dialysis Benefits
- Home Peritoneal Dialysis
- Home Hemodialysis
- Talk to a Home Dialysis Advocate
- Kidney Transplant
- In-Center Hemodialysis
- Choosing Not to Treat
Life On Dialysis
- Staying Healthy
- Dialysis Medications
- Avoiding Infections
- Staying Active
- Dialysis Travel Services
- Insurance and Managing Costs
- Your Emotional Health While on Dialysis
- Tips for Loved Ones
- Join the Kidney Care Community
- Login to PatientHub
Recipes & Nutrition
- Eating Well with CKD
- Eating Well on Dialysis
- Fluid Management
Thrive Central
- Diet & Nutrition
Fresenius Kidney Care South St. Petersburg
Also known as SOUTH ST. PETERSBURG
Dialysis Center
OPEN TODAY until 5:00 PM
650 34th St S Saint Petersburg , Florida 33711

Hours of Operation
Services offered in the dialysis center.
- In-center hemodialysis
- KidneyCare 365 classes
Amenities available
Use this 10-digit identification number for insurance purposes.
NPI: 1598878274
Nearby locations
Fresenius kidney care american dialysis center, fresenius kidney care st. petersburg, fresenius kidney care pinellas park dialysis, different names, same great cares.
Fresenius Kidney Care dialysis centers are part of Fresenius Medical Care North America (FMCNA). Some centers may be known as Fresenius Kidney Care or Fresenius Medical Care, as well as other names. Every center listed in our dialysis finder results is part of the Fresenius Kidney Care dialysis network.
Starting dialysis? Take a free class

CONSIDER THE FREEDOM OF HOME DIALYSIS
Saint-Petersburg Pasteur Institute

The history of the Saint-Petersburg Pasteur Institute dates back to 1908, when the first serodiagnostic and bacteriological laboratory was set up on the Bolshoi Prospekt of Petrogradskaya; officially becoming the Institut Pasteur in St. Petersburg on May 5, 1923, in commemoration of the 100 th birth anniversary of Louis Pasteur.
The Saint-Petersburg Pasteur Institute conducts a wide range of infection studies, with a permanent relationship with practical work and an integrated approach in the methodology: the ability to solve all scientific problems by one’s own means; from the detection of the etiological agent to the creation of vaccines and sera.
In the 1920’s, the Saint-Petersburg Pasteur Institute began to produce vaccines against rabies, smallpox, typhoid fever, cholera and diphtheria as well as sera for treatment of dangerous infections. In addition, the institute participated actively in the development and implementation of international and national programs for poliomyelitis and measles eradication.
The Institute establishes and operates national and regional reference centers for monitoring enteric fever, Yersinia infections, viral hepatitis, HIV and AIDS and rickettsia diseases and also updates and further develops its methods of collaboration with public health institutions.
Currently, the Saint-Petersburg Pasteur Institute is conducting fundamental and applied research in the field of epidemiology, microbiology and biotechnology in order to ensure the sanitary and epidemiological well-being of the population of the country.
For more information, visit the Saint-Petersburg Pasteur Institute official website .
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |

IMAGES
VIDEO
COMMENTS
In this lab, students will get dialysis tubing and fill them with varying concentrations of sugar water solution. They will measure the initial mass of the tubes, and then soak the dialysis tubing overnight in distilled water. The following day they will measure the new mass, and see how water moved across the dialysis tubing membrane.
Selective Permeability of Dialysis Tubing Lab: Explained
Part of NCSSM CORE collection: This video shows the flow of water through dialysis tubing into a sample of molasses. http://www.dlt.ncssm.eduPlease attribute...
Visking Tubing demonstration: Osmosis. This is a visual way of demonstrating a semi-permeable membrane.AIM: To investigate osmosis using dialysis tubing (Vis...
8. After blotting the dialysis tubing dry, use an electronic balance to determine the mass, to the resolution of the balance, of the dialysis tubing containing the sucrose solution. Record the mass, in grams, in the Data Analysis section of the lab. 9. Note the time and place the sealed dialysis tubing in the cup containing the water. 10.
Procedure. Cut about 6 inches of dialysis tubing and soften it in water. Tie one end of the dialysis tubing in a double knot to make a leak proof bag. Secure the buret to a stand. Slide the open end of the dialysis bag around the 50 mL buret and pull the bag up so that the bag and tubing overlap for about one inch.
3. Osmosis - Dialysis Tubing (To be done by each group) In this experiment, you will investigate the effect of solute concentration on the rate of osmosis. Bags made of dialysis tubing will be filled with either distilled water or varying concentrations of sucrose solutions. The pores of the dialysis tubing are permeable to water.
You can construct a model cell by using the dialysis tubing. Dialysis tubing behaves much like a cell membrane. To create a model of a cell, place the dialysis tubing in water until it is thoroughly soaked. Remove the soaked tubing from the water and tightly twist one end several times and either tie with string or tie a knot in the tubing.
6. Trim excess dental floss and dialysis tubing from the ends of your sealed dialysis bag. 7. Add a few drops of Lugol's iodine to your beaker of 250 mL of water until it appears a pale yellow. 8. Place your dialysis bag into the beaker and wait 30-40 minutes. Review the background information and fill in the Start of Experiment section of ...
It also illustrates the mechanics of diffusion and osmosis by which a cell will attempt to create homeostasis, or equilibrium between its inner and outer environments. PREPARATION - BY LAB TECHNICIAN. Cut the dialysis tubing into 15cm lengths and soak for 15 minutes in a beaker filled with room temperature distilled water.
Purpose: The purpose of this lab will be to introduce students to osmosis and diffusion using chicken eggs, since they are visible to the naked eye. This demonstration is "the hook" that can be used as a reference point throughout the rest of the unit. ... Pour the "blood" into the dialysis tubing and tie another knot at the top of the tubing ...
Biology. Osmosis occurs when different concentrations of water are separated by a differentially permeable membrane. One example of a differentially permeable membrane within a living cell is the plasma membrane. This experiment demonstrates osmosis by using dialysis membrane, a differentially permeable cellulose sheet that permits the passage ...
dialysis tubing. Silver nitrate solution (brown color) was added to both tubes. If milky white precipitate was formed, that means sodium chloride was present. Data: Conclusion: According to our data, we can confirm that the process of osmosis and diffusion had occurred. Through this experiment, we were able to learn that osmosis
Very Simple Diffusion and Osmosis Experiment The concept of cellular transport ... Students are given 2 pieces of dialysis tubing. One is filled with a starch solution and the other is filled with a glucose solution. Each is placed into a cup containing tap water. The cup that contains the starch tube also has iodine added to the water in the cup.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright ...
For the experiment with the starch and iodine indicator, note whether there was a color change in the fluid in the artificial cell. Also consider whether there was a color change in the water in the beaker, and what both of these observations say about the properties of the dialysis tubing membrane.
Dialysis technicians must: Obtain a formal dialysis technician certification from a nationally recognized organization. Recertify every three to four years. Complete at least 30 hours of continuing education. Prove at least 3000 hours of work experience in the dialysis field within the last certification period.
The maze is made of opaque white plexiglass. The first design of the rat maze is a square 42 cm × 42 cm arena with 30 cm high walls. The maze consists of 9 square compartments (3 × 3) divided by interior partitions. Each compartment is a 14 × 14 cm square, formed by removable barrier walls.
We would like to show you a description here but the site won't allow us.
The history of the Saint-Petersburg Pasteur Institute dates back to 1908, when the first serodiagnostic and bacteriological laboratory was set up on the Bolshoi Prospekt of Petrogradskaya; officially becoming the Institut Pasteur in St. Petersburg on May 5, 1923, in commemoration of the 100 th birth anniversary of Louis Pasteur.. The Saint-Petersburg Pasteur Institute conducts a wide range of ...