Search the site
Links to social media channels


The Need for Whole-ecosystem Experiments
Why do you need to conduct experiments on a whole lake? Why not just use test tubes and aquariums in the lab?
IISD-ELA Research Scientist Scott Higgins answers these questions and explains the concept of whole-ecosystem research.
Whole-ecosystem experiments are especially useful because they take into account the vast complexity of interactions between the natural environment and the large numbers of species present in most ecosystems. Experiments done in the laboratory are very useful, but often do not scale well to the ecosystem level because they frequently include only one or a few species that one might find in nature.
Policy Analysis details
You might also be interested in, planning for the future.
A guide to help increase climate resilience at a watershed scale in Manitoba's integrated watershed management plans (IWMP).
August 30, 2024
The Case of Eco-Certification in Manitoba's Commercial Fisheries
Manitoba commercial fisheries stand to benefit from obtaining eco-certification.
August 23, 2024
Building Natural Infrastructure Capacity Across Professions and Skilled Trades in the Canadian Prairies
As the demand for natural infrastructure continues to grow across the Canadian Prairies, it's essential to build more professional capacity in the skilled trades.
August 19, 2024
10 Ecosystem Project Ideas
- Freebies , Planning , Science

When you think about ecosystem project ideas, do you immediately think about dioramas in a shoebox, like this one I found on Pinterest?

Don’t get me wrong, dioramas are a great way for students to demonstrate their learning but it’s also the most common way. If you are like me, you are always looking for unique ways for students to express what they learned. That’s why I have a variety of ecosystem project ideas!

Create Your Own Ecosystems or Habitats.
Have your students work in groups, research, and then create an ecosystem together. It can be something as simple as collecting pond water, organisms, and plants. You could also have students create individual habitats instead of an entire ecosystem. We created our own habitats and the students really enjoyed it. Together we discussed the importance of meeting our living things’ needs and a healthy environment. We had a habitat for ants, fish, worms, and so much more.

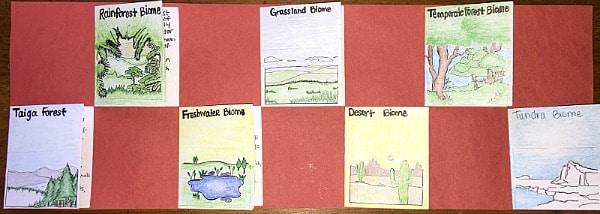
Create a Flap Book.
Provide students with a 12 x 9 strip of construction paper and several index cards (one per ecosystem you are studying). Have students name, draw, and color the ecosystem on the outside of the index card, and on the inside provide valuable information about the ecosystem inside. When you are done, it will look like this:
Create an Imaginary Ecosystem.
Have students create their own ecosystem but still requiring the characteristics of ecosystems such as needing to have both living and nonliving factors, populations, communities, and so on. Have students determine the food chains and much more. It will definitely require some creative thinking on their part, but it will definitely be fun!
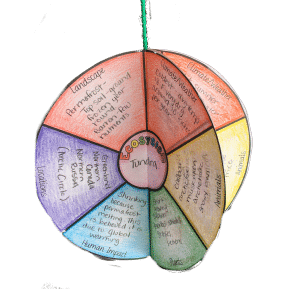
Create an Ecosystem Mobile.
Students love creating mobiles and they make for a cute display. If you can’t find hangers to make mobiles, you can easily use other materials such as sticks (yes, sticks from trees.), dowels (found in craft stores), or paper towel rolls. When creating an ecosystem mobile , you can have students again use index cards like in the example above, designing the outside and describing the ecosystem on the inside. You could also have students get creative and design something that represents that ecosystem, such as a raindrop for the rainforest. Students will love this ecosystem project idea!

Read Around the Room.
Set out many books about ecosystems around the room and students are sure to get excited! Have different locations representing different ecosystems and then move students around from station to station. If you want, you can have a student record in a chart or on one big piece of chart paper what they learned about that ecosystem. There are many great books out there on ecosystems.
Create a Scavenger Hunt.
What student doesn’t love a scavenger hunt? To create an ecosystem scavenger hunt , you would just place information about each ecosystem around your room in different locations. For instance in one spot you may have information about deserts and in another location information about grasslands. Then create a few questions for students to answer regarding each ecosystem. Students move around the room reading about each ecosystem and hunt for those questions. It’s a great way to sneak in some reading and just another ecosystem project idea.

Create an Accordion Book.
Can you tell I’m a crafty, foldable kind of gal? I just love hands-on activities and foldables. I think I wrote about this a little in my Going Wild for Ecosystems post. Drag out some construction paper or copy paper and have students fold it in half. Then have them draw the ecosystem at the top and write about its characteristics at the bottom of the half sheet. When finished, you end up with an ecosystem accordion foldable . (See image below).

Do this with each half for however number of ecosystems you are studying. Then connect them all by gluing them (or taping) side by side. (see image above).
Create a Circle Book.
Are you looking for an ecosystem project idea that is easy-peasy? These circle books have been my latest obsession. I’ve even got some created that I haven’t uploaded yet! But just like any of the above, you don’t have to head to my store to purchase them, you could easily create them yourself! Provide each student with one circle per ecosystem you would like them to represent. Then on each circle have them illustrate the ecosystem on the top and describe its characteristics on the bottom. (Sensing a theme?) Then fold each circle in half back to back and glue them together to form your ecosystem circle book .
Project Based Learning.
Are you looking for a way to get in a little PBL? Why not have students design their own ecosystem zoo ? (This is a shameless plug!) This project integrates area, perimeter, geometry, and STEM learning in your science classroom. Students work through a series of steps, including research, to design and build a model of their own ecosystem zoo! It’s differentiated and can easily be adapted!

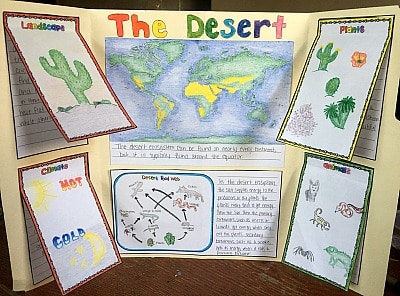
Triboard Display.
Why not have your students create a display similar to a science fair? In this display, students would take a regular file folder (see image below) and attach pieces that describe the landscape, climate, plants, animals, and food chain/web of the ecosystem. Then have students place a world map in the middle and color all the locations in the world where their ecosystem can be found. This can also be done on a larger scale with an actual tri board.
Want to save a little money? Check out the Ecosystem Bundle! It combines many of my ecosystem resources together at a discounted price.
GRAB THE FREEBIE!
Want to grab the ecosystem tri-board display activity above for free?
Grab The FREE Printable Here!
This is just a small sampling of some ecosystem project ideas. If you’re looking to save time, you can find many of these items inexpensively prepared for you in my store here , though you can also create many of these ideas yourself. And if you’re looking to save money, I’ve got a great bargain with my ecosystem bundle —eight whole products that can easily stand alone or be chained together!

Check out these related products!

- ecosystems , freebie , Life Science , Projects
FIND IT NOW!
Check me out on tpt.

CHECK THESE OUT

5th Grade Math Workshop Growing Bundle- 9 Units

Three Types of Rocks and Minerals with Rock Cycle Circle Book
Want to save time?
COPYRIGHT © 2016-2024. The Owl Teacher | Privacy page | Disclosure Page | Shipping | Returns/Refunds
BOGO on EVERYTHING!
Search Filters:
Main Navigation
Search Cornell
Cornell Institute for Biology Teachers
- Labs & Activities
- Workshops & Events
- Equipment Lending Library
- Connect with Cornell
Labs & Activities
Browse labs & activities:.
- 2012 CIBT Alumni Workshop (19)
- Animals (18)
- Ecology (25)
- Elementary School (12)
- Evolution (10)
- Forensics (3)
- Genetics (7)
- High School (49)
- Human Health (11)
- Inquiry/Scientific Method (30)
- Insects (7)
- Microbiology (12)
- Middle School (32)
- Molecular Biology (12)
- Physical Sciences (10)
- Physiology (10)
- Plants (19)
- Recently Updated! (6)

Abuse-a-Cyst- University of Utah
2012 cibt alumni workshop, high school, inquiry/scientific method, middle school.
Brine shrimp populations survive in some of the harshest environments. Subject brine shrimp cysts to extreme conditions then try to hatch them to see just how tough they are! Downloads Abuse a Cyst Lab (University of Utah)
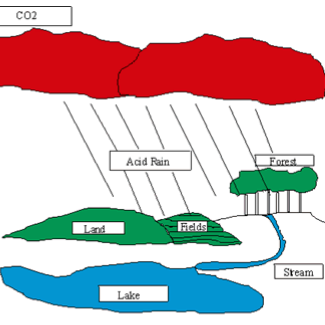
Acid Rain Lab- Katherine Betrus Derrico
Students will design and conduct an experiment to test the effect of acid rain on the germination of seeds. They will utilize the data from their experiment to explain their conclusions, and also read a passage on acid rain. Downloads Acid Rain Lab Rubric (Katherine Betrus Derrico) Acid Rain Lab… read more of the article entitled “Acid Rain Lab- Katherine Betrus Derrico”
Battle-jar Galactica- Matt Downing
Microbiology.
In this investigation students will study the types of bacteria that grow during the formation of sauerkraut, identify some characteristics of each, as well as research the type of respiratory pathway used by the organisms to break down the cabbage to get their energy. Downloads pH Questions (Matt Downing) Bacteria… read more of the article entitled “Battle-jar Galactica- Matt Downing”
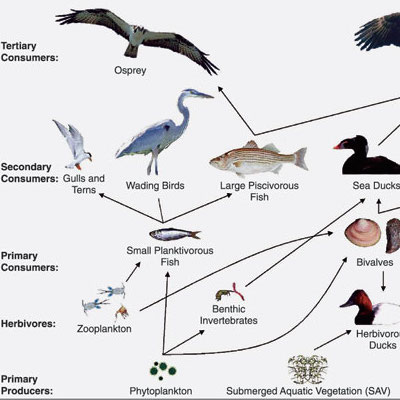
Biomagnification Lab- Todd Shuskey
This lab demonstrates how contaminants can accumulate in organisms within a food web by using paper cutouts and M&M®s candies to simulate fish, osprey, and DDT. Students can see how the contamination levels increase as the trophic level increases. Downloads Biomagnification Lab Pictures (in color) Biomagnification Lab Pictures (in black and… read more of the article entitled “Biomagnification Lab- Todd Shuskey”

Bottle Ecosystem- Tim Downs
Physical sciences.
The objective of this lab is to put together a suitable habitat (ecosystem) that will allow one or two guppies to survive to the end of the school year and beyond. Students will make observations of their ecosystems for the three weeks. The ecosystem in this experiment will be closed,… read more of the article entitled “Bottle Ecosystem- Tim Downs”

Bouquet of Flowers
Recently updated.
This series of four different lab activities all relate to flower reproduction. They have been designed to relate to each other and to stand alone. Name that Pollinator focuses on adaptations for successful pollination. Both pollen and pollen vectors are examined. Observing, data gathering, making measurements through the microscope, and… read more of the article entitled “Bouquet of Flowers”

Comparative Skulls
What can a skull tell you? A lot! If you look at a skull for clues about its origin, not only can you identify what species it might be from, but you can learn many details about the original animal. In this lab, students will determine what clues to analyze in… read more of the article entitled “Comparative Skulls”
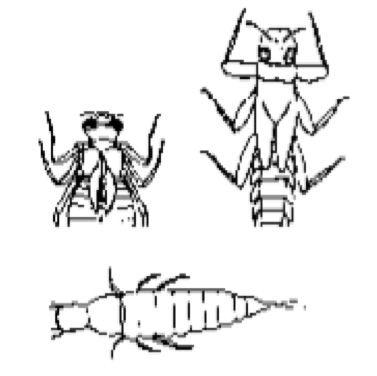
Comparing Aquatic Communities
Teams of students measure physical and chemical characteristics of different sites in streams and/or ponds and collect benthic invertebrate organisms. They interpret patterns in the structure of the biological community at each site in light of the abiotic (physical and chemical) and biotic nature of the environment. Downloads Comparing Aquatic… read more of the article entitled “Comparing Aquatic Communities”
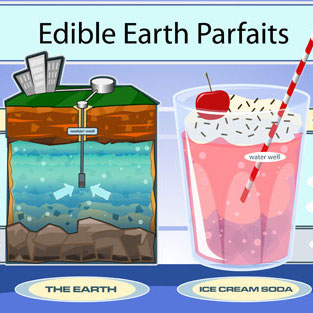
Edible Earth Parfaits- Groundwater Foundation
Elementary school.
This activity uses soda, ice cream, sprinkles, colored sugars, and food coloring to represent the layers of Earth and aquifers under the surface. Students are instructed to “drill a well” with a straw and “pump the well” by sucking on the straw, as they watch the decline in the water… read more of the article entitled “Edible Earth Parfaits- Groundwater Foundation”
Food Chain Game- Delta Education
In this activity, students investigate food chains by assuming the roles of animals that are part of a food chain. Downloads

Goldenrod Galls
This investigation examines natural selection and coevolution using goldenrod (Solidago canadensis), its stem gall insect (Eurosta solidaginis), and associated parasites, parasitoids, and predators that feed upon the stem gall insect (i.e., Eurytoma obtusiventris, Eurytoma gigantea, Mordellistena unicolor, and birds). Through measurements of gall size and an investigation of events occurring… read more of the article entitled “Goldenrod Galls”

Lichens on Tree Trunks- Scott LaGreca
Students will learn to recognize moss and lichens, identify various trees, record observations using a mapping technique, use a compass, and think about the conditions mosses and lichens need to grow. They will identify and mark trees with mosses and lichens growing on their trunks, and try to figure out… read more of the article entitled “Lichens on Tree Trunks- Scott LaGreca”

Mark-Recapture- Nancy Wright
This lab presents a popular method often used to estimate the population size of a single species of highly mobile animals, such as insects or vertebrates. Students use other students in the school as their population and the Lincoln-Peterson method to determine population size. “Real ecologists” also use this method… read more of the article entitled “Mark-Recapture- Nancy Wright”

Medical Importance of Biodiversity- Mary Keymel
Human health.
Students assume the role of an ethnobotanist for a start-up pharmaceutical company, who is about to journey to the rainforest, coral reef, or another natural source of medicine in the world. Their mission is to catalog 1 plant or animal species that may be useful to medical research. They will… read more of the article entitled “Medical Importance of Biodiversity- Mary Keymel”

Mollusk Dichotomous Key
In this lab, students will be introduced to the concept of a dichotomous key through the use of preliminary activities modeled by the teacher. They will then learn about the ecology and biology of selected marine mollusks, before putting their dichotomous key reading skills to the test on 8 or… read more of the article entitled “Mollusk Dichotomous Key”
-->Cornell University --> ©2014 ↑
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 28 August 2023
Understanding and applying biological resilience, from genes to ecosystems
- Rose Thorogood 1 , 2 na1 ,
- Ville Mustonen 2 , 3 , 4 , 5 na1 ,
- Alexandre Aleixo 6 ,
- Pedro J. Aphalo 2 , 7 ,
- Fred O. Asiegbu 7 , 8 ,
- Mar Cabeza 2 , 9 ,
- Johannes Cairns 2 , 4 ,
- Ulrika Candolin 2 ,
- Pedro Cardoso 6 , 10 ,
- Jussi T. Eronen 9 , 11 , 12 ,
- Maria Hällfors 2 , 13 , 14 ,
- Iiris Hovatta 15 , 16 , 17 ,
- Aino Juslén 6 , 14 ,
- Andriy Kovalchuk 8 , 18 nAff25 ,
- Jonna Kulmuni 2 , 19 ,
- Liisa Kuula 15 ,
- Raisa Mäkipää 20 ,
- Otso Ovaskainen 2 , 21 , 22 ,
- Anu-Katriina Pesonen 15 ,
- Craig R. Primmer 2 , 5 ,
- Marjo Saastamoinen 1 , 2 , 13 ,
- Alan H. Schulman 5 , 7 , 20 ,
- Leif Schulman 6 , 14 ,
- Giovanni Strona 2 , 13 , 23 &
- Jarno Vanhatalo 2 , 13 , 24
npj Biodiversity volume 2 , Article number: 16 ( 2023 ) Cite this article
8176 Accesses
5 Citations
61 Altmetric
Metrics details
- Biodiversity
- Community ecology
- Ecological modelling
- Ecosystem ecology
- Evolutionary ecology
- Molecular ecology
- Palaeoecology
The natural world is under unprecedented and accelerating pressure. Much work on understanding resilience to local and global environmental change has, so far, focussed on ecosystems. However, understanding a system’s behaviour requires knowledge of its component parts and their interactions. Here we call for increased efforts to understand ‘biological resilience’, or the processes that enable components across biological levels, from genes to communities, to resist or recover from perturbations. Although ecologists and evolutionary biologists have the tool-boxes to examine form and function, efforts to integrate this knowledge across biological levels and take advantage of big data (e.g. ecological and genomic) are only just beginning. We argue that combining eco-evolutionary knowledge with ecosystem-level concepts of resilience will provide the mechanistic basis necessary to improve management of human, natural and agricultural ecosystems, and outline some of the challenges in achieving an understanding of biological resilience.
Similar content being viewed by others
Ecological changes with minor effect initiate evolution to delayed regime shifts
The value of long-term ecological research for evolutionary insights

The long-term restoration of ecosystem complexity
Introduction.
The Anthropocene is characterised by the pervasive impact of human activity on all aspects of life on earth 1 . Human-driven climate change and overexploitation of natural resources, as well as increasing human population densities and urbanisation, are placing progressively larger areas under human influence and disturbances such as increased and/or more variable temperatures (and associated events such as droughts and fires), direct anthropogenic alterations (e.g. pollution, land-use changes, habitat fragmentation), and introduction of invasive species 2 . Even the world’s topology has changed, as global movement of individuals and goods erodes biogeographical barriers 3 . These environmental changes put ecosystems under unprecedented and accelerating pressures, inducing regime shifts 4 , causing loss of ecosystem services 5 , and even changing the course of evolution 6 . There is therefore an urgent need to determine why some species, communities or ecosystems decay while others persist or adapt 7 , and then implement this knowledge for improved management practices that can reverse or mitigate damage 8 .
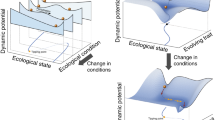
In ecology, ‘resilience’ has attracted great interest as a concept that describes the capacity of a system to respond to disturbance (Table 1 , Fig. 1 inset, following ref. 9 ; see ref.s 10 , 11 for recent in-depth reviews of definitions). Ecosystems may show strong ‘resistance’ with minimal perturbation in state or function. Or, if perturbed (i.e. low resistance), ecosystems may over time ‘recover’ and move back towards their previous state, or even benefit from the disturbance. Systems with low recovery potential, on the other hand, may shift abruptly (i.e. a tipping point) into a new and possibly stable state (i.e. a regime shift). Resilience has therefore typically been studied theoretically and empirically by considering how a system returns to its previous state (‘engineering resilience’ 12 ) or by the amount of disturbance absorbed before it tips into a different state (‘ecological resilience’ 13 ). However, translating the concept of resilience into an understanding of the mechanisms or properties that determine how much an ecosystem can absorb or resist a disturbance, or what shapes the trajectory of its recovery back to a previous or new stable state, remains challenging 10 , 14 . In part, this may be because resilience has typically been studied at the level of the ecosystem 15 , 16 which reduces our power to identify how and why resistance and/or recovery responses occur 17 : understanding the behaviour and interactions of a system’s component parts is essential to understand and forecast ecology 18 . On the other hand, while studying lower biological levels in isolation makes it easier to measure properties that might comprise a system’s resilience (e.g. population size, individual fecundity, genetic diversity; see Fig. 1 inset), reductionist approaches can hinder detection of connections between seemingly isolated biological events 19 . How can we deal with this complexity to identify the critical drivers and indicators of resistance and recovery?
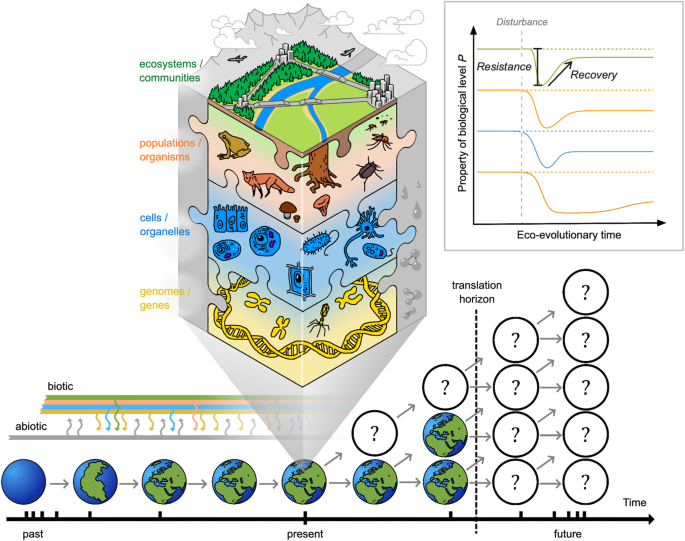
Biological resilience (mechanisms and processes across biological levels that enable systems to resist disturbance and/or recover over time back to a steady state after perturbations) is mediated by connections within and among levels of organisation (simplified to genes and genomes, cells and organelles, organisms and populations, communities and ecosystems; depicted by multi-coloured shading and lines), and recognises that the present state (expanded in centre of figure) is shaped by ecological and evolutionary responses to past biotic (multi-coloured) and abiotic (grey) disturbance and selection (note that time is represented by a log-scale). Resistance (change) and recovery (time, state and rate) can be measured using properties of different biological levels (inset) to provide a ‘common currency’ for integration, and then enhance the translation horizon (vertical dashed line, close in time) by providing more readily measurable indicators and improving accuracy of forecast outcomes (grey arrows and question marks within circles). Note that the resistance and recovery trajectories of biological levels to a disturbance event may differ in both amplitude and temporal scale (inset), and that ‘recovery’ is also sometimes referred to in the literature as a measure of resilience (e.g. refs. 11 , 27 ).
Here we propose that this can be achieved by adopting a ‘biological resilience’ framework (Fig. 1 ) where we: (1) test ecosystem-level resilience concepts (i.e. resistance and recovery responses, state changes) across lower levels of biological organisation; and (2) harness knowledge provided by the eco-evolutionary history of adaptation to past perturbations to better understand resilience from the bottom up. In doing so, biological resilience acknowledges that processes occurring within and between components across biological levels, from genes to communities, shape how systems resist disturbance or recover from perturbations. This framework stands out from recent calls to encourage analysis of resilience across systems and scales, and from ecosystems to populations (e.g. refs. 16 , 17 ) as we explicitly acknowledge the crucial role of eco-evolutionary history. Furthermore, investigating how biological levels themselves respond over time to a disturbance event (e.g. from changes in cellular processes to genetic adaptation via measures of gene or allelic diversity) would provide scope for a common ‘language’ and integration of data to dive deeper into uncovering the mechanisms and processes that afford resilience from individuals to communities and ecosystems. We first (i) explore how the eco-evolutionary past provides context for present and future resistance and recovery responses, and then (ii) discuss why it is necessary to consider how abiotic and biotic disturbance events can affect biological levels differently to detect mechanisms and underlying processes. Next, we (iii) outline three testable hypotheses to kick-start research into resilience across levels of biological organisation, from genes to cells, individuals, populations and communities. Collecting and integrating large amounts of data about how every biological component responds to a disturbance is often considered unrealistic. However, here we (iv) identify new opportunities emerging from the ongoing infusion of big data into ecology and evolutionary biology and stress the need to combine these data with experimental approaches to (v) enable advances in translating research into practice. Each of these steps is beginning to be investigated (examples across taxa, biological levels, and ecological context are given throughout) but they lack an overarching framework that brings all of them together. Our aim here is therefore not to cover all aspects of how resilience is, or could be, studied in-depth, but to extend recent calls to move from ecosystems to species (e.g. ref. 16 ) and encourage discussion of why and how ‘resilience thinking’ could be applied across biological levels.
(Re)Placing resilience into an eco-evolutionary context
When Holling introduced ecological resilience in his landmark paper 13 , he briefly suggested that a system’s resilience is a product of its evolutionary history (1973:p.18). Most research conducted since, however, has lacked an evolutionary perspective 14 , 20 . Therefore, much of the discussion, theory and examples of resilience in ecology lack a long time horizon and largely ignore how past environments influence current (or future) resistance and recovery responses 21 . Similarly, eco-evolutionary biologists rarely study how a system’s resilience might be conferred by processes that occur within or across the biological levels that form the focus of their studies 22 , despite research programmes often having a shared interest in determining how particular measurable traits or variables vary in response to a stressor or disturbance event (e.g. ref. 23 ). This disconnect among fields may be because much of the work on resilience describes patterns at the ecosystem level 14 , whereas studies of evolutionary processes rarely scale to complex communities 24 . Indeed, focusing on how ecology and evolution shape patterns and processes within individuals and populations has attracted criticism for being too narrow to address large ecological problems 20 , 25 . Nevertheless, here we argue that adopting a ‘common currency’ of studying resistance and recovery across biological levels will improve integration of eco-evolutionary theory with resilience (see Box 1 ) and provide information from the evolutionary past to improve our power to estimate both present and future states.
Box 1 Integrating ecology and evolution to understand biological resilience
Evolutionary mechanisms (mutation, drift, migration, natural selection) generate changes in allele frequencies from one generation to another (i.e. microevolution) and, given sufficient time or conditions, can lead to large-scale changes that transcend species boundaries (i.e. macroevolution). Similarly, processes that influence ecology (e.g. density, connectivity, competition, species interactions) at smaller scales (e.g. within populations, communities) give rise to large-scale macroecological patterns (e.g. biodiversity and ecosystem function). Darwin made no distinction between micro and macro scales, nor did he (or Wallace) separate ecology from evolutionary processes (see ref. 20 ). Over the 20th century, however, research in ecology and evolution specialised to specific scales and processes that presents a major challenge for understanding ecological patterns and processes 20 . Adopting a biological resilience framework necessitates reintegration. How might this be achieved?
(i) Harness existing and emerging approaches
The combination of theory, modelling and empirical approaches of eco-evolutionary dynamics provides a potential solution to reintegrate ecological and evolutionary processes across biological levels and scales 85 , 136 , 137 and detect relevant responses to environmental change (e.g. refs. 23 , 138 ). Here, phenotypic and genotypic variation coupled with rapid evolution play a key role to explain how populations scale up to influence species interactions and ecological communities (including their structure, function, and dynamics), as well as influence how selection pressures are responded to and genomes are inherited. Work in this rapidly developing field is scaling up from population-level studies 139 to analyse how evolutionary processes impact ecological dynamics (and vice versa ) in communities and even ecosystems 136 , with explicit acknowledgement that interactions and feedback also occur across non-adjacent biological levels (see Fig. 1 in refs. 136 , 140 , and see ref. 141 for a review of available models) – as we propose here for biological resilience. Studies of eco-evolutionary dynamics are possible in both the lab and the field 85 and are expanding in scope towards a landscape perspective 24 , 51 , 142 . Taking an eco-evolutionary approach to consider feedbacks on ecosystem-level processes is also now beginning to attract attention, suggesting that evolutionary changes in the variation of traits may play an important role in shaping how and when ecosystems reach tipping points and possibly irreversible ecosystem change 22 .
(ii) Recognise conceptual similarities
Understanding biological resilience will require a step change to move from describing either macro- or micro- scale patterns to demonstrating how evolutionary and ecological processes shape short- and longer-term responses to environmental change. Fortunately, eco-evolutionary dynamics and resilience in ecology rely on similar landscape-based frameworks to conceptualise and mathematically explore predictions. Resilience is typically described by a ‘stability landscape’ where valleys in the landscape represent alternative stable states and disturbance events create wobbles that may push systems over the hilltops between valleys (see ref. 10 ). In principle, these landscapes can be described by mathematical functions and may be measured by identifying the critical state variables that describe its dimensions, although in practice it remains very challenging to identify alternative stable states available in the past or the future 10 . Similarly, evolutionary biology makes use of ‘adaptive landscapes’ where fitness functions are described according to phenotypic traits (or genotypes) to conceptualise and predict the strength and direction of selection. Populations or species are described as ‘climbing’ towards ‘adaptive peaks’ of trait/genotype combinations with the highest fitness, where ‘adaptive valleys’ of lower fitness inhibit movement across the landscape. Emerging topics of research include integrating environmental variables to understand past, current and future movement among adaptive peaks (e.g. refs. 143 , 144 ). Although the axes of adaptive and resilience landscapes are at different biological levels (typically populations and ecosystems, respectively) and the location of stable states are inverse (‘peaks’ in adaptive landscapes, ‘valleys’ in resilience landscapes), in both cases the population or system of interest is expected to oscillate and move across the landscape in response to ecological change. Considerable effort is now going into translating these landscapes from metaphor to useful predictive tools (e.g. resilience landscapes 10 , adaptive landscapes 143 ) meaning the time is right to bridge the gap.
(iii) Identify shared terminology
A lack of common language is a widely recognised barrier to disciplinary integration, and this is further exacerbated when fields share jargon but differ in definitions, or when definitions of key terms are easily confused (e.g. ref. 145 ). This is a problem for integrating evolutionary biology with resilience across biological levels as ecologists and evolutionary biologists share terms but use them differently (Table 1 ). For example, ‘resistance’ is used in resilience (e.g. ref. 11 ) to describe how much a system is perturbed by a disturbance event (i.e. a rate) whereas biologists studying pathogens, parasites and antagonistic coevolution define resistance as a strategy to prevent or limit infection by an enemy (i.e. a trait, or suite of traits; e.g. ref. 146 ). ‘Tolerance’ on the other hand describes how much a host can prolong its survival or recover its reproductive success, given infection 147 . This perhaps has analogies to ‘recovery’ back to a stable state in resilience (see Introduction, Fig. 1 ), although time to recover is less of a focus in studies of tolerance than resilience. Providing in depth equations is beyond the scope of this conceptual overview, and even amongst existing studies of resilience, there is variation in how resistance and recovery parameters are measured (see ref. 11 for an overview of studies of resilience in forest trees, soil communities, and watersheds). The first step for comprehensive studies utilising information across disciplines is therefore to build a shared glossary, preferably in mathematical terms that relate to the landscapes outlined in (ii), with expectations of how (and when) putative state variables at the different biological levels being measured will respond to the disturbance event of interest.
Using eco-evolutionary theory to read the past from the present state
Estimating components (or attributes) of resilience such as resistance and recovery rely on measures before, during and after disturbance events (see ref. 11 for example equations used in different ecological contexts). This presents a major challenge for understanding resilience, as even if the ‘before disturbance event’ state is contemporaneous or known, it is rare that information is available about what stable states may have been like in the past. Evolutionary genetics provides an approach to help tackle this problem, as past perturbations leave their mark on the genome (i.e. ‘evolutionary memory’ 26 ) which can (i) affect an individual’s capacity to respond, (ii) influence a population’s ability to adapt to changing environmental conditions, and therefore (iii) shape ecological community interactions and potentially ecosystem function now and in the future, even if perturbations are novel to those experienced in the past 27 . At the level of genes, evolutionary history is manifested in variation introduced by mutation and/or migration (gene flow) as well as recombination (new combinations of genetic variation) that is filtered by natural selection or fixed by random genetic drift. Some genetic variants may provide an advantage against future disturbance events, such as through acquired resistance against a parasite, pest or antibiotic encountered in the past 28 . On the other hand, disturbances that result in severe population bottlenecks can result in the loss of potentially beneficial variation and/or fixation of maladapted alleles, and thus have negative effects on resilience 29 . Similarly, past selection that strongly favoured specific alleles may also limit future resilience due to the loss of genetic variation required for new adaptation to take place (e.g. Afrotropical butterfly experiencing climate change induced variation in seasonality 30 ).
The principle of evolutionary parsimony states that species with a shared evolutionary history are likely to have experienced similar selection pressures (e.g. from shared disturbance events) in the past 31 , and therefore possibly convergent responses at different biological levels. It is perhaps not surprising, then, that a recent study harnessing evolutionary history found that current variation in demographic resilience (i.e. responses of population growth and size) was explained more by phylogenetic relatedness among species than recent (~50 years) environmental stochasticity 21 . Incorporating historical global temperature records, species-level functional traits, and rates of phylogenetic diversification is also helping to explain how microevolutionary history induces different macroevolutionary responses to temperature change across angiosperms 32 . Evolutionary history can also be harnessed to understand resilience at the cellular and molecular level, with comparisons of e.g. protein interactomes across the tree of life revealing how these complex networks of molecular interactions evolve greater resilience to a loss of network connections over time 33 . Moving beyond phylogenetic relatedness, there is a rich body of evolutionary theory (e.g. the Coalescent) and simulation frameworks (e.g. SLiM) available to estimate past population sizes and genetic diversity (summarised in ref. 34 ) or the prevalence of deleterious genetic mutations in response to dated environmental events (e.g. ref. 35 ). These tools could be used to model resistance and recovery of populations or species of interest to current and future disturbance scenarios (e.g. ref. 36 and see ref. 37 for a workflow to detect and predict responses to thermal disturbances), or by comparing demographic histories for interacting species (see ref. 34 for an example with great apes, malaria Plasmodium , and the Anopheles mosquito vector), it could soon be possible to gain a deeper perspective on past states of biological levels from populations and single species to communities and ecosystems.
While genetic information will underpin the capability of an organism to respond, there is now also abundant evidence from many taxa that genotypes can generate different phenotypic (including cellular, physiological, morphological, and behavioural) responses depending on environmental conditions (i.e. plasticity, Table 1 ). Such plasticity can enable individuals to resist negative impacts on fitness and consequently buffer (or even increase) populations from possible demographic perturbations (see ref. 38 ), or be maladaptive if it leads individuals to respond inappropriately to previously reliable environmental cues (e.g. ref. 39 ). Phenotypically plastic responses can be modified further depending on the composition, structure and spatial context of the perturbed population or ecological community 40 , and variation in plasticity can influence individual and species interactions and therefore feedback on community composition and ecosystem function 41 , 42 . Although often studied by measuring individual phenotypes or gene expression in response to a specific environmental condition (i.e. representing a disturbance event), it is now acknowledged that plasticity may leave heritable ‘epigenetic’ marks on the genome (i.e. not changes to DNA sequences) that influence the future regulation of gene expression and shape how subsequent generations may resist or recover (e.g. refs. 43 , 44 ). Therefore, phenotypic plasticity that evolved in the past may be ‘read’ now to explain current, and predict future, states and interactions across biological levels. While there is growing interest in testing whether current plasticity plays a significant role in resistance and recovery to e.g. climate warming (heterotrophy of corals 45 , physiology of ectotherms 46 , demographic variation of commercially-important fishes 47 ), there have been few attempts to ‘read the past’ from current plasticity 38 , 48 . Harnessing knowledge about the past to understand biological resilience will likely require integrating phenotypic plasticity, epigenetics and genetic information (e.g. ref. 49 ), meaning there is potential to provide a major advance across diverse fields.

Finding the right scale: effects of disturbance events vary across biological levels
If we can uncover how elements of the system have responded to past disturbance events or state perturbations, then this information will become useful for predicting current and future changes. However, disturbances can be complex and vary in intensity, duration, frequency and spatial extent 50 and the impact of disturbance events on both the degree and timing of any perturbation will vary across biological levels (Fig. 1 ). For example, an adaptive genetic mutation 51 or socially-inherited behaviour 52 enabling a species to exploit its perturbed habitat can in turn, alter community assembly through variation in demography. This may or may not occur contemporaneously with the spread of the genetic mutation, as community changes caused by past disturbances may also determine subsequent community assembly through complex cascading effects on species succession (e.g. the order in which species recolonize an area after a habitat perturbation is important for community assembly 53 ) and potentially ecosystem function. Adopting a biological resilience framework could help to predict these events as incorporating a longer time horizon reveals resilience to be a dynamic and constantly evolving product of long term (co-) evolutionary, ecological and biogeographical processes (e.g. ref. 54 ).
Understanding how these processes operate at different biological levels of organisation will be critical, as the rate of evolution for example is constrained by generation times that vary from minutes (e.g. cells and microbes) to centuries (e.g. trees), reproductive strategy influences opportunities for outcrossing and mutation, and migration can diversify or limit local genotypic and phenotypic variation. However, at present, it remains unclear whether one level in particular will be of greater importance for predicting responses to current and future disturbance, and while it is likely that responses of one level to a given disturbance event will influence how multiple other levels respond, investigations into the carry-over effects of perturbations across biological levels are few and mostly focus on adjacent levels (e.g. changes in population influence response of communities 55 ). The composition, structure and spatial context of a perturbed population or ecological community also needs to be taken into account 40 . Range-edge populations, for example, can be comprised of a different set of individual response-types than those found in the range core (e.g. spatial sorting 56 ) and potentially set up cascades of change across other biological levels (e.g. reduced genetic diversity 57 ), and fragmented habitats influence the degree to which species can reduce their exposure to perturbations by shifting, shrinking or expanding their range via dispersal 58 , or by modifying physiological or behavioural responses 59 . Spatial context also has fundamental implications for longer-term adaptation to environmental change as it shapes gene flow 60 . Integrating past and present distributions and habitats is therefore likely to be a key, albeit challenging, aspect to understand biological resilience. Nevertheless, using evolutionary history as a ‘natural experiment’ and integrating information about adaptation explicitly into a resilience framework could provide a previously untapped resource for predicting how ecological systems respond to disturbance events.
A biological resilience framework generates testable hypotheses
It is clear that determining how different biological levels resist and recover and buffer other levels from perturbations will be complex, and that harnessing available information from the past is not straightforward. However, theory and mathematical models lay the foundations for identifying what to measure from experimental and empirical systems and how to extract these observations from real data (Box 1 ). Much of the theoretical work on resilience has made use of complex dynamic system models (e.g. ref. 61 ), but simpler approaches to calculate resilience are available (e.g. ref. 15 ), and efforts to incorporate evolutionary perspectives into models of ecosystem-level responses (e.g. tipping points 22 , warning signals 54 , species coexistence 62 ) and model complex interactive processes across biological levels (e.g. network models 63 ) are beginning. Furthermore, there is growing theory surrounding the ecological and evolutionary dynamics of resistance (e.g. antibiotics 64 ) and rapid genetic adaptation to ecological change (e.g. ref. 65 ) that could provide useful approaches to bridge resistance and recovery responses across biological levels. A long-term problem in ecological modelling, however, is that theoretical models are good for understanding causality, but difficult to test critically with data, whereas statistical models are correlative, and thus may not identify the relevant underlying mechanisms even if they fit the present data well. Nevertheless, considering perturbations across biological levels in terms of eco-evolutionary form and function helps generate hypotheses concerning the role of past disturbances in shaping current and future resilience (i.e. resistance and recovery, Fig. 1 ): (i) past experience primes a biological entity to cope with future disturbances of a similar nature. Alternatively, but not necessarily mutually exclusively, (ii) populations and communities exposed to more variable environments and higher levels of disturbance over the long term are expected to be most resilient. However, even these may accrue a resilience debt if the magnitude and frequency of the disturbances differ too much from their historical disturbance regimes 66 . Finally, (iii) even without long-term disturbance histories, rapid adaptation may improve resilience against specific stressors. This may, however, come at the cost of decreased resilience in the longer term because of reduced pre-existing diversity after rapid adaptation or altered species interactions 57 , 67 . Aspects of these hypotheses have already begun to be tested (Table 2 ), but not yet across biological levels within a relevant system.
Approaches to understand biological resilience
Understanding biological resilience will require concerted multidisciplinary research programmes where the effects of a disturbance (or multiple stressors) in terms of resistance and recovery responses are investigated across different levels, and where feedback among levels is also measured explicitly (Table 2 , Fig. 1 ). At present, research into coral reef resilience provides a worked example: surveys and experiments have demonstrated that different coral species exhibit different degrees of resistance and recovery to similar stressors 68 . Comparing the species’ evolutionary history provides some insight into why: a recent study suggests Caribbean corals show lower recovery than Indo-Pacific corals due to an evolutionary bottleneck 2.8 million years ago that favoured large and long-lived species with low rates of recruitment 69 . Efforts to investigate genomic predictors of coral bleaching 70 , and even to assist evolution towards more resilient forms 71 , are also now attracting wide attention 72 . Furthermore, mapping dependencies of coral-fish species based on natural history and fitting structural equation models has recently suggested that coral loss may lead to substantial negative change in fish diversity and biomass worldwide, with effects extending beyond the fish species directly dependent on corals 55 . Salmonid fishes (see Box 2 ) could also provide a model system for similar combinations of approaches to better understand current changes in populations following disturbances (including at the ecosystem level) from fishing, find reliable indicators of the mechanisms that improve recovery, and provide more reliable forecasts of management scenarios.
There are many other studies beyond these examples that report genetic-, phenotypic-, or community-level changes along environmental gradients or responses to natural changes, but far fewer either consider more complex environmental scenarios (e.g. multiple or sequential stressors) or how the effects at one biological level may affect others. As such, much of the current work in understanding biological resilience (even if not yet couched in this terminology) relies on surveys and correlations that are carried out at one level. For example, ‘which genes contribute to more resilient phenotypes?’ 73 , ‘which populations are more resilient to certain perturbations?’ 74 or, ‘which species are most affected by which particular aspects of a perturbation?’ 75 . Furthermore, the results of experiments, particularly into resilience at the cellular 76 or genetic levels 77 , are often not interpreted in a broader ecological context or compared to available data from natural populations 78 . Here we explore how we can move beyond studying the effects of single stressors or single species or levels and progress towards more complex experimental designs and assessments of more complex situations in the wild. Although this survey is not exhaustive, we hope that it provides insight into the range of methodologies used across biological levels to better enable discussion and design of multidisciplinary research.
To enable future studies to cover multiple biological levels, incorporating standardized collection of data and sample material across biological levels (e.g. genetic material, phenotype and community structure) into geographical surveys and long-term studies is a good starting point. If these standardised surveys are conducted over multiple seasons, years, or generations, this long-term monitoring has the potential to facilitate (i) detection of subtle responses and/or subtle perturbations, (ii) replication over time, and (iii) detection of ecological and evolutionary memories 79 . The same recommendation is relevant for “opportunistic” sampling following the (often unexpected) formation of a resilience-relevant gradient/difference. Data for multiple biological levels at sites that have experienced a heat wave for example, or an oil spill or chemical release, can either be compared to those of a nearby site that did not experience the perturbation 80 , or in the event that surveys of the affected sites were conducted prior to the perturbation, a ‘before vs. after’ analysis can be conducted 81 . Second, the prehistoric and palaeocological record is an important potential source of survey data, as it is now becoming tractable to incorporate with extant data (e.g. biotic interactions through food web analyses, process-based models of origin and extinction, and species co-occurrence matrices, ref. 82 ). This paleo-perspective could offer natural experiments: data are available to potentially help explain how community assembly (and disassembly) works when time spans are increased 83 , for example, or how genetic structure and adaptations respond to perturbations ranging from major extinctions to rapid climate change or species invasions over long time periods (e.g. ref. 84 ).
A major challenge for survey approaches mentioned above however is to disentangle the effects of co-varying environmental characteristics (e.g. photoperiod and temperature along a latitudinal gradient, or simultaneous drought and reduced food availability). Therefore, experiments in semi-natural (e.g. in vitro microcosms or outdoor mesocosm setups) or field settings (e.g. ponds/tanks, forest/field plots, enclosures suitable for small mammals, or free-ranging individuals and populations) are an essential third approach to test how resilience occurs across biological levels, and offer an attractive compromise where ‘real-world’ conditions are partly retained but where some manipulation and/or control is nevertheless possible, together with replicates 85 . These experiments can range greatly across organismal scale, geography, and biological levels (e.g. ref. 48 , 86 ), and can also be conducted alongside interventions to mitigate species decline or change in ecosystem function (e.g. conservation actions including introductions of individuals or translocations of populations 87 ), if the selection of individuals or species to be moved is designed to test the relative resilience of different characteristics (e.g. social behaviour 88 , genetic diversity 67 ). Although further removed from ‘real world’ conditions, common garden experiments (i.e. the rearing individuals in a controlled environment under common conditions) could be used to study responses to environmental or anthropogenic stressors by adding ‘treatments’ such as thermal stress, disease, or changes in community (e.g. flour beetles 89 , burying beetles 90 ). Here, environmental differences can be eliminated, or specific environmental factors can be tested so that the extent of resilience that is plastic versus evolutionary (e.g. fish 91 , crops 92 ) can be measured. Resurrection-type experiments (i.e. dormant propagules from ancestral populations) are also a promising approach in taxa where genotypes that have experienced varying conditions in the past are available to test responses under experimental conditions 93 . Experimental designs like these outlined above have been criticised for over-simplifying ecological processes, however taking an experimental approach will be essential to tease apart the relative effects of multiple stressors, either simultaneously, or sequentially, or at different stages of an organism’s life-history. Starting with experimental designs or studies at single biological levels is tractable yet will enable refining hypotheses and study designs for the future study of other biological levels in more complex conditions.
Fourth, eco-evolutionary and environmental Big Data, from the molecular to the ecosystem level, provide a broad and expanding scope, particularly when datasets span space and/or time. At the molecular level, Big Data on genes and genomes (NCBI 94 ) and databases of their function (Gene Ontology GO 95 , Kyoto Encyclopaedia of Genes and Genomes KEGG 96 ) are rapidly increasing. These databases are designed to be taxonomically comparable, or even species-neutral, to enable transfer of functional annotation (molecular function, biological role and cellular location) or gene network information derived from model organisms to inferred orthologues in newly sequenced species. If the current focus on medical science or morphological characters broadens to encompass functions in response to ecological stimuli 97 , then big genomic data will become an even more useful resource for studying the molecular basis of biological resilience. Similarly, finding the most potent data sources for reconstructing time series into the past still requires innovation, but this approach carries considerable promise for analyses of resilience to changes that have already occurred. For example, abiotic data from the last few decades are now openly available (e.g. CORINE 98 , WorldClim 99 , CHELSA 100 ) and big data on species occurrences (GBIF 101 ), traits (TRY 102 ) and abundances through time 103 are becoming available at an increasing rate. Collecting data of changes in the deeper past requires continued efforts in digitising physical collections (museum specimens 104 ) and application and development of new techniques for data extraction and analysis 82 .
At present, most of the global databases (e.g. those mentioned above) at present contain (partially) non-comparable data, and experimental data are rarely combined with observational data despite potential to increase credibility of conclusions 105 . Leveraging big data across biological levels is challenging as it requires intensive upskilling in data integration 106 and ideally coordinated platforms for e.g. different ecosystems, communities, or management areas of interest (e.g. ‘ePlant’ platform 107 for data across multiple levels from Arabidopsis and crop plants, or ‘Metascape’ 108 for multiple -omics assays to understand molecular mechanisms). However, as the resolution and density of data increases, and new algorithms that make use of large-scale computational resources become available, the possibilities to find and match comparable drivers-to-biotic-units cases will increase. In the meantime, existing data can be analysed by taking advantage of newly developed methods that minimise biases in unrelated or uncertain data (e.g. Bayesian approaches 109 ), or when fully comparable data are available, by using mechanistic models that allow moving beyond correlative analyses (e.g. individual-based models 110 ). Artificial intelligence could also begin to be utilised to predict the consequences of ongoing and future change. Although ‘black-box’ neural network approaches are popular, symbolic regression (an approach that finds explicit mathematical formulas to explain linear and non-linear relationships) holds much promise for distiling previously hidden natural laws from available data as it derives simpler and more interpretable equations (e.g. in community ecology 111 ). However, an outstanding issue is the need to incorporate measures of sampling effort as unbalanced sampling may lead to incorrect interpretations if not accounted for in analyses 112 – a problem similar to discriminatory biases in social data applications of machine learning.
Box 2 Investigating biological resilience of salmonid fishes
Numerous species and populations of salmonid fishes have been the focus of intensive monitoring and sampling programmes extending across many decades because of their socioeconomic significance and important ecosystem roles (including as keystone species). By combining existing research across biological levels (including genes, cells, populations and ecosystems) and evaluating the next steps within a biological resilience research framework, here we provide a worked example of the value of considering multiple biological levels when investigating an ecosystem-level perturbation.
The Barents Sea ecosystem is being perturbed by rapid increases in fishing pressure and climate change. Long-term ecological and environmental data together with life-history phenotype and genetic information from a population of Atlantic salmon ( Salmo salar ) from northernmost Europe is now being used to determine how this organism is responding via adaptation and shaping the overall resilience of the ecosystem (Box 2 Figure). Population genetic analyses 148 using a 50-year archive of fish scales have supported the hypothesis that reductions in life-history diversity (i.e. apparent low resistance) were actually an adaptive response to the perturbation 149 . The potential drivers of this response have then been investigated by linking these findings with long-term environmental and salmon prey species ecosystem data. It was discovered that as the abundance of capelin, a fat rich prey, declined so too did the abundance of salmon with a large body size and late-maturing life-history strategy 148 . Molecular biological research has also shown that the large-effect gene linked with the late-maturing life-history strategy and body condition in salmon 150 has important roles in adipocyte production regulation 151 , thus providing connections about biological resilience processes from genes and cells to populations and ecosystems. This example has implications for fisheries management, as prey species abundance was driven primarily by commercial fishing pressure: capelin is a common protein source in domestic animal (including aquaculture salmon) feed. Thus, research across multiple biological levels demonstrated indirect effects of (capelin) fishing on wild salmon life-history diversity.
To move closer to understanding biological resilience, the next steps include determining how selection acts on life-history traits when undergoing an ecosystem-level perturbation (including epigenetic markers on the genome from changes in cellular function, e.g. ref. 152 ), investigating how population-level demographic changes in salmon (including composition according to life-history traits) scale up to influence other ecological interactions within the Barents Sea ecosystem, and measuring the response curves (i.e. resistance and recovery, Fig. 1 ) of genetic diversity, demographic variables (i.e. effective population size), and community composition, before, during and possibly after the perturbation (i.e. depending on potential management scenarios to lessen disturbance on the ecosystem). This could be achieved by targeted experimental approaches and by using Big Data from both long-term surveys mentioned above and ancient DNA to determine response to past known ecological disturbance events (see ref. 153 for an example of herring population dynamics in response to the ‘first example of industrial fishing’ 800 years ago by the Vikings). Demo-genetic individual-based simulations that bring together data from individuals, populations, and communities (e.g. ref. 154 ) could then be a particularly useful method to link data across biological levels and to forecast future scenarios.

Translating biological resilience from research to management and conservation
While there have been many calls to adapt management and conservation of natural resources to improve resilience to environmental change, substantial obstacles remain before this can be realised. First, managers require indicators at levels most appropriate for decision-making. Many of the indicators currently available, however, are system-wide or remain challenging to quantify 15 , 113 , 114 . Indicators based on species diversity and habitat connectivity, for example, allow assessment of large-scale patterns 113 , but they are less helpful for management of more tractable system components. Similarly, current discussions around genetic diversity are often difficult to reconcile with ecosystem health as they operate at different timescales and in many cases the links to ecosystem functioning remain unclear (e.g. see ref. 115 for a discussion of this problem in the ecological restoration of plants). Second, attempts to manage ‘for resilience’ typically focus on avoiding thresholds or tipping points. Rather, managers need to compare alternative choices, assess potential outcomes with greater certainty than is currently possible, and manage adaptively 8 (Weise et al., 2020). Third, management approaches largely aim for current or recent known or assumed historical states, rather than attempting to forecast outcomes according to novel future conditions. This is especially problematic when the time horizon is long 8 , for example in forestry and agriculture where long or uncertain time horizons play a large part in the difficulty to translate recommendations 116 , 117 . Determining how resilience operates at different biological levels has potential to move beyond this stalemate, as the ecological and evolutionary history of components of the system 82 , 118 can be used to better evaluate past states, identify more manageable indicators at tractable biological levels, and predict future states under different management scenarios (e.g. Boxs 2 , 3 ).
Box 3 Applying biological resilience
Here we highlight the broad potential for the applicability of a biological resilience approach by briefly exploring how it could influence translation and management in two divergent examples: (i) forestry and agriculture, and (ii) human health.
(i) Biological resilience in forestry and agriculture
In the past, forest managers have assumed that the climate and other associated factors will remain stable, in spite of the long generation times and individual lifespans of many forest tree species and biomes 117 . However, soil degradation (for example) can occur rapidly compared to the lifespan of the forest and then impact on the ability of trees to withstand other environmental perturbations 155 . Similarly, modern plant breeding selects for yield potential under high and stable resource supply, and generally relies on genetically uniform cultivars. A biological resilience framework, however, encourages a different approach. For example, studies of local adaptation at the population level would help to understand how we can best buffer food and/or timber production against perturbations, perhaps by combining long-term data series and targeted experiments informed by historical farming practices or evolutionary processes 117 . In a context with clear applications for management, Ives and colleagues recently discovered that spatial heterogeneity in crop-harvesting is a major driver of the ecological and evolutionary feedbacks that limit resistance of pea aphids to parasitoid wasps, an important biological control agent 156 . Past perturbations also leave abiotic ‘stress memory’, encoded in DNA methylation and chromatin marks, which may increase resilience over multiple generations 157 , 158 in a process of acquired transgenerational resistance 43 . Similarly, interactions across trophic and biological levels are well-known features of plant growth and health, with key work demonstrating that these also influence resilience (e.g. plant-microbe interactions influence resistance to climate change 159 ). Harnessing this information could lead to improved crop plant and tree breeding programmes (e.g. ref. 160 ), but much of this work remains embedded in model plant systems, such as Arabidopsis . Understanding which features at what biological level are most important to manage (e.g. managing for genetic diversity of monotypic plantations versus diversity of associated mycorrhizal fungi) will require combined approaches and translation of work from model species to natural systems.
(ii) Biological resilience in human health
While ecological systems are increasingly becoming viewed as socio-ecological systems 2 , the idea that the human mind and body can be viewed as a complex ecological system is only just beginning to be recognised 123 , 161 . Understanding how circadian misalignment of sleep/wake cycles leads to a mismatch between abiotic cues and internal cellular functions (e.g. impairment of beta cell function and insulin sensitivity 162 ), and then scales up to affect system health via resilience to disease and other stressors, could help to provide more appropriate guidelines for managing shift work, for example. Recent experiences with COVID-19 also demonstrate the need to consider how resilience operates across biological levels: identifying what makes an individual more resilient to a virus at the cellular level (e.g. vaccine development) is not enough if insufficient people take up the vaccine (i.e. population level), or if the virus itself evolves resistance. Indeed, understanding the biological resilience of viral infections, or cancerous growths for example, to medical interventions could assist in progress with treatment. Genetic heterogeneity is known to negatively affect treatment success in cancer 163 , yet this heterogeneity reflects the selective pressures endured, and the variation accumulated, during the whole history of that cancer and can reveal vulnerabilities to therapy 164 . Furthermore, life-history strategies of cells, such as dormancy, can blunt the effects of therapy (e.g. tuberculosis). This suggests that diversity could be an important component of resilience in human health, but this requires testing in translational models.
Challenges of implementing a biological resilience framework
Here we have argued that understanding and managing for biological resilience requires moving away from the approach of considering function or resilience only at the level of ecosystems, or of focusing studies within a single biological level. We have also stressed how the resilience of the present state not only relies on perturbations experienced in the past (whether contemporary, transgenerational, or deeper in evolutionary time) but that we can also access information about these past responses. Nevertheless, incorporating evolutionary history and complex interactions within and across biological levels is non-trivial, and key challenges exist for modelling complexity and broadening the scope of data collection, as well as setting the temporal and spatial boundaries of the systems or components being studied.
Firstly, in both theoretical and empirical work, we need to identify which connections among what levels are most critical to study. A top-down view of ecosystems works best when considering change over a relatively short period of time, and reduces power for forecasting future responses, either to predicted environmental change or potential management interventions. In ecosystem ecology, species, for example, are normally classified into functional types that leave out valuable information about evolutionary responses to specific perturbations in the past. These responses can however be searched for by mining existing data (e.g. ref. 33 ) or by experiment (e.g. ref. 30 ). Similarly, we need to move beyond research focusing on what makes an individual, or a species, resistant or tolerant to some disturbance event without assessing its relevance to systems or communities. Research in eco-evolutionary dynamics is already beginning to tackle these interactions (see Box 1 ) and adapting this approach to investigate resilience provides a model for moving forwards. While it is not tractable to measure everything, well-controlled experiments can provide critical data to understand the mechanisms that drive biological resilience – or the lack of it. However, as experiments entail at least some simplification of natural complexity, results will need to be linked conceptually to surveys of the relevant organisms and ecosystems.
Considering multiple levels of biological organisation will also necessitate data collection that tracks responses and maximises phylogenetic, functional, spatial and temporal coverage with minimum monetary cost 119 . This is a challenging task for independent research groups as the acquisition of uninterrupted and consistent time series of ecological and environmental data depend on continued funding. Therefore, coordinated multidisciplinary research projects would enhance data collection and optimise funding streams, making it possible to expand the scope from single- to multiple levels. Some types of data are already available to inform about responses to past conditions, but if we are to make better use of existing and future available datasets, these will require high quality metadata annotations including as many potential ecological variables as possible (and not only the ones directly related to the analyses data were collected for) and easy and open access (e.g. following the FAIR principles 120 ).
Providing the evidence necessary to make the case to policy makers is perhaps the most important challenge. For example, accumulating knowledge on ecosystem resilience is yet to change the principles of forestry or cropland management dramatically, which is alarming given that we know many current management practices compromise the ability of future generations to meet their own needs. This may be because resilience is currently difficult to quantify, and a lack of resilience is easier to recognise than a successful management practice. A biological resilience framework could improve identification of ‘resilience indicators’ at scales in which management decisions are made. Tracking genetic diversity at a species level, for example, is a feasible method to collect robust data, and could enable modelling of which actions are likely to be most successful. A critical further step, however, will be improved monitoring of the impact of potential indicators so that we are able to learn from both successful and less successful implementations. Similarly, there are still substantial gaps to bridge between scientists, policymakers and other stakeholders. For example, in commercial farming and forestry widespread adoption of science-led practices depends on short-term economic benefits, so adoption will require policy-based incentives. A deeper understanding of management practices, and co-creation of research questions with stakeholders that will apply management practices, is essential, particularly if we are to implement decisions using an experimental approach.
In summary, biological resilience requires shifting our perspective in eco-evolutionary studies towards investigating terms of resistance versus recovery (the key conceptual outcomes in ecosystem resilience) while also incorporating an eco-evolutionary perspective to better understand ecosystem-level processes (see Fig. 1 , Box 1 ). This requires real multidisciplinary coordinated actions. But we can also begin to take small steps within existing research programmes. Researchers should consider reframing current research to test theory regarding types of responses to disturbance events under study. Or we could consider how influences from evolutionary history may impact ecological responses being detected under current conditions. Although challenging, this approach should provide the advances in data collection, modelling, and testing of hypotheses across levels that are urgently needed to understand and better support resilience in the face of current and future environmental challenges.
Lewis, S. L. & Maslin, M. A. Defining the Anthropocene. Nature 519 , 171–180 (2015).
Article CAS PubMed Google Scholar
Ellis, E. C. Ecology in an anthropogenic biosphere. Ecol. Monogr. 85 , 287–331 (2015).
Article Google Scholar
van Kleunen, M. et al. Global exchange and accumulation of non-native plants. Nature 525 , 100–103 (2015).
Article PubMed Google Scholar
Scheffer, M., Carpenter, S., Foley, J. A., Folke, C. & Walker, B. Catastrophic shifts in ecosystems. Nature 413 , 591–596 (2001).
Foley, J. A. Global consequences of land use. Science 309 , 570–574 (2005).
Sullivan, A. P., Bird, D. W. & Perry, G. H. Human behaviour as a long-term ecological driver of non-human evolution. Nat. Ecol. Evol. 1 , 0065 (2017).
Sutherland, W. J. et al. Identification of 100 fundamental ecological questions. J. Ecol. 101 , 58–67 (2013).
Weise, H. et al. Resilience trinity: safeguarding ecosystem functioning and services across three different time horizons and decision contexts. Oikos 129 , 445–456 (2020).
Helfgott, A. Operationalising systemic resilience. Eur. J. Oper. Res. 268 , 852–864 (2018).
Dakos, V. & Kéfi, S. Ecological resilience: what to measure and how. Environ. Res. Lett. 17 , 043003 (2022).
Yi, C. & Jackson, N. A review of measuring ecosystem resilience to disturbance. Environ. Res. Lett. 16 , 053008 (2021).
Pimm, S. L. The complexity and stability of ecosystems. Nature 307 , 321–326 (1984).
Holling, C. S. Resilience and stability of ecological systems. Ann. Rev. Ecol. Syst. 4 , 1–23 (1973).
Oliver, T. H. et al. Biodiversity and resilience of ecosystem functions. Trends Ecol. Evol. 30 , 673–684 (2015).
Ingrisch, J. & Bahn, M. Towards a comparable quantification of resilience. Trends Ecol. Evol. 33 , 251–259 (2018).
Capdevila, P. et al. Reconciling resilience across ecological systems, species and subdisciplines. J. Ecol. 109 , 3102–3113 (2021).
Gladstone-Gallagher, R. V., Pilditch, C. A., Stephenson, F. & Thrush, S. F. Linking traits across ecological scales determines functional resilience. Trends Ecol. Evol. 34 , 1080–1091 (2019).
Levin, S. A. The problem of pattern and scale in ecology: the Robert H. MacArthur Award lecture. Ecology 73 , 1943–1967 (1992).
Gallagher, R. & Appenzeller, T. Beyond reductionism. Science 284 , 79–79 (1999).
Article CAS Google Scholar
McGill, B. J. et al. Unifying macroecology and macroevolution to answer fundamental questions about biodiversity. Global Ecol. Biogeogr. 28 , 1925–1936 (2019).
Cant, J., Capdevila, P., Beger, M. & Salguero‐Gómez, R. Recent exposure to environmental stochasticity does not determine the demographic resilience of natural populations. Ecol. Lett. 26 , 1186–1199 (2023).
Dakos, V. et al. Ecosystem tipping points in an evolving world. Nat. Ecol. Evol. 3 , 355–362 (2019).
Morris, D. W. Adaptation and habitat selection in the eco-evolutionary process. Proc. R. Soc. B: Biol. Sci. 278 , 2401–2411 (2011).
Tylianakis, J. M. & Maia, L. F. The patchwork of evolutionary landscapes. Nat. Ecol. Evol. 4 , 672–673 (2020).
Carroll, S. P. et al. Applying evolutionary biology to address global challenges. Science 346 , 1245993–1245993 (2014).
Article PubMed PubMed Central Google Scholar
Desai, M. M. Reverse evolution and evolutionary memory. Nat. Genet. 41 , 142–143 (2009).
Donohue, I. et al. Navigating the complexity of ecological stability. Ecol. Lett. 19 , 1172–1185 (2016).
Bartholomé, J. et al. The genetics of exapted resistance to two exotic pathogens in pedunculate oak. New Phytol. 226 , 1088–1103 (2020).
Donelson, J. M. et al. Understanding interactions between plasticity, adaptation and range shifts in response to marine environmental change. Philos. Trans. R. Soc. B 374 , 20180186 (2019).
Oostra, V., Saastamoinen, M., Zwaan, B. J. & Wheat, C. W. Strong phenotypic plasticity limits potential for evolutionary responses to climate change. Nat. Commun. 9 , 1005 (2018).
Hang, D., Torng, E., Ofria, C. & Schmidt, T. M. The effect of natural selection on the performance of maximum parsimony. BMC Evol. Biol. 7 , 1–15 (2007).
Sun, M. et al. Recent accelerated diversification in rosids occurred outside the tropics. Nat. Commun. 11 , 1–12 (2020).
Google Scholar
Zitnik, M., Sosič, R., Feldman, M. W. & Leskovec, J. Evolution of resilience in protein interactomes across the tree of life. Proc. Natl Acad. Sci. USA 116 , 4426–4433 (2019).
Article CAS PubMed PubMed Central Google Scholar
Hecht, L. B., Thompson, P. C. & Rosenthal, B. M. Assessing the evolutionary persistence of ecological relationships: a review and preview. Infect. Genet. Evol. 84 , 104441 (2020).
Mathur, S., Tomeček, J. M., Tarango-Arámbula, L. A., Perez, R. M. & DeWoody, J. A. An evolutionary perspective on genetic load in small, isolated populations as informed by whole genome resequencing and forward-time simulations. Evolution 77 , 690–704 (2023).
Louis, M. et al. Influence of past climate change on phylogeography and demographic history of narwhals, Monodon monoceros . Proc. R. Soc. B 287 , 20192964 (2020).
Cortés, A. J., López-Hernández, F. & Osorio-Rodriguez, D. Predicting thermal adaptation by looking into populations’ genomic past. Front. Genet. 11 , 564515 (2020).
Fox, R. J., Donelson, J. M., Schunter, C., Ravasi, T. & Gaitán-Espitia, J. D. Beyond buying time: the role of plasticity in phenotypic adaptation to rapid environmental change. Philos. Trans. R.Soc. B 374 , 20180174 (2019).
Ghalambor, C. K., McKay, J. K., Carroll, S. P. & Reznick, D. N. Adaptive versus non‐adaptive phenotypic plasticity and the potential for contemporary adaptation in new environments. Funct. Ecol. 21 , 394–407 (2007).
Thebault, E. & Fontaine, C. Stability of ecological communities and the architecture of mutualistic and trophic networks. Science 329 , 853–856 (2010).
Turcotte, M. M. & Levine, J. M. Phenotypic plasticity and species coexistence. Trends Ecol. Evol. 31 , 803–813 (2016).
Hess, C., Levine, J. M., Turcotte, M. M. & Hart, S. P. Phenotypic plasticity promotes species coexistence. Nat. Ecol. Evol. 6 , 1256–1261 (2022).
Holeski, L. M., Jander, G. & Agrawal, A. A. Transgenerational defense induction and epigenetic inheritance in plants. Trends Ecol. Evol. 27 , 618–626 (2012).
Pazzaglia, J., Reusch, T. B. H., Terlizzi, A., Marín‐Guirao, L. & Procaccini, G. Phenotypic plasticity under rapid global changes: the intrinsic force for future seagrasses survival. Evol. Appl. 14 , 1181–1201 (2021).
Grottoli, A. G., Rodrigues, L. J. & Palardy, J. E. Heterotrophic plasticity and resilience in bleached corals. Nature 440 , 1186–1189 (2006).
Seebacher, F., White, C. R. & Franklin, C. E. Physiological plasticity increases resilience of ectothermic animals to climate change. Nat. Clim. Change 5 , 61–66 (2015).
Taylor, B. M. et al. Demographic plasticity facilitates ecological and economic resilience in a commercially important reef fish. J. Anim. Ecol. 88 , 1888–1900 (2019).
Valladares, F. et al. The effects of phenotypic plasticity and local adaptation on forecasts of species range shifts under climate change. Ecol. Lett. 17 , 1351–1364 (2014).
McNamara, J. M., Dall, S. R. X., Hammerstein, P. & Leimar, O. Detection vs. selection: integration of genetic, epigenetic and environmental cues in fluctuating environments. Ecol. Lett. 19 , 1267–1276 (2016).
Barnosky, A. D. et al. Approaching a state shift in Earth’s biosphere. Nature 486 , 52–58 (2012).
Legrand, D. et al. Eco-evolutionary dynamics in fragmented landscapes. Ecography 40 , 9–25 (2017).
Hämäläinen, L., M. Rowland, H., Mappes, J. & Thorogood, R. Social information use by predators: expanding the information ecology of prey defences. Oikos 2022 , e08743 (2022).
Fukami, T. Historical contingency in community assembly: integrating niches, species pools, and priority effects. Ann. Rev. Ecol. Evol. Syst. 46 , 1–23 (2015).
Baruah, G., Clements, C. F. & Ozgul, A. Eco‐evolutionary processes underlying early warning signals of population declines. J. Anim. Ecol. 89 , 436–448 (2020).
Strona, G. et al. Global tropical reef fish richness could decline by around half if corals are lost. Proc. R. Soc. Lond. B 288 , 20210274 (2021).
CAS Google Scholar
Massot, M., Legendre, S., Fédérici, P. & Clobert, J. Climate warming: a loss of variation in populations can accompany reproductive shifts. Ecol. Lett. 20 , 1140–1147 (2017).
Sgrò, C. M., Lowe, A. J. & Hoffmann, A. A. Building evolutionary resilience for conserving biodiversity under climate change. Evol. Appl. 4 , 326–337 (2011).
Fahrig, L. Effects of habitat fragmentation on biodiversity. Ann. Rev. Ecol. Evol. Syst. 34 , 487–515 (2003).
Baguette, M. & Van Dyck, H. Landscape connectivity and animal behavior: functional grain as a key determinant for dispersal. Landsc. Ecol. 22 , 1117–1129 (2007).
Anderson, C. D. et al. Considering spatial and temporal scale in landscape-genetic studies of gene flow. Mol. Ecol. 19 , 3565–3575 (2010).
Clark, A. T. et al. General statistical scaling laws for stability in ecological systems. Ecol. Lett. 24 , 1474–1486 (2021).
Yamamichi, M., Gibbs, T. & Levine, J. M. Integrating eco‐evolutionary dynamics and modern coexistence theory. Ecol. Lett. 25 , 2091–2106 (2022).
Cavigelli, S., Leips, J., Xiang, Q. Y., Lemke, D. & Konow, N. Next steps in integrative biology: mapping interactive processes across levels of biological organization. Integr. Comp. Biol. 61 , 2066–2074 (2021).
Meredith, H. R. et al. Applying ecological resistance and resilience to dissect bacterial antibiotic responses. Sci. Adv. 4 , eaau1873 (2018).
Waldvogel, A. et al. Evolutionary genomics can improve prediction of species’ responses to climate change. Evol. Lett. 4 , 4–18 (2020).
Waples, R. S., Beechie, T. & Pess, G. R. Evolutionary history, habitat disturbance regimes, and Anthropogenic changes: what do these mean for resilience of Pacific salmon populations?. Ecol. Soc. 14 , art3 (2009).
Stange, M., Barrett, R. D. H. & Hendry, A. P. The importance of genomic variation for biodiversity, ecosystems and people. Nat. Rev. Genet. 22 , 89–105 (2021).
Hughes, T. P., Graham, N. A., Jackson, J. B., Mumby, P. J. & Steneck, R. S. Rising to the challenge of sustaining coral reef resilience. Trends Ecol. Evolu. 25 , 633–642 (2010).
Roff, G. Evolutionary history drives biogeographic patterns of coral reef resilience. Bioscience 71 , 26–39 (2021).
Fuller, Z. L. et al. Population genetics of the coral Acropora millepora : toward genomic prediction of bleaching. Science 369 , eaba4674 (2020).
van Oppen, M. J. H., Oliver, J. K., Putnam, H. M. & Gates, R. D. Building coral reef resilience through assisted evolution. Proc. Natl Acad. Sci. USA 112 , 2307–2313 (2015).
Bay, R. A. & Guerrero, L. Can genomes predict coral bleaching? Science 369 , 249–250 (2020).
Papakostas, S. et al. A proteomics approach reveals divergent molecular responses to salinity in populations of European whitefish ( Coregonus lavaretus ). Mol. Ecol. 21 , 3516–3530 (2012).
Thom, D. et al. The climate sensitivity of carbon, timber, and species richness covaries with forest age in boreal–temperate North America. Glob. Change Biol. 25 , 2446–2458 (2019).
Strayer, D. L. Alien species in fresh waters: ecological effects, interactions with other stressors, and prospects for the future. Freshw. Biol. 55 , 152–174 (2010).
López-Maury, L., Marguerat, S. & Bähler, J. Tuning gene expression to changing environments: from rapid responses to evolutionary adaptation. Nat. Rev. Genet. 9 , 583–593 (2008).
Kokko, H. et al. Can evolution supply what ecology demands? Trends Ecol. Evol. 32 , 187–197 (2017).
Verhagen, I., Tomotani, B. M., Gienapp, P. & Visser, M. E. Temperature has a causal and plastic effect on timing of breeding in a small songbird. The J. Exp. Biol. 223 , jeb218784 (2020).
Grant, P. R. et al. Evolution caused by extreme events. Philos. Trans. R. Soc. B: Biol. Sci. 372 , 20160146 (2017).
Ellegren, H., Lindgren, G., Primmer, C. R. & Møller, A. P. Fitness loss and germline mutations in barn swallows breeding in Chernobyl. Nature 389 , 593–596 (1997).
Bergen, E. et al. The effect of summer drought on the predictability of local extinctions in a butterfly metapopulation. Conserv. Biol. 34 , 1503–1511 (2020).
Fraser, D. et al. Investigating biotic interactions in deep time. Trends Ecol. Evol. 36 , 61–75 (2020).
Lyons, S. K. et al. Holocene shifts in the assembly of plant and animal communities implicate human impacts. Nature 529 , 80–83 (2016).
Frisch, D. et al. A millennial-scale chronicle of evolutionary responses to cultural eutrophication in Daphnia. Ecol. Lett. 17 , 360–368 (2014).
Hendry, A. P. A critique for eco‐evolutionary dynamics. Funct. Ecol. 33 , 84–94 (2019).
Carpenter, S. R. et al. Early warnings of regime shifts: a whole-ecosystem experiment. Science 332 , 1079–1082 (2011).
Franks, V. R. et al. Changes in social groups across reintroductions and effects on post-release survival. Anim. Conserv. 23 , 443–454 (2020).
Goldenberg, S. Z. et al. Increasing conservation translocation success by building social functionality in released populations. Glob. Ecol. Conserv. 18 , e00604 (2019).
Koch, E. L. & Guillaume, F. Additive and mostly adaptive plastic responses of gene expression to multiple stress in Tribolium castaneum . PLoS Genet. 16 , e1008768 (2020).
Sun, S.-J. & Kilner, R. M. Temperature stress induces mites to help their carrion beetle hosts by eliminating rival blowflies. ELife 9 , e55649 (2020).
Papakostas, S. et al. Gene pleiotropy constrains gene expression changes in fish adapted to different thermal conditions. Nat. Commun. 5 , 4071 (2014).
Bustos‐Korts, D. et al. Exome sequences and multi‐environment field trials elucidate the genetic basis of adaptation in barley. Plant J. 99 , 1172–1191 (2019).
Franks, S. J. et al. The resurrection initiative: storing ancestral genotypes to capture evolution in action. BioScience 58 , 870–873 (2008).
National Library of Medicine (US) & National Center for Biotechnology Information. National Center for Biotechnology Information (NCBI) . https://www.ncbi.nlm.nih.gov/ (2020).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. Nat. Genet. 25 , 25–29 (2000).
Kanehisa, M., Sato, Y., Furumichi, M., Morishima, K. & Tanabe, M. New approach for understanding genome variations in KEGG. Nucleic Acids Res. 47 , D590–D595 (2019).
Primmer, C. R., Papakostas, S., Leder, E. H., Davis, M. J. & Ragan, M. A. Annotated genes and nonannotated genomes: cross-species use of Gene Ontology in ecology and evolution research. Mol. Ecol. 22 , 3216–3241 (2013).
European Union, Copernicus Land Monitoring Service 2018, European Environment Agency. CORINE Land Cover . https://land.copernicus.eu/pan-european/corine-land-cover (2023).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37 , 4302–4315 (2017).
Karger, D. N. et al. Climatologies at high resolution for the earth’s land surface areas. Sci. Data 4 , 170122 (2017).
GBIF.org. GBIF Home Page . https://www.gbif.org (2020).
Kattge, J. et al. TRY plant trait database – enhanced coverage and open access. Glob. Change Biol. 26 , 119–188 (2020).
Ovaskainen, O. et al. Chronicles of nature calendar, a long-term and large-scale multitaxon database on phenology. Sci. Data 7 , 47 (2020).
The NOW Community. NOW—New and Old Worlds: Database of fossil mammals. Zenodo https://zenodo.org/record/4268068 (2020),
Kotta, J. et al. Integrating experimental and distribution data to predict future species patterns. Sci. Rep. 9 , 1821 (2019).
Barone, L., Williams, J. & Micklos, D. Unmet needs for analyzing biological big data: a survey of 704 NSF principal investigators. PLoS Comput. Biol. 13 , e1005755 (2017).
Waese, J. et al. ePlant: visualizing and exploring multiple levels of data for hypothesis generation in plant biology. Plant Cell 29 , 1806–1821 (2017).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10 , 1523 (2019).
Ovaskainen, O., & Abrego, N. Joint Species Distribution Modeling with Applications in R . (Cambridge University Press, 2020).
DeAngelis, D. L. & Grimm, V. Individual-based models in ecology after four decades. F1000Prime Rep. 6 , 39 (2014).
Cardoso, P. et al. Automated discovery of relationships, models, and principles in ecology. Front. Ecol. Evol. 8 , 530135 (2020).
Foster, S. D. et al. Effects of ignoring survey design information for data reuse. Ecol. Appl. 31 , e02360 (2021).
Dunbar, W. et al. in Managing Socio-ecological Production Landscapes and Seascapes for Sustainable Communities in Asia: Mapping and Navigating Stakeholders, Policy and Action (Saito, O., Subramanian, S. M., Hashimoto, S. & Takeuchi, K.) 93–116 (Springer Singapore, 2020).
Standish, R. J. et al. Resilience in ecology: abstraction, distraction, or where the action is? Biol. Conserv. 177 , 43–51 (2014).
Kettenring, K. M., Mercer, K. L., Reinhardt Adams, C. & Hines, J. Application of genetic diversity-ecosystem function research to ecological restoration. J. Appl. Ecol. 51 , 339–348 (2014).
Dhankher, O. P. & Foyer, C. H. Climate resilient crops for improving global food security and safety. Plant Cell Environ. 41 , 877–884 (2018).
Millar, C. I., Stephenson, N. L. & Stephens, S. L. Climate change and forests of the future: managing in the face of uncertainty. Ecol. Appl. 17 , 2145–2151 (2007).
Yang, Q., Fowler, M. S., Jackson, A. L. & Donohue, I. The predictability of ecological stability in a noisy world. Nat. Ecol. Evol. 3 , 251–259 (2019).
Cardoso, P. & Leather, S. R. Predicting a global insect apocalypse. Insect Conser. Divers. 12 , 263–267 (2019).
Wilkinson, M. D. et al. The FAIR guiding principles for scientific data management and stewardship. Sci. Data 3 , 160018 (2016).
Molinelli, E. J. et al. Perturbation biology: inferring signaling networks in cellular systems. PLoS Comput. Biol. 9 , e1003290 (2013).
Jansen, R. C. Studying complex biological systems using multifactorial perturbation. Nat. Rev. Genet. 4 , 145–151 (2003).
Billman, G. E. Homeostasis: the underappreciated and far too often ignored central organizing principle of physiology. Front. Physiol. 11 , 200 (2020).
Harmange, G., et al. Disrupting cellular memory to overcome drug resistance. Preprint at bioRxiv https://doi.org/10.1101/2022.06.16.496161 (2022).
Stockmaier, S., Ulrich, Y., Albery, G. F., Cremer, S. & Lopes, P. C. Behavioural defences against parasites across host social structures. Funct. Ecol. 37 , 809–820 (2023).
Craine, J. M. et al. Global diversity of drought tolerance and grassland climate-change resilience. Nat. Clim. Change 3 , 63–67 (2013).
Rivera, H. E. et al. A framework for understanding gene expression plasticity and its influence on stress tolerance. Mol. Ecol. 30 , 1381–1397 (2021).
Júnior, E. C. B., Rios, V. P., Dodonov, P., Vilela, B. & Japyassú, H. F. Effect of behavioural plasticity and environmental properties on the resilience of communities under habitat loss and fragmentation. Ecol. Modell. 472 , 110071 (2022).
Leivesley, J. A. et al. Survival costs of reproduction are mediated by parasite infection in wild Soay sheep. Ecol. Lett. 22 , ele.13275 (2019).
Krause, S. M. B. et al. Environmental legacy contributes to the resilience of methane consumption in a laboratory microcosm system. Sci. Rep. 8 , 8862 (2018).
Barnosky, A. D. et al. Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science 355 , eaah4787 (2017).
de Mazancourt, C., Johnson, E. & Barraclough, T. G. Biodiversity inhibits species’ evolutionary responses to changing environments. Ecol. Lett. 11 , 380–388 (2008).
Simberloff, D. et al. Impacts of biological invasions: what’s what and the way forward. Trends Ecol. Evol. 28 , 58–66 (2013).
Steane, D. A. et al. Genome-wide scans detect adaptation to aridity in a widespread forest tree species. Mol. Ecol. 23 , 2500–2513 (2014).
Bell, G. Evolutionary rescue and the limits of adaptation. Philos. Trans. R. Soc. B: Biol. Sci. 368 , 20120080 (2013).
Ware, I. M. et al. Feedbacks link ecosystem ecology and evolution across spatial and temporal scales: empirical evidence and future directions. Funct. Ecol. 33 , 31–42 (2019).
Bassar, R. D., Coulson, T., Travis, J. & Reznick, D. N. Towards a more precise—and accurate—view of eco‐evolution. Ecol. Lett. 24 , 623–625 (2021).
Hanski, I. Eco‐evolutionary dynamics in a changing world. Ann. N. Y. Acad. Sci. 1249 , 1–17 (2012).
Schoener, T. W. The newest synthesis: understanding the interplay of evolutionary and ecological dynamics. Science 331 , 426–429 (2011).
Palkovacs, E. P. & Hendry, A. P. Eco-evolutionary dynamics: Intertwining ecological and evolutionary processes in contemporary time. F1000 Biol. Rep. 2 , 1 (2010).
Govaert, L. et al. Eco‐evolutionary feedbacks—theoretical models and perspectives. Funct. Ecol. 33 , 13–30 (2019).
Nadeau, C. P. & Urban, M. C. Eco-evolution on the edge during climate change. Ecography 42 , 1280–1297 (2019).
Coblentz, K. E., & DeLong, J. P. Ecological boundaries and constraints on viable eco‐evolutionary pathways. Oikos https://doi.org/10.1111/oik.09893 (2023).
Yanniris, C. & Frankel, V. M. Bridging valleys: expanding the adaptive landscape concept beyond theoretical space: with applications in ecology and evolution. Ideas Ecol. Evol. 11 , 10–18 (2018).
Kelly, R. et al. Ten tips for developing interdisciplinary socio-ecological researchers. Socio-Ecol. Pract. Res. 1 , 149–161 (2019).
Svensson, E. I. & Råberg, L. Resistance and tolerance in animal enemy–victim coevolution. Trends Ecol. Evol. 25 , 267–274 (2010).
Roy, B. A. & Kirchner, J. W. Evolutionary dynamics of pathogen resistance and tolerance. Evolution 54 , 51–63 (2000).
CAS PubMed Google Scholar
Czorlich, Y., Aykanat, T., Erkinaro, J., Orell, P. & Primmer, C. R. Rapid evolution in salmon life history induced by direct and indirect effects of fishing. Science 376 , 420–423 (2022).
Czorlich, Y., Aykanat, T., Erkinaro, J., Orell, P. & Primmer, C. R. Rapid sex-specific evolution of age at maturity is shaped by genetic architecture in Atlantic salmon. Nat. Ecol. Evol. 2 , 1800–1807 (2018).
Debes, P. V. et al. Polygenic and major‐locus contributions to sexual maturation timing in Atlantic salmon. Mol. Ecol. 30 , 4505–4519 (2021).
Halperin, D. S., Pan, C., Lusis, A. J. & Tontonoz, P. Vestigial-like 3 is an inhibitor of adipocyte differentiation. J. Lipid Res. 54 , 473–481 (2013).
Sävilammi, T. et al. Cytosine methylation patterns suggest a role of methylation in plastic and adaptive responses to temperature in European grayling ( Thymallus thymallus ) populations. Epigenetics 16 , 271–288 (2020).
Atmore, L. M. et al. Population dynamics of Baltic herring since the Viking Age revealed by ancient DNA and genomics. Proc. Natl Acad. Sci. USA 119 , e2208703119 (2022).
Lamarins, A. et al. Importance of interindividual interactions in eco-evolutionary population dynamics: the rise of demo-genetic agent-based models. Evol. Appli. 15 , 1988–2001 (2022).
Swinfield, T. et al. Imaging spectroscopy reveals the effects of topography and logging on the leaf chemistry of tropical forest canopy trees. Glob. Change Biol. 26 , 989–1002 (2020).
Ives, A. R. et al. Self-perpetuating ecological–evolutionary dynamics in an agricultural host–parasite system. Nat. Ecol. Evol. 4 , 702–711 (2020).
Chang, Y. et al. Epigenetic regulation in plant abiotic stress responses. J. Int. Plant Biol. 62 , 563–580 (2020).
Friedrich, T., Faivre, L., Bäurle, I. & Schubert, D. Chromatin-based mechanisms of temperature memory in plants. Plant, Cell Environ. 42 , 762–770 (2019).
Rudgers, J. A. et al. Climate disruption of plant-microbe interactions. Ann. Rev. Ecol. Evol. Syst. 51 , 561–586 (2020).
Messier, C. et al. The functional complex network approach to foster forest resilience to global changes. For. Ecosyst. 6 , 21 (2019).
Bernstein, A. All creatures great and small. BMJ https://doi.org/10.1136/bmj.l2385 (2019).
Mason, I. C., Qian, J., Adler, G. K. & Scheer, F. A. J. L. Impact of circadian disruption on glucose metabolism: implications for type 2 diabetes. Diabetologia 63 , 462–472 (2020).
Maley, C. C. et al. Genetic clonal diversity predicts progression to esophageal adenocarcinoma. Nat. Genet. 38 , 468–473 (2006).
Alexandrov, L. B., Nik-Zainal, S., Siu, H. C., Leung, S. Y. & Stratton, M. R. A mutational signature in gastric cancer suggests therapeutic strategies. Nat. Commun. 6 , 8683 (2015).
Download references
Acknowledgements
This manuscript is a contribution by members of the HiLIFE (Helsinki Institute for Life Science) Grand Challenge programme in Understanding Biological Resilience (BIORESILIENCE), established after external review by the HiLIFE Scientific Council and funded by the Academy of Finland funding instrument PROFI1 (awarded to the University of Helsinki). This initiative brought together 40+ researchers from diverse fields including ecology, evolution, and conservation to neurobiology, veterinary medicine, and cancer biology with the intention to spark discussion and begin novel collaborative projects—many of which were restricted by the COVID-19 pandemic. We are grateful to Jenni Villa and Unni Pulliainen for their project coordination efforts, Mikhail Shubin for his assistance with drawing figures, and helpful discussion and comments on the manuscript from Pol Capdevila. Other funding provided to: R.T.: HiLIFE start-up grant (funded by Academy of Finland PROFI1) and Academy of Finland grant no. 133803; J.C.: Jenny and Antti Wihuri Foundation; P.C.: Kone Foundation; J.K.: HiLIFE Fellows scheme (funded by Academy of Finland PROFI1) and Academy of Finland grant no. 328961; L.K.: Academy of Finland grant no. 12871741; R.M.: Academy of Finland grant no. 312912; O.O.: Academy of Finland grant no. 309581 and Norges Forskningsråd (Research Council of Norway) no. 223257; C.R.P.: HiLIFE Fellows scheme (funded by Academy of Finland PROFI1) and Academy of Finland grant no. 314254. All authors from the Research Centre for Ecological Change were funded by the Jane and Aatos Erkko Foundation.
Author information
Andriy Kovalchuk
Present address: Onego Bio Ltd, Helsinki, Finland
These authors contributed equally: Rose Thorogood, Ville Mustonen.
Authors and Affiliations
HiLIFE Helsinki Institute of Life Science, University of Helsinki, Helsinki, Finland
Rose Thorogood & Marjo Saastamoinen
Research Programme in Organismal & Evolutionary Biology, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland
Rose Thorogood, Ville Mustonen, Pedro J. Aphalo, Mar Cabeza, Johannes Cairns, Ulrika Candolin, Maria Hällfors, Jonna Kulmuni, Otso Ovaskainen, Craig R. Primmer, Marjo Saastamoinen, Giovanni Strona & Jarno Vanhatalo
Department of Computer Science, Faculty of Science, University of Helsinki, Helsinki, Finland
Ville Mustonen
Helsinki Institute for Information Technology, University of Helsinki, Helsinki, Finland
Ville Mustonen & Johannes Cairns
Institute of Biotechnology, HiLIFE Helsinki Institute for Life Science, University of Helsinki, Helsinki, Finland
Ville Mustonen, Craig R. Primmer & Alan H. Schulman
LUOMUS Finnish Museum of Natural History, University of Helsinki, Helsinki, Finland
Alexandre Aleixo, Pedro Cardoso, Aino Juslén & Leif Schulman
Viikki Plant Science Centre, University of Helsinki, Helsinki, Finland
Pedro J. Aphalo, Fred O. Asiegbu & Alan H. Schulman
Department of Forest Sciences, Faculty of Agriculture and Forestry, University of Helsinki, Helsinki, Finland
Fred O. Asiegbu & Andriy Kovalchuk
HELSUS Helsinki Institute of Sustainability Science, University of Helsinki, Helsinki, Finland
Mar Cabeza & Jussi T. Eronen
CE3C - Centre for Ecology, Evolution and Environmental Changes, CHANGE—Global Change and Sustainability Institute, Faculty of Sciences, University of Lisbon, 1749-016, Lisbon, Portugal
Pedro Cardoso
Research Programme in Ecosystems and Environment, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland
Jussi T. Eronen
BIOS Research Unit, Helsinki, Finland
Research Centre for Ecological Change, Faculty of Biological and Environmental Sciences, University of Helsinki, Helsinki, Finland
Maria Hällfors, Marjo Saastamoinen, Giovanni Strona & Jarno Vanhatalo
Syke Finnish Environment Institute, Helsinki, Finland
Maria Hällfors, Aino Juslén & Leif Schulman
SleepWell Research Program, Faculty of Medicine, University of Helsinki, Helsinki, Finland
Iiris Hovatta, Liisa Kuula & Anu-Katriina Pesonen
Department of Psychology and Logopedics, Faculty of Medicine, University of Helsinki, Helsinki, Finland
Iiris Hovatta
Neuroscience Center, HiLIFE Helsinki Institute for Life Science, University of Helsinki, Helsinki, Finland
VTT Technical Research Centre of Finland Ltd, Espoo, Finland
Department of Evolutionary and Population Biology, Institute for Biodiversity and Ecosystem Dynamics, University of Amsterdam, Amsterdam, The Netherlands
Jonna Kulmuni
Natural Resources Institute Finland (Luke), Helsinki, Finland
Raisa Mäkipää & Alan H. Schulman
Centre for Biodiversity Dynamics, Department of Biology, Norwegian University of Science and Technology, Trondheim, Norway
Otso Ovaskainen
Department of Biological and Environmental Science, University of Jyväskylä, Jyväskylä, Finland
European Commission, Joint Research Centre, Directorate D – Sustainable Resources, Ispra, Italy
Giovanni Strona
Department of Mathematics and Statistics, Faculty of Science, University of Helsinki, Helsinki, Finland
Jarno Vanhatalo
You can also search for this author in PubMed Google Scholar
Contributions
All authors were involved in conceptualisation, writing—original draft preparation, and writing—review and editing. R.T. and V.M. were responsible for preparation of the final version.
Corresponding author
Correspondence to Rose Thorogood .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Thorogood, R., Mustonen, V., Aleixo, A. et al. Understanding and applying biological resilience, from genes to ecosystems. npj biodivers 2 , 16 (2023). https://doi.org/10.1038/s44185-023-00022-6
Download citation
Received : 16 March 2022
Accepted : 07 August 2023
Published : 28 August 2023
DOI : https://doi.org/10.1038/s44185-023-00022-6
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
Riparian plant-soil-microbial c:n:p stoichiometry: are they conserved at plant functional group level.
Environmental Science and Pollution Research (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Anthropocene newsletter — what matters in anthropocene research, free to your inbox weekly.
Click through the PLOS taxonomy to find articles in your field.
For more information about PLOS Subject Areas, click here .
Loading metrics
Open Access
Peer-reviewed
Research Article
Evaluation of bioavailable 137 Cs transfer from forest litter to Scarabaeidae beetle ( Protaetia orientalis ) through a breeding experiment in Fukuhshima
Roles Conceptualization, Investigation, Visualization, Writing – original draft
Affiliation Environmental Impact Assessment Section, Fukushima Branch, National Institute for Environmental Studies, Miharu, Fukushima, Japan
Roles Formal analysis, Visualization, Writing – original draft
* E-mail: [email protected]
Roles Conceptualization, Writing – review & editing
Affiliations Environmental Impact Assessment Section, Fukushima Branch, National Institute for Environmental Studies, Miharu, Fukushima, Japan, Savannah River Ecology Laboratory, University of Georgia, Aiken, South Carolina, United States of America, Department of Animal Science, Faculty of Agriculture, Iwate University, Morioka, Iwate, Japan
Roles Investigation
Roles Supervision, Writing – review & editing
- Jaeick Jo,
- Yumiko Ishii,
- Rie Saito,
- Asuka Tanaka,
- Seiji Hayashi

- Published: September 6, 2024
- https://doi.org/10.1371/journal.pone.0310088
- Reader Comments
Following the Fukushima Daiichi Nuclear Power Plant accident in 2011, most of the released 137 Cs remained in the litter and surface soil of the adjacent forest floor. However, 137 Cs absorption by large soil invertebrates near this site has not been estimated. The aim of this study was to understand the role of soil macroinvertebrates in 137 Cs uptake from forest litter into forest ecosystems. Breeding experiments were conducted using scarab beetle larvae ( Protaetia orientalis ). Dissection experiments revealed that 85% of the total 137 Cs was concentrated in the digestive tract of larvae, while a low proportion was absorbed into the skin and muscle tissues. The 137 Cs absorption rate, indicating the transfer of 137 Cs from consumed litter to larval tissue, was low (0.39%). 137 Cs concentrations decreased to one-fourth from larva to imago, possibly due to excretion from the digestive tract and during eclosion. In the elimination experiment, biological half-lives were 0.26–0.64 and 0.11–0.47 days and 3.35–48.30 and 4.01–17.70 days for the digestive tract and muscle/skin tissues in the fast and slow components, respectively, corresponding to 137 Cs discharge from the gastrointestinal tract and physiological clearance. In the sequential extraction experiment, litter digestion by flower chafer larvae significantly reduced the bioavailable fraction of 137 Cs including water-soluble, exchangeable, oxidized, and organic forms, from 23.2% in litter to 17.7% in feces. Residual 137 Cs was not reduced by digestion, probably because it was fixed in soil clay. Our study on breeding experiments of the Scarabaeidae beetle confirmed the low bioavailability of 137 Cs in the litter in Fukushima. However, litter feeders may play an important role in transferring 137 Cs to higher trophic levels in the forest ecosystem by extracting the bioavailable fraction of the vast stock of 137 Cs on the forest floor.
Citation: Jo J, Ishii Y, Saito R, Tanaka A, Hayashi S (2024) Evaluation of bioavailable 137 Cs transfer from forest litter to Scarabaeidae beetle ( Protaetia orientalis ) through a breeding experiment in Fukuhshima. PLoS ONE 19(9): e0310088. https://doi.org/10.1371/journal.pone.0310088
Editor: Mohamad Syazwan Mohd Sanusi, Universiti Teknologi Malaysia, MALAYSIA
Received: December 26, 2023; Accepted: August 24, 2024; Published: September 6, 2024
Copyright: © 2024 Jo et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: All relevant data are within the manuscript and its Supporting information files.
Funding: This work was financially supported by the research program on Disaster Environment, an internal budget of National Institute for Environmental Studies. The internal budget was originally issued by the Ministry of Environment, Japan ( http://www.nies.go.jp/shinsai/index-e.html ). The funders of this paper had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Introduction
The Fukushima Daiichi Nuclear Power Plant accident that occurred in March 2011 released large quantities of radiocesium ( 137 Cs and 134 Cs) into the environment. Because the half-life of 137 Cs (30.2 yr) is longer than that of 134 Cs (2.06 yr), it persists for a longer time [ 1 ]. In forested areas, 137 Cs is initially trapped in the leaves and branches of the forest canopy and subsequently migrates downward to the forest floor through litterfall and rain [ 2 , 3 ]. Most of the total 137 Cs stays in the upper part of the mineral soil layer (0–5 cm depth) [ 4 – 6 ] because 137 Cs is fixed on frayed edge sites in the interlayers of illite and micaceous clay minerals. This retention is nearly irreversible and limits the vertical migration of 137 Cs into deeper forest soil layers [ 7 ]. However, some 137 Cs remains in a bioavailable form in the surface forest floor layer for a long time because of the cycling process of uptake and defoliation by trees and 137 Cs accumulation by fungal activities [ 8 – 11 ]. 137 Cs in organic matter can serve as a major source of 137 Cs cycling in the forest ecosystem [ 6 , 12 ].
Detritivores, such as earthworms and many coleopteran larvae, inhabiting organic surface layers that are highly contaminated with radionuclides are important decomposers in ecosystem material cycles and may also contribute to the movement of 137 Cs in the forest floor [ 13 , 14 ]. Detritivores may play a major role in 137 Cs uptake from the forest floor into forest ecosystems because they are consumed by various animals at higher trophic levels, such as birds, mammals, and fish. In forest insect communities, detritivorous insects have higher 137 Cs concentrations than herbivorous insects [ 15 ]. In headwater streams, fish consume both aquatic and terrestrial insects. Thus, they can become contaminated with 137 Cs through consuming 137 Cs-contaminated detritivorous insects [ 16 ]. However, some studies that assessed the transfer of 137 Cs from litter to detritivores suggested that the bioavailability of 137 Cs in these organisms is extremely low. For example, 95% of 137 Cs in earthworms is present in the digestive tract and is mostly excreted without being transferred to other parts of the body tissues [ 13 ]. Further, the leachability of 137 Cs, or its bioavailability, in substrates is determined by its chemical form and has been evaluated in extraction experiments [ 11 , 12 , 17 ]. Manaka et al. (2019, 2020) reported that relatively mobile 137 Cs in organic matter rapidly decreased in the months following the Fukushima Daiichi Nuclear Power Plant accident [ 12 ] but its proportion has since remained constant [ 11 ]. Therefore, 137 Cs bioavailability in litter and uptake into detritivores should be quantitatively evaluated to understand their role in 137 Cs dynamics in forest ecosystems and the contamination of organisms of higher trophic levels, such as fish. To the best of our knowledge, comparable 137 Cs absorption has not been estimated for other large soil invertebrates.
Thus, this study investigated the transfer of 137 Cs into larvae of flower chafer ( Protaetia orientalis ) (Coleoptera: Scarabaeidae), which is commonly distributed throughout Japan. Among several species tested in preliminary experiments, P . orientalis was selected for the study due to its prevalence as a dominant detritivore litter feeder in the forests of Fukushima and its demonstrated suitability for laboratory breeding. The larvae feed on a mixture of decomposed leaf litter and fermented wood approximately 10 months before imago emergence. First, we conducted breeding experiments to evaluate the uptake and excretion of 137 Cs by flower chafer larvae. 137 Cs distribution in the muscle, digestive tract, and skin was investigated via dissection. Additionally, the 137 Cs absorption rate at which 137 Cs in the litter was transferred to the larval tissue, was calculated. 137 Cs excretion in flower chafer larvae was investigated using elimination experiments. Second, sequential extraction was conducted to determine the proportion of bioavailable 137 Cs within the litter. Comparison with the absorption rates from the breeding experiments allowed us to investigate how much bioavailable 137 Cs in the sequential extractions was transferred to the larvae. Furthermore, by comparing the sequential extraction results between the litter and feces, we revealed the physicochemical forms of 137 Cs in the litter that the larvae could absorb through digestion.
Materials and methods
Litter preparation.
The decomposed litter used in the rearing experiment was collected in August 2020 from a deciduous broadleaf forest located in the upper reaches of the Ota River in Minamisōma City, Fukushima Prefecture. The site is approximately 30 km away from Tokyo Electric Power Company’s Fukushima Daiichi Nuclear Power Plant. This site is the forest where we have been studying the transfer of 137 Cs from aquatic and terrestrial insects to fish that consume them [ 18 ]. The litter was collected from relatively flat areas alongside headwater stream, where broadleaf litter accumulated. Dry streams or slope area were excluded to minimize the effects of wash-off due to water flow. The decomposed litter was obtained from the partially decomposed organic layer (F-H layer, including fermentation and humus sublayers) after removing fresh litter from the surface. Sampling of approximately 1.5 kg of litter was performed at 10 different locations and the collected litter was mixed. The litter was brought to the laboratory, dried in an oven at 60 °C for 7 d, ground and homogenized using an impact mill (IFM-800DG; Iwatani Ltd., Tokyo, Japan), and sieved through a 1-mm mesh sieve. Because there is a restriction on the quantity of radiocesium material that can be brought into the laboratory according to the safety management regulations for sample handling personnel established by the institute, the litter was used after mixing with 137 Cs-free commercially available substrate, which was made from powdered decayed oak wood (spawning first; Fortech Ltd., Wakayama, Japan) at a ratio of 1:1.
Preparation of flower chafer
We collected flower chafer imagines from May to September 2020 using traps at the institution property in Miharu Town, Fukushima Prefecture ( Fig 1A ). Protaetia orientalis is a common and known pest species [ 19 ]. It is not listed as a nationally endangered species under the Law for the Conservation of Endangered Species of Wild Flora and Fauna of Japan ( https://www.env.go.jp/en/nature/biodiv/law.html ). Therefore, permissions are not required for its collection and experimentation in the study area. The collected imagines were placed in a cage with a commercial 137 Cs-free substrate for oviposition and their offspring were used for the breeding experiment. The larvae were reared for 3–5 months at 20 °C. Subsequently, the third and last instar larvae were used for the rearing experiment ( Fig 1B ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
Pictures of (A) Imago and (B) larva of flower chafer, and (C) the U-8 container used for the rearing experiment.
https://doi.org/10.1371/journal.pone.0310088.g001
Breeding experiment
The design of the breeding experiment is shown in Fig 2 . Before starting the breeding experiment, 250 g of litter moistened with water was placed in a plastic container ( Fig 1C , U8 container, d = 50 mm; h = 62 mm) and the 137 Cs activity concentration of the litter was measured. Twenty third-instar larvae were reared for 30 d, with one larva in each U8 container kept in a laboratory under dark conditions at 20 °C. After one month, the larvae were removed from the U8 containers and fixed in 70% ethanol. The fixed larvae were cleaned by removing body surface contaminants using a brush. Subsequently, their muscle, skin, and digestive tract were dissected using dissecting scissors and the dissected body parts were dried at 60 °C for approximately 2 d. We prepared samples of each individual larvae, obtaining a total of 20 samples of each body part. After the dry weights were measured using a balance (XSE205 DualRange; Mettler Toledo Ltd., Tokyo, Japan), samples were cut into small pieces using dissecting scissors and stored in flat-bottom test tubes (diameter = 15 mm and height = 57 mm) until subsequent germanium (Ge) γ -ray spectrometry analyses. The larval feces left in pellet form in the U8 container were separated from the remaining leaf litter using a sieve with a mesh size of 1 mm. The feces and remaining leaf litter were dried at 60 °C for approximately 2 d, the dry weights were measured and homogenized, and the samples were stored in U8 containers until Ge γ -ray spectrometry analyses. Some larvae were reared until they reached the imago stage in the U8 containers by using the same litter; however, many larvae died before reaching the imago stage, resulting in four samples for prepupae/pupae and three samples of imagines.
https://doi.org/10.1371/journal.pone.0310088.g002
Estimating the biological half-life of 137 Cs
Approximately 60 third-instar larvae were reared for one month using the same litter containing 137 Cs as used in the breeding experiment in three cages (33 × 23 × 19 cm, 20 larvae per cage). They were then moved to a commercial 137 Cs-free substrate in three cages (33 × 23 × 19 cm, 20 larvae per cage) to evaluate the elimination of 137 Cs from the larvae. Subsequently, the larvae of six individuals were preserved in 70% ethanol for 0, 1, 3, 6, 12, 24, 48, 96, and 192 h. The larvae were then dissected, dried, weighed, homogenized, and stored in flat-bottom test tubes until Ge γ -ray spectrometry analyses, following the same procedures as those used for the breeding experiments.
Sequential extraction of 137 Cs
After larval consumption, the sequential extraction method was adopted for the litter and fecal pellets. Twelve replicates of litter were subsampled from the litter prepared for the rearing experiment and 12 replicates of fecal pellets were prepared from 12 of the 20 individuals used for the rearing experiments. The homogenized litter and fecal samples after Ge γ-ray spectrometry analyses were dried at 60 °C for approximately 1 d before subsample.
Sequential extraction has been applied to evaluate the chemical forms of 137 Cs in soil, sediments, and litter [ 11 , 12 , 17 , 24 ]. We conducted a 137 Cs extraction experiment based on Takechi et al. (2020); however, we added a water-soluble extraction step as a first step. We sequentially extracted 137 Cs, which exists in a (1) water-soluble form, (2) ion-exchangeable form ( 137 Cs that can be exchanged by ammonium ions), (3) oxide form ( 137 Cs that is bound to iron and manganese oxides and extracted by reductive dissolution), (4) organic form ( 137 Cs that is bound to organic matter and extracted by oxidizing and decomposing organic matter), and (5) fixed form ( 137 Cs that is trapped in minerals and is scarcely dissolved). The litter and fecal pellet samples were used for the following extraction procedure after determining 137 Cs activity concentration by Ge γ -ray spectrometry analyses. The steps can be described in detail below:
- Step (1): Water-soluble form. Three grams (dry weight) of each sample were placed in a centrifuge tube and 30 mL of pure water was added. Three fecal pellet samples weighed less than 3 g (2.87–2.96 g) owing to insufficient sample volume. In these cases, the solvent was added at a ratio of 1 (sample):10 (solvent). The tube was stirred (150 rpm) at room temperature (25 °C) for 16 h using shaking incubators (PRAS, 12-R-FF, Preci Co., Ltd.) and then centrifuged at 7,931 rpm for 30 min (7000, KUBOTA) to separate the supernatant and residue. The supernatant was spontaneously filtered through a filter paper (Whatman 42, Global Life Science Technologies Japan K.K., pore size 2.5 μm) and the extract acquired after filtering was transferred to a U8 container. The residue was rinsed with 30 mL pure water, centrifuged at 7,931 rpm for 30 min, and the resultant supernatant was discarded. In each subsequent extraction, the residue was rinsed with the same volume of pure water as the extraction solution added in each step.
- Step (2): Ion-exchangeable form. The residue from the previous step was mixed with 30 mL of 1 mol/L ammonium nitrate solution (1 mol/L NH 4 NO 3 ). The resultant suspension was stirred, centrifuged, and filtered, and the acquired extract was separated under the same conditions as described in Step (1).
- Step (3): Oxide form. The residue of the previous step was mixed with 60 mL of 0.5 mol/L hydroxyl amine hydrochloride (HONH 2 -HCl, containing 2.5% v/v 2.0 mol/L HNO 3 ). The suspension was stirred, centrifuged, and filtered, and the extract was separated under the same conditions as described in Step (1).
- Step (4): Organic form. The residue of the previous step was mixed with 12 mL of hydrogen peroxide (H 2 O 2 36%), adjusted to pH 2.0 using 1% HNO 3, and left at room temperature (20°C) for approximately 1 h until the reaction settled. The container (with a loose lid) containing the sample was placed in a thermostatic bath (Thermominder, SX-10N, TAITEC) at 85 °C and heated for 1 h while shaking it occasionally. Thereafter, the liquid was heated without a lid until the volume was less than 4 mL. This process was repeated two times. After the sample had cooled to room temperature, 36 mL of 1.0 mol/L NH 4 NO 3 adjusted to pH 2.0 using concentrated nitric acid was added. The suspension was stirred, centrifuged, and filtered, and the extract was separated under the same conditions as described in Step (1).
- Step (5): Residuals. Finally, the residue was transferred to a U8 container and thoroughly dried at 105 °C, and the dry weights were measured. The extracts obtained from Steps (1)–(4) were transferred to a U8 container and weighed.
137 Cs measurements
The radioactivity of the flower chafer samples was measured using a high-purity germanium (HPGe) coaxial detector (Canberra GCW6023; Mirion Technologies, Canberra Ltd., Tokyo, Japan). The leaf litter and fecal samples from the breeding experiment and the extract and residue from the sequential extraction experiment were stored in U8 containers, and 137 Cs activity concentrations were measured using HPGe (Canberra GCW6023; Mirion Technologies, Canberra Ltd., Tokyo, Japan, and SEG-EMS GEM 30–70; SEIKO EG&G Ltd., Tokyo, Japan). Most samples were measured for <10% of counting error, except for small quantities of dissected flower chafer samples in flat-bottomed test tubes, which were measured for <15% of counting error. The minimum detectable amount of 137 Cs above the detection limit was about 0.07 Bq in the well-type HPGe coaxial detector. The standardized mixed sources ( 134 Cs and 137 Cs) for calibrating the detectors were MX035 (Japan Radioisotope Association, Tokyo, Japan) for the well-type HPGe coaxial detectors and MX033U8PP (Japan Radioisotope Association, Tokyo, Japan) for the U8 container. The standardized mixed sources were measured with varying filling quantities. Measurement efficiencies for 134 Cs (604.66 and 795.76 keV) and 137 Cs (661.66 keV) were obtained, and three efficiency calibration curves were created for different filling heights. The concentration of 137 Cs was then calculated using the comparative method based on these curves. Gamma Studio software (SEIKO EG&G, Tokyo, Japan) and Spectrum Explorer (Mirion Technologies, Canberra Ltd., Tokyo, Japan) were used to analyze the γ -ray spectra. The sample activities were corrected for radioactive decay until sample collection. 137 Cs activity concentrations were expressed as 137 Cs concentrations on a dry weight basis. S1 and S2 Tables show the 137 Cs activity concentrations (Bq/kg) and total 137 Cs (Bq) of the breeding experiment and sequential extraction experiment, respectively.
Statistical analysis and calculation of assimilation rate and concentration ratio
For the breeding experiment, the distribution of both 137 Cs concentrations and log-transformed 137 Cs concentrations were not normal by the Shapiro-Wilk normality test and not equivalent across samples by the Levene’s Test for Homogeneity of Variance. Therefore, multiple comparisons were made using pairwise Wilcoxon rank-sum tests for 137 Cs concentrations in litter before breeding, feces, dissected tissues of flower chafers (digestive tract, skin, and muscle), and whole-body larvae with Bonferroni adjustments for p values. The 137 Cs concentrations in whole-body larvae were calculated using the weight and concentration of each tissue in the dissection experiment assuming that the total 137 Cs in whole-body larvae is equivalent to the sum of 137 Cs in each body part. The absorption rate of 137 Cs in flower chafer larvae during the breeding experiment was calculated for each individual ( Fig 2 ). The total 137 Cs in the litter consumed by the larvae was calculated by subtracting the total 137 Cs in the remaining litter after breeding from the total 137 Cs in the provided litter (A). The total 137 Cs assimilated in the larval tissue was calculated as the sum of 137 Cs in the larval skin and muscle (B). The absorption rate at which 137 Cs in the diet was transferred to flower chafer larval tissue at the end of the experiment was calculated as (B/A) × 100. 137 Cs concentrations in whole-body larvae, prepupae/pupae, and imagines were compared using the pairwise Wilcoxon rank-sum test. Additionally, the concentration ratio (CR) was calculated as follows: CR = 137 Cs concentration in flower chafer (Bq/g) / 137 Cs concentration in litter (Bq/g) for whole-body larvae, prepupae/pupae, and imagines.
Based on the results of the sequential extraction experiment, the bioavailable fraction of 137 Cs (percentage of the sum of water-soluble, exchangeable, oxide, and organic forms to the total 137 Cs contained in the sample) between the litter and feces excreted from larvae was compared using pairwise Wilcoxon rank-sum tests. The quantity of extracted 137 Cs from the sample per gram in water-soluble, exchangeable, oxide, and organic forms and the remaining 137 Cs were also compared between the litter and feces. Because the distributions of the log-transformed total quantities of the extract were not normal and did not have equivalence variance across samples, we applied pairwise Wilcoxon rank-sum tests. All statistical analyses and calculations were performed using R ver. 4.3.1. [ 23 ].
Results and discussion
Uptake and absorption of 137 cs into flower chafer larvae.
The results of the dissection experiment showed that the 137 Cs concentration in the digestive tract of the larvae was significantly higher than that in the skin and muscle tissues ( p < 0.001, Fig 3A ). The calculated distribution of 137 Cs in larvae showed that most 137 Cs was localized in the digestive tract of the larvae (85.1 ± 9.7% of the total 137 Cs), whereas the muscle and skin included only 10.9 ± 6.9% and 3.9 ± 3.1% of the total 137 Cs, respectively ( Fig 3B ). This 137 Cs distribution was comparable to that of earthworms showing 95% of the total 137 Cs in the digestive tract [ 13 ]. Most 137 Cs concentrations in detritivorous aquatic insects also have been described by their digestive tract contents, the removal of which has been observed to significantly reduce 137 Cs concentrations [ 20 , 25 ].
(A) 137 Cs concentrations in flower chafer larvae after the breeding experiment. Litter (provided as diet), and feces, digestive tract, skin, muscle, and whole-body of larvae are shown (N = 20). Different letters beside each box indicate significant differences in the 137 Cs concentrations based on the Wilcoxon rank-sum tests. (B) Percentage of 137 Cs distributed in each organ of larvae for total 137 Cs in flower chafer larvae (N = 20). Each violin represents the distribution of an individual measure, and the dots represent the means.
https://doi.org/10.1371/journal.pone.0310088.g003
No statistically significant changes in 137 Cs concentrations were detected between the litter and feces ( p = 0.25, Fig 3A ), possibly because the 137 Cs decrease due to transfer from litter to larvae was marginal. Additionally, fecal samples may have been contaminated with the surrounding litter powders when the larvae were discharged, which is indicated by the 137 Cs concentrations in feces being significantly higher than those in the digestive tract contents obtained by dissection ( p = 0.018).
The localization of 137 Cs in the digestive tract indicates that most of the 137 Cs ingested by flower chafer larvae may be excreted in feces, with little 137 Cs absorbed into the body tissues. Moreover, the 137 Cs absorption rate estimated in this study was extremely low. The total 137 Cs in the litter consumed during one month of breeding (A) was 132.0 ± 49.8 Bq and that transferred to the body tissues of muscle and skin (B) was 0.42 ± 0.1 Bq; thus, the absorption rate was 0.39 ± 0.2%. Few comparable studies have investigated 137 Cs absorption by soil macroinvertebrates [ 13 ]. However, 137 Cs absorption in fish has been estimated via feeding experiments, in which fish were fed with radiocesium-labeled diets; notably, 137 Cs absorption varied greatly depending on the food items. 137 Cs absorption in brown trout was 55% from Chironormidae sp. Larvae, 48% from freshwater amphipods, 76% from freshwater snails, 23% from Ephemeroptera sp. Larvae, 82% from zooplankton, and 66% from brown trout muscle [ 26 ]. Much lower 137 Cs absorption in bluegill was reported from detritus (3%) and sediment-fed Chironomus larvae (7%–16%), indicating that fish can absorb most of the 137 Cs associated with tissues and absorb marginal quantities of 137 Cs from detritus and clay in Chironomus larvae [ 27 ]. 137 Cs absorption in flower chafer larvae in this study was lower than that in fish, indicating significantly lower absorption rates for soil macroinvertebrates from detritus containing clay minerals. 137 Cs absorption can be affected by the physicochemical forms of 137 Cs in the litter and the quantity of clay minerals; however, future studies are needed to clarify this relationship.
By rearing flower chafers to the imago stage, 137 Cs concentrations were substantially reduced throughout the developmental stages ( Table 1 ). Compared to the concentrations in whole-body larvae, 137 Cs concentrations were reduced by 50% in prepupae/pupae ( p = 0.06, marginal due to small sample size) and 72% in imagines ( p < 0.01). The larva discharges most of its digestive tract content by purging the gut before pupation. Subsequently, the digestive tract structure of scarab beetles completely changes during the pupal stage, and the cuticle of the digestive tract and the remaining contents are excreted during eclosion [ 28 ]. These metamorphosis processes may eliminate 137 Cs from the digestive tract. The 137 Cs concentrations in imagines were approximately equal to those assimilated in the skin and muscle tissues of the larvae, suggesting that the imago 137 Cs concentration was determined by the 137 Cs assimilated into the tissues during the larval stage ( Table 1 ). The calculated whole body 137 Cs CRs for larvae, prepupa/pupa, and imago were 0.27 ± 0.11, 0.13 ± 0.03, and 0.06 ± 0.02, respectively ( Table 1 ). These CR values were almost consistent with the CR value (0.13 ± 0.10) determined previously for forest detritivorous insects (all CRs were calculated for the imago stage) [ 15 ], thus, confirming that the transferred 137 Cs concentration evaluated in our breeding experiment was within the range observed in the field.
Standard deviations are shown in parentheses.
https://doi.org/10.1371/journal.pone.0310088.t001
Estimation of the biological half-life of 137 Cs
In the elimination experiment, 137 Cs concentrations decreased at different rates in the digestive tract, muscles, and skin tissues ( Fig 4 ; Table 2 ). 137 Cs in the digestive tract was rapidly eliminated during the first 2 d. This result was consistent with a previous finding that reported that the contents were completely excreted from the digestive tract within 47 h in third-instar scarab larvae ( Trypoxylus dichotomus ) [ 29 ]. After 2 d, when the digestive tract contents were assumed to have been replaced by clean litter, 137 Cs in the digestive tract remained and decayed slowly. This can be attributed to litter powder trapped by the walls of the digestive tract. Although 137 Cs in the digestive tract gradually decreased, it was expected to remain in the tract until it was completely excreted by pupation and eclosion, as has been shown in breeding experiments up to the imago stage. The estimated biological half-life of 137 Cs in the fast component by excretion of digestive tract contents (0.26–0.64 d; T b1 for digestive tract in Table 2 ) was slightly slower than those estimated for earthworms (0.10–0.33 d; summarized in Table 1 in Tanaka et al. 2018) and Trichopteran larvae (0.22–0.37 d; Fujino et al. 2018).
The lines represent the prediction of the fitted two-component model ( Table 2 ).
https://doi.org/10.1371/journal.pone.0310088.g004
For biological half-life, T b , 95% confidence intervals are shown in parentheses.
https://doi.org/10.1371/journal.pone.0310088.t002
Contrastingly, 137 Cs in skin and muscle tissues decreased rapidly during the first day and a relatively constant decrease of 137 Cs followed until Day 8. The two-component model indicated that different pools of 137 Cs were likely involved in 137 Cs elimination [ 21 , 30 ]. The fast component may be small pools of 137 Cs that are easily discharged within 1 d and the slow component may be 137 Cs that is assimilated into tissues and gradually discharged. The estimated biological half-life of the slow component (4.0–17.6 d; T b2 for muscle and skin in Table 2 ) was relatively faster than that estimated in earthworms (15–54 d; summarized in Table 1 in Tanaka et al. 2018), although the direct comparisons were difficult due to the short experimental period of this study.
137 Cs whole-body burdens have been used to estimate the half-life in earthworms [ 13 ] and invertebrates [ 20 , 21 , 31 ]. In these estimated two-component models, short-lived and long-lived components were interpreted to represent gut clearance and physiological clearance of assimilated 137 Cs, respectively. However, our dissection experiment revealed 137 Cs kinetics more comprehensively; particularly, digestive tract and physiological clearance were represented by a two-component model respectively consisting of multiple 137 Cs pools.
Bioavailable 137 Cs by sequential extractions
We calculated the recovery rate by dividing the sum of 137 Cs extracted in each fraction by the total 137 Cs present in the pre-extraction samples. This yielded a recovery rate of 94.7 ± 7.9%, confirming the validity of the sequential extraction analysis of this study. Fig 5 shows the results of the sequential extraction of litter and feces after conducting the breeding experiment. Because 137 Cs dissolution primarily occurs by ion exchange, reductive dissolution of Fe and Mn oxides, and microbial degradation of organic matter, these fractions (water-soluble, exchangeable, oxide, and organic forms) were considered as the bioavailable fraction [ 17 ].
The proportions shown are the means of 12 samples for litter and feces.
https://doi.org/10.1371/journal.pone.0310088.g005
The previous study conducting the sequential extraction experiments of litter in Fukushima reported that relatively mobile 137 Cs remained in constant proportions from 2012 to 2019, which were assumed to be co-extracted with soluble fats, waxes, oils, holocellulose, and acid-soluble lignin [ 11 ]. These forms of 137 Cs may be extracted as bioavailable 137 Cs in this experiment, although direct comparison is not possible due to differences in extraction solvents. Litter digestion by flower chafer significantly reduced bioavailable fraction from 23.2 ± 2.3% in litter to 17.7 ± 2.5% in feces ( p < 0.001). Additionally, the quantity of extracted 137 Cs was significantly lower in the feces than in the litter for all forms of bioavailable 137 Cs ( Fig 6A ); the reduction was 45%, 22%, 29%, and 24% for water-soluble, exchangeable, oxidized, and organic forms, respectively. This suggested that litter feeding by flower chafer larvae may absorb 137 Cs through the digestive process not only in an easy-elution form (water soluble and exchangeable) but also in oxide and organic forms.
Extracted bioavailable fraction of 137 Cs from (A) water-soluble (WS), exchangeable (EX), oxidized (OX), and OR (organic) forms from litter (L) and feces (F) (N = 12). (B) 137 Cs remaining in residuals. Each violin represents the distribution of an individual measure, and the dots show the means. The p -values show the result of the Wilcoxon rank-sum tests.
https://doi.org/10.1371/journal.pone.0310088.g006
Scarab larvae consume cellulose from various sources, including plant roots, soil organic matter, and decaying wood, and extract nutrients and energy from these sources [ 32 ]. The degradation mechanism of these components in the digestive tract has been well-studied, especially in the scarab larvae of Pachnoda ephippiata [ 32 , 33 ]. Particularly, their midgut is highly alkaline (pH 12) and the most easily digestible proteins are mobilized and absorbed by endogenous proteinases. The digestive tract contents are then passed into a hindgut fermentation chamber containing diverse microorganisms, which degrade cellulose under anaerobic conditions. The digestive system of scarab larvae may promote 137 Cs elution and absorption in oxide and organic forms present in the litter. In contrast, in our study, digestion did not reduce the quantity of residual 137 Cs in the feces compared to that in the litter ( Fig 6B ). More than 70% of the 137 Cs remained as residuals, which may include 137 Cs fixed in clay minerals [ 6 , 7 , 34 ]. In addition, the distribution of radiocesium-bearing microparticles (CsMPs) has been reported for the Ota River watershed where the litter was collected; therefore, CsMPs can also contribute to 137 Cs in the residuals [ 18 ]. The 137 Cs in clay minerals and CsMPs is assumed to be unavailable for biological uptake during digestion. Further investigation for the quantity of clay minerals and CsMPs present in the litter is needed because it is likely to have a significant impact on both the results of the sequential extraction and the 137 Cs absorption rate in flower chafer larvae.
The digestion process by detritivores potentially influences the bioavailability of 137 Cs in the litter via decomposition [ 14 , 35 , 36 ]. Our results showed that digestion by larvae reduced the bioavailable fraction of 137 Cs in the litter. This was contrary to the findings of previous studies that have suggested that digestion of litter by scarab larvae ( Trypoxylus dichotomus ) significantly increases the exchangeable fraction of KCl in the litter [ 14 ]. This inconsistency could be attributed to differences in the litter provided. Ishii et al. (2018) fed larvae with intact litter and concluded that physical decomposition increased 137 Cs elution in feces. However, the larvae in our experiment were fed with finely crushed litter; thus, the effect of physical decomposition was marginal, but the chemical digestion process may have reduced the bioavailable 137 Cs in the feces. The effect of the ingestion of flower chafer larvae on the bioavailability of 137 Cs in intact litter under natural conditions remains unclear. Therefore, further studies are required to evaluate the impact of feeding by detritivores on the bioavailability of 137 Cs in forest floor litter.
The low bioavailability of 137 Cs in the litter observed in this study was consistent with earlier observations that reported low absorption 137 Cs in the detritus of earthworms [ 13 ]. However, detritus feeders may play an important role in 137 Cs input into forest ecosystems by extracting the bioavailable fraction of 137 Cs stock that is found in abandance on the forest floor. Furthermore, 137 Cs in the skin and muscle tissues of detritivores is probably present in a highly bioavailable form that is susceptible to absorption by predators, such as fish. Therefore, it is likely that more bioavailable 137 Cs accumulates at higher trophic levels in the food web. In the aquatic food web, the sequential extraction results indicated that the ratio of easy-elution forms (exchangeable and carbonate forms) was higher in planktivorous fish (almost 100%) than in plankton (40%–80%) [ 37 ]. Thus, estimating the bioavailability of 137 Cs in detritivore and higher trophic organisms would be useful for understanding 137 Cs dynamics through the detrital food chain.
Furthermore, future work to estimate the quantity of 137 Cs uptake by detritivores against stock in the forest floor is needed to elucidate the contribution of detritivores to the contamination of organisms in the forest ecosystem. In addition, the bioavailability and absorption rates of 137 Cs in forest litter may be influenced by factors such as tree species and the quantity of fixed 137 Cs in clay minerals. Therefore, the 137 Cs availability and absorption rates estimated in this study may not fully represent the broad contaminated area across Fukushima Prefecture. Estimating these parameters among different regions with varying tree species and other detritivorous insect species, would contribute to a more comprehensive understanding of the role of detritivores in the dynamics of 137 Cs in forest ecosystems.
Our study quantitatively demonstrated the low bioavailability of 137 Cs in forest floor litter and lower absorption rates of 137 Cs in flower chafer larvae. Although 23% of the 137 Cs in the litter was in the bioavailable form as evaluated during the sequential extraction, the breeding experiment showed that only 0.4% of 137 Cs was absorbed into the larvae. Flower chafer larvae absorbed 137 Cs through digestion, not only in an easy-elution form (water-soluble and exchangeable) but also in oxide and organic forms. Our study indicated that detritivores may play a role in extracting the bioavailable fraction of 137 Cs remaining in large quantities on the forest floor. Further studies are needed to evaluate the contribution of detritivores to 137 Cs transfer into organisms at higher trophic levels and forest ecosystems.
Supporting information
https://doi.org/10.1371/journal.pone.0310088.s001
https://doi.org/10.1371/journal.pone.0310088.s002
Acknowledgments
We would like to express our appreciation to all collaborators who have investigated radiocesium contamination in insects and freshwater fish in the Ota River. We also would like to thank Mr. Arai for his assistance with the germanium gamma-ray spectrometry analyses and for providing valuable comments on this manuscript.
- View Article
- Google Scholar
- PubMed/NCBI
- 9. Hashimoto S, Komatsu M, Miura S. Behavior of Radiocesium in the Forest. In: Hashimoto S, Komatsu M, Miura S, editors. Forest radioecology in Fukushima: radiocesium dynamics, impact, and future. Springer; 2021. pp. 21–46. https://doi.org/10.1007/978-981-16-9404-2_3
- 19. Morimoto K, Nakane T, Ohbayashi K, Nomura S, Kurosawa Y. Iconographia insectorum Japonicorum colore naturali edita vol. II. Hokuryukan Co., LTD., Tokyo 2007.
- 21. Tostowaryk TM. Assimilation and elimination of cesium by freshwater invertebrates. Colorado State University. 1999. https://mountainscholar.org/handle/10217/37344
- 23. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2022. https://www.R-project.org/
Samsung Newsroom's videos will no longer be supported on Internet Explorer. Please try a different type of web browser .
- SmartThings
- Custom range
- Content Type
- Press Release
Galaxy Book4 Edge, Samsung’s Next-Gen AI PC, Now Available With 15-Inch Display
All the power of copilot+ pcs, complete with access to galaxy ai features through the connected galaxy ecosystem, in a compact and lightweight design.

Samsung Electronics today announced the Galaxy Book4 Edge (15-inch), the latest addition to Samsung’s Galaxy Book lineup, now powered by Qualcomm’s cutting-edge Snapdragon ® X Plus 8-core platform. This new Copilot+ PC seamlessly integrates advanced AI capabilities, which elevate productivity and creativity to new heights.
Enhanced connectivity with the Samsung Galaxy ecosystem allows for one easy and efficient workflow across multiple devices, and further improves access to trailblazing Galaxy AI 1 tools. All of these innovations are housed in a slim design featuring a 15.6-inch FHD display, a long-lasting battery with Super-Fast Charging and Samsung’s multi-layer Samsung Knox security. The Galaxy Book4 Edge (15-inch) is available in select markets starting October in an iconic Sapphire Blue finish.
“Galaxy Book4 Edge series changed the game by bringing together Samsung Galaxy’s seamless mobile and PC integration with AI-driven productivity and creativity, and now it’s available at another great size to encompass every user’s unique preferences and needs,” said Dr. Hark-Sang Kim, EVP & Head of New Computing R&D Team, Mobile eXperience Business at Samsung Electronics. “With every expansion of our next-generation AI PC portfolio, we’re fueling AI innovation while also providing access to impactful AI tools for all.”
Portable Design With All-Day Battery
The Galaxy Book4 Edge (15-inch) is one of the most portable 15.6-inch laptops available. The thin and lightweight chassis features a stunning FHD display with anti-glare technology to ensure a comfortable viewing experience in any lighting condition. The slim design doesn’t sacrifice connectivity for portability and features a wide array of ports, including USB-C, USB-A, HDMI, headphone/microphone and a Micro SD slot. A long-lasting battery 2 with Super-Fast Charging 3 supports prolonged work sessions and keeps the device ready to use whenever inspiration strikes.
Next-Level Computing Powered by Snapdragon ® X Plus
Thanks to the industry-leading AI processing power and responsive performance of the Snapdragon ® X Plus 8-core platform, Galaxy Book4 Edge (15-inch) can take full advantage of the wide array of Copilot+ PC capabilities. 4
- Cocreator can bring ideas to life in a snap with minimal effort.
- Windows Studio Effects, combined with a high-definition camera and Wi-Fi 7 5 readiness, enables clear communication of visual ideas.
- Streamline note-taking or break communication barriers with Live Captions. 6
“Snapdragon ® X Plus 8-core redefines possibilities with responsive performance and groundbreaking on-device AI processing,” said Kedar Kondap, SVP & GM, Compute and Gaming, Qualcomm Technologies, Inc. “With incredible power efficiency, it’s designed to keep pace with dynamic lifestyles and get users in the flow — whether it’s crafting presentations on the move or kickstarting creative projects — pushing productivity to new heights.

A Mobile AI Ecosystem Like No Other
Connection to Samsung’s ever-growing Galaxy device ecosystem unlocks an intuitive working environment, where files can be transferred with unprecedented ease, and productivity is not interrupted by access limitations. Users can work seamlessly across PC and phone at any time, letting technology do the heavy lifting. Microsoft Phone Link 7 provides access to your Galaxy phone on your PC, 8 enabling use of popular Galaxy AI phone features such as Live Translate, Photo Assist and Circle to Search with Google, 9 opening up new ways to communicate, create and discover.
As with all smartphones and tablets in the Galaxy ecosystem, Galaxy Book4 Edge (15-inch) is secured by Samsung Knox , Galaxy’s multi-layer security platform that safeguards critical information and protects against vulnerabilities with end-to-end secure hardware, real-time threat detection and collaborative protection. And as a Copilot+ PC, you know your computer is secure, as Windows 11 brings layers of security — from malware protection, to safeguarded credentials, to data protection and more trustworthy apps.
Availability
The Galaxy Book4 Edge (15-inch) will be available in select markets including France, Germany, Italy, Korea, Spain, the UK and the U.S. starting October, 10 in an iconic Sapphire Blue color that is bold, refined and unique to the Galaxy Book4 Edge series. For more information about Galaxy Book4 Edge, please visit: https://www.samsung.com/galaxy-book/ .
Specifications
|
| |
| 356.6 x 229.7 x 15.0mm | |
| 1.50 kg | |
| Windows 11 Home | |
| 15.6-inch*, 16:9 FHD (1920×1080), 300nits, 60Hz, Anti-Glare. | |
| Snapdragon X Plus 8-core | |
| Qualcomm Hexagon™ NPU with Qualcomm AI Engine up to 45 TOPS | |
| Qualcomm Adreno™ GPU | |
| Bluetooth v5.3 / Wi-Fi 7 | |
| Sapphire Blue | |
| 16 GB | |
| 256 GB / 512 GB | |
|
| |
| 2MP (1080p FHD) | |
| Dual Microphones / Stereo Speaker (1.5W*2), Dolby Atmos | |
| Pro keyboard with Numeric key | |
| 61.2 Wh (Typical) | |
| 65W USB Type-C Adapter | |
| 2 x USB-C (4.0), HDMI 2.1 (Supports 4K@60Hz), USB-A (3.2), Micro SD, Headphone out/Microphone-in Combo, Security Slot | |
* Specifications may vary by market. * All functionality, features, specifications and other product information provided in this document including, but not limited to, the benefits, design, pricing, components, performance, availability and capabilities of the product are subject to change without notice. * Snapdragon, Qualcomm, Hexagon and Adreno are trademarks or registered trademarks of Qualcomm Incorporated. Snapdragon, Qualcomm Hexagon and Qualcomm Adreno are products of Qualcomm Technologies, Inc. and/or its subsidiaries.
1 Samsung does not make any promises, assurances or guarantees as to the accuracy, completeness or reliability of the output provided by AI features. Samsung Galaxy phone (One UI 6.1 or later) and Link to Windows/Microsoft Phone Link connection required for Galaxy AI features on PC. Samsung and/or Microsoft account login may be required to use/connect select Samsung AI features on PC. Feature availability may vary by application, market, network provider, Android OS/One UI version and/or model. Galaxy AI features will be provided for free until the end of 2025 on supported Samsung Galaxy devices. Different terms may apply for AI features provided by third parties. 2 For 15-inch model. Up to 26-hours video playback. Actual battery life may vary depending on model, network environment, usage patterns and other factors. 3 For 15-inch model. Device charges up to 45% in 30 minutes. Battery capacity and amount of charge after a 30-minute charge may vary by configuration. 4 Recall may not be available on Galaxy Book4 Edge at launch. Recall’s availability is dependent on Microsoft’s release schedule. Optimized for select languages (English, Chinese (simplified), French, German, Japanese and Spanish. Content-based and storage limitations apply. Learn more: https://aka.ms/copilotpluspcs . 5 Actual speed may vary by market, carrier and user environment. Wi-Fi 7 availability may vary due to OS version, market, location, network conditions and other factors. Wi-Fi 7 wireless network routers required and sold separately. 6 Currently supports translation for video and audio subtitles into English from 40+ languages. See https://aka.ms/nextgenaipcs . 7 Users must be signed into the same Microsoft account. PC (Microsoft Phone Link app) requires Windows 10 or above. Microsoft Phone Link recommends Samsung Galaxy device to be on the same Wi-Fi network as the PC. Some mobile apps may restrict content to be shared on other screens. Some related features may vary by model. 8 Copilot in Windows has specific system requirements and is available in select global markets. Functionality may be limited. Learn more: https://aka.ms/copilotpluspcs . 9 Results may vary depending on visual matches. Requires internet connection. Users may need to update Android to the latest version. Product functionality may be dependent on your app and device settings. Some functions may not be compatible with certain apps. Availability of the service varies by market and language. Accuracy of results is not guaranteed. 10 Availability and timing will vary by market.
TAGS Galaxy AI Galaxy Book4 Edge Galaxy Book4 Series Samsung Knox Snapdragon® X Plus 8-core
Products > Mobile
Press Resources > Press Release
Samsung-Mobile-Galaxy-Book4-Edge-15inch_dl1.jpg
Samsung-Mobile-Galaxy-Book4-Edge-15inch_dl2.jpg
Samsung-Mobile-Galaxy-Book4-Edge-15inch_dl3.jpg
- Embrace AI With Galaxy Book5 Pro 360: The First in Samsung’s Lineup of New Powerhouse AI PCs
- Samsung Releases the Galaxy Book4 Edge, Its First Copilot+ PC, in Global Markets
- Galaxy Book4 Edge: Samsung’s Next-Gen AI PC Expands the Galaxy AI Ecosystem
- [Interview] Elevating the PC Experience: Samsung and Intel Explore the Galaxy Book4 Series and Future of AI PCs
For any issues related to customer service, please go to Customer Support page for assistance. For media inquiries, please click Media Contact to move to the form.
Get daily updates from Samsung Newsroom
- Media Contact
- SAMSUNG.COM
- Terms of Use
- Privacy and Cookies
Copyright© 2010-2024 SAMSUNG All Rights Reserved.
- People & Culture
- More stories
- TVs & Displays
- Home Appliances
- Network Solutions
- Semiconductors
- More Products
- Citizenship
- Environments
- Sustainability

IMAGES
VIDEO
COMMENTS
Long-term ecosystem-level experiments, in which the environment is manipulated in a controlled manner, are important tools to predict the responses of ecosystem functioning and composition to future global change. We present the results of a meta-analysis performed on the results of long-term ecosystem-level experiments near Toolik Lake, Alaska ...
These experiments are the Jena Experiment, established in 2002 in Jena, Germany (hereafter, the Jena Experiment) 10,32, and the BioDIV experiment, established in 1994 at the Cedar Creek Ecosystem ...
These two experiments have the advantage of being stand-level, ecosystem experiments in established forests that are readily comparable with ecosystem-scale models. Both experiments ran for more ...
Biodiversity-ecosystem functioning (BEF) experiments address ecosystem-level consequences of species loss by comparing communities of high species richness with communities from which species have been gradually eliminated. BEF experiments originally started with microcosms in the laboratory and with grassland ecosystems.
Conceptual illustration of varying levels of ecosystem model complexity for an ecosystem model with an objective of maximizing the abundance of species s 1 and s 2. Solid lines indicate ...
Large, full ecosystem level experiments with sometimes ground-breaking treatments, such as the TEMPEST flooding experiment (Hopple et al., 2023), are also not replicated but provide invaluable insights into ecosystem functioning. An alternative example is the unique Biosphere 2 setup, which allowed for a fully traceable experiment on a "whole ...
Two-level experimental manipulations of a single global change factor (e.g. ambient atmospheric [CO 2] vs elevated [CO 2]) would not capture nonlinear responses to a global change factor, as indicated by the black dots in (a-d). Instead, multilevel experiments are needed to identify the shape of response functions.
Whole-ecosystem experiments are especially useful because they take into account the vast complexity of interactions between the natural environment and the large numbers of species present in most ecosystems. Experiments done in the laboratory are very useful, but often do not scale well to the ecosystem level because they frequently include ...
Long-term ecological experiments are experiments that have been undertaken at an ecosystem level for more than six years or are funded for at least six years into the future. These are controlled, replicated, manipulative experiments that are being undertaken in the field under real-world conditions.
Experimental manipulations of entire ecosystems have been conducted in lakes, catchments, streams, and open terrestrial and marine environments. Experiments have addressed applied problems of ecosystem management and complex responses of communities and ecosystems to perturbations. In the course of some experiments, environmental indicators and ...
Fun science experiments to explore everything from kitchen chemistry to DIY mini drones. Easy to set up and perfect for home or school. Browse the collection and see what you want to try first! As humans we are part of the environment. With over 7.5 billion of us on Earth, our combined actions also have a big impact on the environment.
After 25 years of biodiversity experiments, it is clear that higher biodiversity (B) plant communities are usually more productive and often have greater ecosystem functioning (EF) than lower diversity communities. However, the mechanisms underlying this positive biodiversity-ecosystem functioning (BEF) relationship are still poorly understood.
Whole-ecosystem experiments are especially useful because they take into account the vast complexity of interactions between the natural environment and the large numbers of species present in most ecosystems. Experiments done in the laboratory are very useful, but often do not scale well to the ecosystem level because they frequently include ...
Long-term ecosystem-level experiments, in which the environment is manipulated in a controlled manner, are important tools to predict the responses of ecosystem functioning and composition to ...
Despite great advances, experiments concerning the response of ecosystems to climate change still face considerable challenges, including the high complexity of climate change in terms of ...
Yummy STEM Project. Make a Hygrometer to Measure Humidity - STEM activity. Hydroponics in a 2-Liter Soda Bottle - STEM activity. Dive into the natural world with these environmental science experiments. Explore ecosystems, conservation, and climate change. Explore classic and cutting-edge high school science experiments in this collection ...
The NGSS Standards for ecosystems span several elementary grade levels. ... Each of these lessons is a way that you can teach your students about food webs, food chains, and ecosystems. By doing experiments and activities like those mentioned above, your students will better understand how energy flows through an ecosystem and how everything is ...
Use these free STEM lessons and activities to talk about habitats, ecosystems, food webs, and more as you explore biodiversity with K-12 students. Biodiversity, the " biological diversity " of our planet, is key to human survival. There are an estimated 8.7 million species of plants and animals on Earth, but biodiversity is more than just a ...
10 Ecosystem Project Ideas. Create Your Own Ecosystems or Habitats. Have your students work in groups, research, and then create an ecosystem together. It can be something as simple as collecting pond water, organisms, and plants. You could also have students create individual habitats instead of an entire ecosystem.
This lab demonstrates how contaminants can accumulate in organisms within a food web by using paper cutouts and M&M®s candies to simulate fish, osprey, and DDT. Students can see how the contamination levels increase as the trophic level increases. Downloads Biomagnification Lab Pictures (in color) Biomagnification Lab Pictures (in black and…
These experiments can range greatly across organismal scale, geography, and biological levels (e.g. ref. 48,86), and can also be conducted alongside interventions to mitigate species decline or ...
An ideal experiment would involve randomly manipulating wildlife populations; however, this would rarely be feasible or ethical in practice. As a result, valuations of ecosystems have mostly relied on nonexperimental settings (16, 25). The lack of experimental or quasi-experimental variation limits our ability to interpret valuation studies ...
Our study on breeding experiments of the Scarabaeidae beetle confirmed the low bioavailability of 137Cs in the litter in Fukushima. However, litter feeders may play an important role in transferring 137Cs to higher trophic levels in the forest ecosystem by extracting the bioavailable fraction of the vast stock of 137Cs on the forest floor.
Next-Level Computing Powered by Snapdragon ® X Plus. Thanks to the industry-leading AI processing power and responsive performance of the Snapdragon ® X Plus 8-core platform, Galaxy Book4 Edge (15-inch) can take full advantage of the wide array of Copilot+ PC capabilities. 4 . Cocreator can bring ideas to life in a snap with minimal effort.