
- Science Notes Posts
- Contact Science Notes
- Todd Helmenstine Biography
- Anne Helmenstine Biography
- Free Printable Periodic Tables (PDF and PNG)
- Periodic Table Wallpapers
- Interactive Periodic Table
- Periodic Table Posters
- Science Experiments for Kids
- How to Grow Crystals
- Chemistry Projects
- Fire and Flames Projects
- Holiday Science
- Chemistry Problems With Answers
- Physics Problems

Unit Conversion Example Problems
- Chemistry Worksheets
- Biology Worksheets
- Periodic Table Worksheets
- Physical Science Worksheets
- Science Lab Worksheets
- My Amazon Books

Unit conversion problems constitute the first week of nearly every science course. Measurement is a basic skill in science, but not all measurement methods are the same. People measure the same thing in different ways and use different units. To communicate between scientists, there has to be a method of converting between the different measurement units.
This is a collection of unit conversion example problems to help you learn the general method and mindset of converting units. Most of these include an example going the opposite direction (for example: cm to m and m to cm).
How to Do Unit Conversions
- How to Convert Between Units – Ladder Method
Use the ladder method or dimensional analysis to tackle conversions between units. See how to use a conversion factor to step-by-step reach the desired end units. This method is especially useful for measurements with more than one conversion like kilometers/hour to meters/sec. The L/min to m3/hr Conversion Example is a conversion example which uses the Ladder Method.
Length Unit Conversion Example Problems
- Feet to Inches (ft to in) Conversion
- Inches to Centimeters (in to cm) Conversion
- Miles to Kilometers (mi to km) Conversion
- Meters to Yards (and back again)
- Nanometers to Angstroms (nm to Å) Conversion
Area Unit Conversion Example Problems
- Square Centimeter to Square Meter (cm 2 to m 2 ) Conversion
Volume Unit Conversion Example Problems
- General Volume Example – Step by step conversion between 3 linear measurements to find the volume.
- Cubic Centimeters to Cubic Meters (cm 3 to m 3 ) Conversion
- Cubic Centimeters to Liters (cm 3 to L) Conversion
- Cubic Feet to Liters (ft 3 to L) Conversion
- Cubic Feet to Cubic Meters (ft 3 to m 3 )
- Cubic Inches to Cubic Feet (in 3 to ft 3 ) Conversion
- Cubic Inches to Liters (in 3 to L) Conversion
- Gallon to Liter (gal to L) Conversion
- Fluid Ounce to Milliliter (fl oz to mL) Conversion
- Quarts to Liters (and Liters to Quarts)
Mass Unit Conversion Example Problems
- Grams to Kilograms (g to Kg) Conversion
- Pounds to Kilograms (lbs to Kg) Conversion
- Pounds to Grams (lb to g)
- Grams to Ounces (g to oz)
- Ounces to Grams (oz to g)
Temperature Unit Conversion Example Problems
- Celsius to Fahrenheit (°C to °F) Conversion
- Celsius to Kelvin (°C to K) Conversion
- How to Convert Fahrenheit to Celsius
- Fahrenheit to Celsius (°F to °C) Example
- Fahrenheit to Kelvin (°F to K) Conversion
- Kelvin to Celsius (K to °C) Conversion
- When does Celsius Equal Fahrenheit?
- When does Fahrenheit Equal Kelvin?
Pressure Unit Conversion Example Problems
- Atmospheres to Kilopascals (atm to kPa) Conversion
- Atmospheres to Pounds per Square Inch (atm to PSI) Conversion
- PSI to atm (pounds per square inch to atmospheres)
- Millimeters of mercury to millibars (mmHg to mbar) Conversion
- Millimeters of mercury to kilopascals (mmHg t kPa) Conversion
Mole and Concentration Conversion Example Problems
- Grams to Moles (g to mol) Conversion
Time Conversion Examples
- Days to Years Conversion
Related Posts
- TPC and eLearning
- What's NEW at TPC?
- Read Watch Interact
- Practice Review Test
- Teacher-Tools
- Request a Demo
- Get A Quote
- Subscription Selection
- Seat Calculator
- Ad Free Account
- Edit Profile Settings
- Metric Conversions Questions
- Metric System Questions
- Metric Estimation Questions
- Significant Digits Questions
- Proportional Reasoning
- Acceleration
- Distance-Displacement
- Dots and Graphs
- Graph That Motion
- Match That Graph
- Name That Motion
- Motion Diagrams
- Pos'n Time Graphs Numerical
- Pos'n Time Graphs Conceptual
- Up And Down - Questions
- Balanced vs. Unbalanced Forces
- Change of State
- Force and Motion
- Mass and Weight
- Match That Free-Body Diagram
- Net Force (and Acceleration) Ranking Tasks
- Newton's Second Law
- Normal Force Card Sort
- Recognizing Forces
- Air Resistance and Skydiving
- Solve It! with Newton's Second Law
- Which One Doesn't Belong?
- Component Addition Questions
- Head-to-Tail Vector Addition
- Projectile Mathematics
- Trajectory - Angle Launched Projectiles
- Trajectory - Horizontally Launched Projectiles
- Vector Addition
- Vector Direction
- Which One Doesn't Belong? Projectile Motion
- Forces in 2-Dimensions
- Being Impulsive About Momentum
- Explosions - Law Breakers
- Hit and Stick Collisions - Law Breakers
- Case Studies: Impulse and Force
- Impulse-Momentum Change Table
- Keeping Track of Momentum - Hit and Stick
- Keeping Track of Momentum - Hit and Bounce
- What's Up (and Down) with KE and PE?
- Energy Conservation Questions
- Energy Dissipation Questions
- Energy Ranking Tasks
- LOL Charts (a.k.a., Energy Bar Charts)
- Match That Bar Chart
- Words and Charts Questions
- Name That Energy
- Stepping Up with PE and KE Questions
- Case Studies - Circular Motion
- Circular Logic
- Forces and Free-Body Diagrams in Circular Motion
- Gravitational Field Strength
- Universal Gravitation
- Angular Position and Displacement
- Linear and Angular Velocity
- Angular Acceleration
- Rotational Inertia
- Balanced vs. Unbalanced Torques
- Getting a Handle on Torque
- Torque-ing About Rotation
- Properties of Matter
- Fluid Pressure
- Buoyant Force
- Sinking, Floating, and Hanging
- Pascal's Principle
- Flow Velocity
- Bernoulli's Principle
- Balloon Interactions
- Charge and Charging
- Charge Interactions
- Charging by Induction
- Conductors and Insulators
- Coulombs Law
- Electric Field
- Electric Field Intensity
- Polarization
- Case Studies: Electric Power
- Know Your Potential
- Light Bulb Anatomy
- I = ∆V/R Equations as a Guide to Thinking
- Parallel Circuits - ∆V = I•R Calculations
- Resistance Ranking Tasks
- Series Circuits - ∆V = I•R Calculations
- Series vs. Parallel Circuits
- Equivalent Resistance
- Period and Frequency of a Pendulum
- Pendulum Motion: Velocity and Force
- Energy of a Pendulum
- Period and Frequency of a Mass on a Spring
- Horizontal Springs: Velocity and Force
- Vertical Springs: Velocity and Force
- Energy of a Mass on a Spring
- Decibel Scale
- Frequency and Period
- Closed-End Air Columns
- Name That Harmonic: Strings
- Rocking the Boat
- Wave Basics
- Matching Pairs: Wave Characteristics
- Wave Interference
- Waves - Case Studies
- Color Addition and Subtraction
- Color Filters
- If This, Then That: Color Subtraction
- Light Intensity
- Color Pigments
- Converging Lenses
- Curved Mirror Images
- Law of Reflection
- Refraction and Lenses
- Total Internal Reflection
- Who Can See Who?
- Lab Equipment
- Lab Procedures
- Formulas and Atom Counting
- Atomic Models
- Bond Polarity
- Entropy Questions
- Cell Voltage Questions
- Heat of Formation Questions
- Reduction Potential Questions
- Oxidation States Questions
- Measuring the Quantity of Heat
- Hess's Law
- Oxidation-Reduction Questions
- Galvanic Cells Questions
- Thermal Stoichiometry
- Molecular Polarity
- Quantum Mechanics
- Balancing Chemical Equations
- Bronsted-Lowry Model of Acids and Bases
- Classification of Matter
- Collision Model of Reaction Rates
- Density Ranking Tasks
- Dissociation Reactions
- Complete Electron Configurations
- Elemental Measures
- Enthalpy Change Questions
- Equilibrium Concept
- Equilibrium Constant Expression
- Equilibrium Calculations - Questions
- Equilibrium ICE Table
- Intermolecular Forces Questions
- Ionic Bonding
- Lewis Electron Dot Structures
- Limiting Reactants
- Line Spectra Questions
- Mass Stoichiometry
- Measurement and Numbers
- Metals, Nonmetals, and Metalloids
- Metric Estimations
- Metric System
- Molarity Ranking Tasks
- Mole Conversions
- Name That Element
- Names to Formulas
- Names to Formulas 2
- Nuclear Decay
- Particles, Words, and Formulas
- Periodic Trends
- Precipitation Reactions and Net Ionic Equations
- Pressure Concepts
- Pressure-Temperature Gas Law
- Pressure-Volume Gas Law
- Chemical Reaction Types
- Significant Digits and Measurement
- States Of Matter Exercise
- Stoichiometry Law Breakers
- Stoichiometry - Math Relationships
- Subatomic Particles
- Spontaneity and Driving Forces
- Gibbs Free Energy
- Volume-Temperature Gas Law
- Acid-Base Properties
- Energy and Chemical Reactions
- Chemical and Physical Properties
- Valence Shell Electron Pair Repulsion Theory
- Writing Balanced Chemical Equations
- Mission CG1
- Mission CG10
- Mission CG2
- Mission CG3
- Mission CG4
- Mission CG5
- Mission CG6
- Mission CG7
- Mission CG8
- Mission CG9
- Mission EC1
- Mission EC10
- Mission EC11
- Mission EC12
- Mission EC2
- Mission EC3
- Mission EC4
- Mission EC5
- Mission EC6
- Mission EC7
- Mission EC8
- Mission EC9
- Mission RL1
- Mission RL2
- Mission RL3
- Mission RL4
- Mission RL5
- Mission RL6
- Mission KG7
- Mission RL8
- Mission KG9
- Mission RL10
- Mission RL11
- Mission RM1
- Mission RM2
- Mission RM3
- Mission RM4
- Mission RM5
- Mission RM6
- Mission RM8
- Mission RM10
- Mission LC1
- Mission RM11
- Mission LC2
- Mission LC3
- Mission LC4
- Mission LC5
- Mission LC6
- Mission LC8
- Mission SM1
- Mission SM2
- Mission SM3
- Mission SM4
- Mission SM5
- Mission SM6
- Mission SM8
- Mission SM10
- Mission KG10
- Mission SM11
- Mission KG2
- Mission KG3
- Mission KG4
- Mission KG5
- Mission KG6
- Mission KG8
- Mission KG11
- Mission F2D1
- Mission F2D2
- Mission F2D3
- Mission F2D4
- Mission F2D5
- Mission F2D6
- Mission KC1
- Mission KC2
- Mission KC3
- Mission KC4
- Mission KC5
- Mission KC6
- Mission KC7
- Mission KC8
- Mission AAA
- Mission SM9
- Mission LC7
- Mission LC9
- Mission NL1
- Mission NL2
- Mission NL3
- Mission NL4
- Mission NL5
- Mission NL6
- Mission NL7
- Mission NL8
- Mission NL9
- Mission NL10
- Mission NL11
- Mission NL12
- Mission MC1
- Mission MC10
- Mission MC2
- Mission MC3
- Mission MC4
- Mission MC5
- Mission MC6
- Mission MC7
- Mission MC8
- Mission MC9
- Mission RM7
- Mission RM9
- Mission RL7
- Mission RL9
- Mission SM7
- Mission SE1
- Mission SE10
- Mission SE11
- Mission SE12
- Mission SE2
- Mission SE3
- Mission SE4
- Mission SE5
- Mission SE6
- Mission SE7
- Mission SE8
- Mission SE9
- Mission VP1
- Mission VP10
- Mission VP2
- Mission VP3
- Mission VP4
- Mission VP5
- Mission VP6
- Mission VP7
- Mission VP8
- Mission VP9
- Mission WM1
- Mission WM2
- Mission WM3
- Mission WM4
- Mission WM5
- Mission WM6
- Mission WM7
- Mission WM8
- Mission WE1
- Mission WE10
- Mission WE2
- Mission WE3
- Mission WE4
- Mission WE5
- Mission WE6
- Mission WE7
- Mission WE8
- Mission WE9
- Vector Walk Interactive
- Name That Motion Interactive
- Kinematic Graphing 1 Concept Checker
- Kinematic Graphing 2 Concept Checker
- Graph That Motion Interactive
- Two Stage Rocket Interactive
- Rocket Sled Concept Checker
- Force Concept Checker
- Free-Body Diagrams Concept Checker
- Free-Body Diagrams The Sequel Concept Checker
- Skydiving Concept Checker
- Elevator Ride Concept Checker
- Vector Addition Concept Checker
- Vector Walk in Two Dimensions Interactive
- Name That Vector Interactive
- River Boat Simulator Concept Checker
- Projectile Simulator 2 Concept Checker
- Projectile Simulator 3 Concept Checker
- Hit the Target Interactive
- Turd the Target 1 Interactive
- Turd the Target 2 Interactive
- Balance It Interactive
- Go For The Gold Interactive
- Egg Drop Concept Checker
- Fish Catch Concept Checker
- Exploding Carts Concept Checker
- Collision Carts - Inelastic Collisions Concept Checker
- Its All Uphill Concept Checker
- Stopping Distance Concept Checker
- Chart That Motion Interactive
- Roller Coaster Model Concept Checker
- Uniform Circular Motion Concept Checker
- Horizontal Circle Simulation Concept Checker
- Vertical Circle Simulation Concept Checker
- Race Track Concept Checker
- Gravitational Fields Concept Checker
- Orbital Motion Concept Checker
- Angular Acceleration Concept Checker
- Balance Beam Concept Checker
- Torque Balancer Concept Checker
- Aluminum Can Polarization Concept Checker
- Charging Concept Checker
- Name That Charge Simulation
- Coulomb's Law Concept Checker
- Electric Field Lines Concept Checker
- Put the Charge in the Goal Concept Checker
- Circuit Builder Concept Checker (Series Circuits)
- Circuit Builder Concept Checker (Parallel Circuits)
- Circuit Builder Concept Checker (∆V-I-R)
- Circuit Builder Concept Checker (Voltage Drop)
- Equivalent Resistance Interactive
- Pendulum Motion Simulation Concept Checker
- Mass on a Spring Simulation Concept Checker
- Particle Wave Simulation Concept Checker
- Boundary Behavior Simulation Concept Checker
- Slinky Wave Simulator Concept Checker
- Simple Wave Simulator Concept Checker
- Wave Addition Simulation Concept Checker
- Standing Wave Maker Simulation Concept Checker
- Color Addition Concept Checker
- Painting With CMY Concept Checker
- Stage Lighting Concept Checker
- Filtering Away Concept Checker
- InterferencePatterns Concept Checker
- Young's Experiment Interactive
- Plane Mirror Images Interactive
- Who Can See Who Concept Checker
- Optics Bench (Mirrors) Concept Checker
- Name That Image (Mirrors) Interactive
- Refraction Concept Checker
- Total Internal Reflection Concept Checker
- Optics Bench (Lenses) Concept Checker
- Kinematics Preview
- Velocity Time Graphs Preview
- Moving Cart on an Inclined Plane Preview
- Stopping Distance Preview
- Cart, Bricks, and Bands Preview
- Fan Cart Study Preview
- Friction Preview
- Coffee Filter Lab Preview
- Friction, Speed, and Stopping Distance Preview
- Up and Down Preview
- Projectile Range Preview
- Ballistics Preview
- Juggling Preview
- Marshmallow Launcher Preview
- Air Bag Safety Preview
- Colliding Carts Preview
- Collisions Preview
- Engineering Safer Helmets Preview
- Push the Plow Preview
- Its All Uphill Preview
- Energy on an Incline Preview
- Modeling Roller Coasters Preview
- Hot Wheels Stopping Distance Preview
- Ball Bat Collision Preview
- Energy in Fields Preview
- Weightlessness Training Preview
- Roller Coaster Loops Preview
- Universal Gravitation Preview
- Keplers Laws Preview
- Kepler's Third Law Preview
- Charge Interactions Preview
- Sticky Tape Experiments Preview
- Wire Gauge Preview
- Voltage, Current, and Resistance Preview
- Light Bulb Resistance Preview
- Series and Parallel Circuits Preview
- Thermal Equilibrium Preview
- Linear Expansion Preview
- Heating Curves Preview
- Electricity and Magnetism - Part 1 Preview
- Electricity and Magnetism - Part 2 Preview
- Vibrating Mass on a Spring Preview
- Period of a Pendulum Preview
- Wave Speed Preview
- Slinky-Experiments Preview
- Standing Waves in a Rope Preview
- Sound as a Pressure Wave Preview
- DeciBel Scale Preview
- DeciBels, Phons, and Sones Preview
- Sound of Music Preview
- Shedding Light on Light Bulbs Preview
- Models of Light Preview
- Electromagnetic Radiation Preview
- Electromagnetic Spectrum Preview
- EM Wave Communication Preview
- Digitized Data Preview
- Light Intensity Preview
- Concave Mirrors Preview
- Object Image Relations Preview
- Snells Law Preview
- Reflection vs. Transmission Preview
- Magnification Lab Preview
- Reactivity Preview
- Ions and the Periodic Table Preview
- Periodic Trends Preview
- Chemical Reactions Preview
- Intermolecular Forces Preview
- Melting Points and Boiling Points Preview
- Bond Energy and Reactions Preview
- Reaction Rates Preview
- Ammonia Factory Preview
- Stoichiometry Preview
- Nuclear Chemistry Preview
- Gaining Teacher Access
- Task Tracker Directions
- Conceptual Physics Course
- On-Level Physics Course
- Honors Physics Course
- Chemistry Concept Builders
- All Chemistry Resources
- Users Voice
- Tasks and Classes
- Webinars and Trainings
- Subscription
- Subscription Locator
- 1-D Kinematics
- Newton's Laws
- Vectors - Motion and Forces in Two Dimensions
- Momentum and Its Conservation
- Work and Energy
- Circular Motion and Satellite Motion
- Thermal Physics
- Static Electricity
- Electric Circuits
- Vibrations and Waves
- Sound Waves and Music
- Light and Color
- Reflection and Mirrors
- Measurement and Calculations
- Elements, Atoms, and Ions
- About the Physics Interactives
- Task Tracker
- Usage Policy
- Newtons Laws
- Vectors and Projectiles
- Forces in 2D
- Momentum and Collisions
- Circular and Satellite Motion
- Balance and Rotation
- Electromagnetism
- Waves and Sound
- Atomic Physics
- Forces in Two Dimensions
- Work, Energy, and Power
- Circular Motion and Gravitation
- Sound Waves
- 1-Dimensional Kinematics
- Circular, Satellite, and Rotational Motion
- Einstein's Theory of Special Relativity
- Waves, Sound and Light
- QuickTime Movies
- About the Concept Builders
- Pricing For Schools
- Directions for Version 2
- Measurement and Units
- Relationships and Graphs
- Rotation and Balance
- Vibrational Motion
- Reflection and Refraction
- Teacher Accounts
- Kinematic Concepts
- Kinematic Graphing
- Wave Motion
- Sound and Music
- About CalcPad
- 1D Kinematics
- Vectors and Forces in 2D
- Simple Harmonic Motion
- Rotational Kinematics
- Rotation and Torque
- Rotational Dynamics
- Electric Fields, Potential, and Capacitance
- Transient RC Circuits
- Light Waves
- Units and Measurement
- Stoichiometry
- Molarity and Solutions
- Thermal Chemistry
- Acids and Bases
- Kinetics and Equilibrium
- Solution Equilibria
- Oxidation-Reduction
- Nuclear Chemistry
- Newton's Laws of Motion
- Work and Energy Packet
- Static Electricity Review
- NGSS Alignments
- 1D-Kinematics
- Projectiles
- Circular Motion
- Magnetism and Electromagnetism
- Graphing Practice
- About the ACT
- ACT Preparation
- For Teachers
- Other Resources
- Solutions Guide
- Solutions Guide Digital Download
- Motion in One Dimension
- Work, Energy and Power
- Chemistry of Matter
- Measurement and the Metric System
- Names and Formulas
- Algebra Based On-Level Physics
- Honors Physics
- Conceptual Physics
- Other Tools
- Frequently Asked Questions
- Purchasing the Download
- Purchasing the Digital Download
- About the NGSS Corner
- NGSS Search
- Force and Motion DCIs - High School
- Energy DCIs - High School
- Wave Applications DCIs - High School
- Force and Motion PEs - High School
- Energy PEs - High School
- Wave Applications PEs - High School
- Crosscutting Concepts
- The Practices
- Physics Topics
- NGSS Corner: Activity List
- NGSS Corner: Infographics
- About the Toolkits
- Position-Velocity-Acceleration
- Position-Time Graphs
- Velocity-Time Graphs
- Newton's First Law
- Newton's Second Law
- Newton's Third Law
- Terminal Velocity
- Projectile Motion
- Forces in 2 Dimensions
- Impulse and Momentum Change
- Momentum Conservation
- Work-Energy Fundamentals
- Work-Energy Relationship
- Roller Coaster Physics
- Satellite Motion
- Electric Fields
- Circuit Concepts
- Series Circuits
- Parallel Circuits
- Describing-Waves
- Wave Behavior Toolkit
- Standing Wave Patterns
- Resonating Air Columns
- Wave Model of Light
- Plane Mirrors
- Curved Mirrors
- Teacher Guide
- Using Lab Notebooks
- Current Electricity
- Light Waves and Color
- Reflection and Ray Model of Light
- Refraction and Ray Model of Light
- Teacher Resources
- Subscriptions

- Newton's Laws
- Einstein's Theory of Special Relativity
- About Concept Checkers
- School Pricing
- Newton's Laws of Motion
- Newton's First Law
- Newton's Third Law
Chemistry: Units and Measurement
Metric Math Conversion Problems
Related Pages Introduction to the Metric System Metric Worksheets Measurement Games
In these lessons, we will look into some methods that can be used for metric conversion problems. The first method uses the metric table and can also be regarded as a shortcut method. The second method uses the metric staircase and the third method uses the unit fraction method. Scroll done the page for free metric worksheets.
The following diagrams show some metric conversions for lengths and weights. Scroll down the page for examples and solutions.
| Metric Conversion Methods | ||
|---|---|---|
Metric Length Conversions (km, m, cm) Metric Mass Conversions (kg, g) Metric Capacity Conversions (L, cL)
Metric Length Word Problems Metric Capacity Word Problems Metric Mass Word Problems Measurement Word Problems Customary Measurement Word Problems Multi-Step Measurement Word Problems
Convert using a Metric Table or Shortcut Method
The metric system uses prefixes to denote multiple of 10.
The following table shows some common prefixes of metric units and how to convert between them.
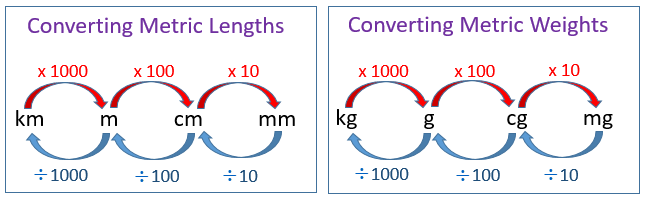
Each prefix differs by a multiple of 10 from the next prefix. When converting between the different units of measure, we look at the number of “jumps” between the prefixes of the two units and then multiply or divide by the powers of 10 accordingly (moving to the right would mean to multiply and moving to the left would mean to divide).
Example: Convert 3 km to m
Solution: There are 3 “jumps” to the right from kilometer to meter.
So, we multiply by 10 3 = 1000
3 km = 3 × 1,000 = 3,000 m
Example: Convert 20 mm to cm
Solution: There is 1 “jump” to the left from millimeter to centimeter
So, we divide by 10.
20 mm = 20 ÷ 10 = 2 cm
How to convert to different metric units of measure for length, capacity, and mass? The metric system is widely used because it is based on the powers of 10. This allows for easy conversion between smaller and larger units. The basic unit of length is the meter (m). It is just over 1 yard. The basic unit of weight is the gram (g). One gram is the mass of 1 cubic centimeter of water. The basic unit of capacity or volume is liter (l). A liter equals 1000 cubic centimeters. Examples: Complete each conversion
- 6.3km = ____ m
- 62,300cm = ___ hm
- 37,200g = ___ kg
- 1.73hg = ___ dg
- 93.7L = ___ mL
- 62,300cL = ___ kL
How to perform metric conversion using the table or shortcut method?
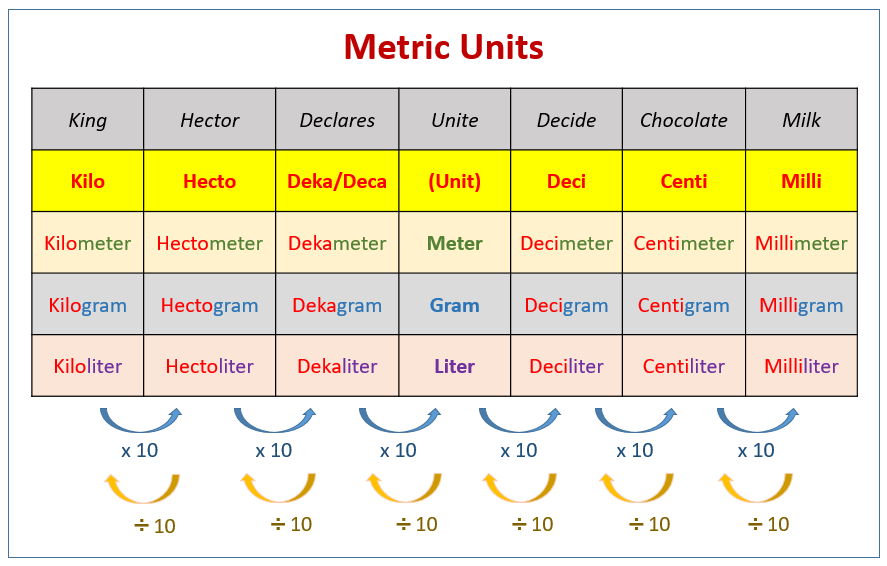
Use the mnemonic “King Henry Drinks Ucky Dark Chocolate Milk”
- 0.521 mm to m
- 9501 g to kg
- 40.6 L to mL
- 1457 dm to hm
- 53.45 dag to mg
- .4 kL to mL
How to convert metric units of weight without using a calculator? The presentation focuses on conversion between kg, g, and mg. Imagine a 1 kilogram(kg) tablet which is made up of a 1000 smaller gram(g) tablets which is in turn made up of 1000 even smaller milligram (mg) tablets. Big to small: multiply Small to big: divide
How to multiply and divide by 1000 in conversion of metric units? This tutorial explains answers to exercises in converting metric units of weight. The exercises involve multiplying and dividing without a calculator.
Metric Staircase or Ladder Method
How to use the metric staircase to convert units within the metric system? Another method similar to the table method is to use the metric staircase. It is also called the ladder method.
Use the mnemonic “Kangaroo Helps Dingo Because Dingo Can’t Multiply”
How to use the metric staircase to convert between metric measurements? When you go up the stairs to the left, move the decimal to the left. When you go down the stairs to the right, move the decimal to the right.
Convert using the Unit Fraction Method
In this method, we multiply by a unit fraction in order to perform the metric conversion.
Common Metric Length Conversions Using Unit Fractions
Common Metric Weight Conversions Using Unit Fractions
Metric Measurement Worksheets Metric Measurement Conversion Metric Measurement Word Problems
Adding Measurements Subtracting Measurements Measurement Word Problems
Metric Length Worksheets Printable & Online Metric Length Conversions (km, m, cm) Metric Length Word Problems (+, -)
Convert m to cm Convert m to km and m
Adding lengths (km, m, cm, mm) Subtracting Lengths (km, m, cm, mm) Multiplying Lengths (km, m, cm, mm) Dividing Lengths (km, m, cm, mm)
Interactive Metric Length Conversion (mm, cm, m, km)
Metric Weight Worksheets Printable & Online Metric Mass Conversions (kg, g) Metric Mass Word Problems (+, -)
Adding and Subtracting Weights (kg, g, mg) Multiplying Weights (kg, g, mg) Dividing Weights (kg, g, mg)
Interactive Metric Weight Conversion (kg, g, mg)
Metric Area, Volume Worksheets Metric Area Conversion
Metric Capacity Conversions (L, cL) Metric Capacity Word Problems (+, -)
Metric Volume Conversion

We welcome your feedback, comments and questions about this site or page. Please submit your feedback or enquiries via our Feedback page.
1.3 Unit Conversion
Learning objectives.
By the end of this section, you will be able to:
- Use conversion factors to express the value of a given quantity in different units.
It is often necessary to convert from one unit to another. For example, if you are reading a European cookbook, some quantities may be expressed in units of liters and you need to convert them to cups. Or perhaps you are reading walking directions from one location to another and you are interested in how many miles you will be walking. In this case, you may need to convert units of feet or meters to miles.
Let’s consider a simple example of how to convert units. Suppose we want to convert 80 m to kilometers. The first thing to do is to list the units you have and the units to which you want to convert. In this case, we have units in meters and we want to convert to kilometers . Next, we need to determine a conversion factor relating meters to kilometers. A conversion factor is a ratio that expresses how many of one unit are equal to another unit. For example, there are 12 in. in 1 ft, 1609 m in 1 mi, 100 cm in 1 m, 60 s in 1 min, and so on. Refer to Appendix B for a more complete list of conversion factors. In this case, we know that there are 1000 m in 1 km. Now we can set up our unit conversion. We write the units we have and then multiply them by the conversion factor so the units cancel out, as shown:
Note that the unwanted meter unit cancels, leaving only the desired kilometer unit. You can use this method to convert between any type of unit. Now, the conversion of 80 m to kilometers is simply the use of a metric prefix, as we saw in the preceding section, so we can get the same answer just as easily by noting that
since “kilo-” means 10 3 (see Table 1.2 ) and 1 = −2 + 3 . 1 = −2 + 3 . However, using conversion factors is handy when converting between units that are not metric or when converting between derived units, as the following examples illustrate.
Example 1.2
Converting nonmetric units to metric.
- Calculate average speed. Average speed is distance traveled divided by time of travel. (Take this definition as a given for now. Average speed and other motion concepts are covered in later chapters.) In equation form, Average speed = Distance Time . Average speed = Distance Time .
- Substitute the given values for distance and time: Average speed = 10 mi 20 min = 0.50 mi min . Average speed = 10 mi 20 min = 0.50 mi min .
- Convert miles per minute to meters per second by multiplying by the conversion factor that cancels miles and leave meters, and also by the conversion factor that cancels minutes and leave seconds: 0.50 mile min × 1609 m 1 mile × 1 min 60 s = ( 0.50 ) ( 1609 ) 60 m/s = 13 m/s . 0.50 mile min × 1609 m 1 mile × 1 min 60 s = ( 0.50 ) ( 1609 ) 60 m/s = 13 m/s .
Significance
- Be sure the units in the unit conversion cancel correctly. If the unit conversion factor was written upside down, the units do not cancel correctly in the equation. We see the “miles” in the numerator in 0.50 mi/min cancels the “mile” in the denominator in the first conversion factor. Also, the “min” in the denominator in 0.50 mi/min cancels the “min” in the numerator in the second conversion factor.
- Check that the units of the final answer are the desired units. The problem asked us to solve for average speed in units of meters per second and, after the cancellations, the only units left are a meter (m) in the numerator and a second (s) in the denominator, so we have indeed obtained these units.
Check Your Understanding 1.2
Light travels about 9 Pm in a year. Given that a year is about 3 × 10 7 s , 3 × 10 7 s , what is the speed of light in meters per second?
Example 1.3
Converting between metric units.
- Be sure to cancel the units in the unit conversion correctly. We see that the gram (“g”) in the numerator in 7.86 g/cm 3 cancels the “g” in the denominator in the first conversion factor. Also, the three factors of “cm” in the denominator in 7.86 g/cm 3 cancel with the three factors of “cm” in the numerator that we get by cubing the second conversion factor.
- Check that the units of the final answer are the desired units. The problem asked for us to convert to kilograms per cubic meter. After the cancellations just described, we see the only units we have left are “kg” in the numerator and three factors of “m” in the denominator (that is, one factor of “m” cubed, or “m 3 ”). Therefore, the units on the final answer are correct.
Check Your Understanding 1.3
We know from Figure 1.4 that the diameter of Earth is on the order of 10 7 m, so the order of magnitude of its surface area is 10 14 m 2 . What is that in square kilometers (that is, km 2 )? (Try doing this both by converting 10 7 m to km and then squaring it and then by converting 10 14 m 2 directly to square kilometers. You should get the same answer both ways.)
Unit conversions may not seem very interesting, but not doing them can be costly. One famous example of this situation was seen with the Mars Climate Orbiter . This probe was launched by NASA on December 11, 1998. On September 23, 1999, while attempting to guide the probe into its planned orbit around Mars, NASA lost contact with it. Subsequent investigations showed a piece of software called SM_FORCES (or “small forces”) was recording thruster performance data in the English units of pound-force-seconds (lbf-s). However, other pieces of software that used these values for course corrections expected them to be recorded in the SI units of newton-seconds (N-s), as dictated in the software interface protocols. This error caused the probe to follow a very different trajectory from what NASA thought it was following, which most likely caused the probe either to burn up in the Martian atmosphere or to shoot out into space. This failure to pay attention to unit conversions cost hundreds of millions of dollars, not to mention all the time invested by the scientists and engineers who worked on the project.
Check Your Understanding 1.4
Given that 1 lbf (pound-force) is 4.45 N, were the numbers being output by SM_FORCES too big or too small?
This book may not be used in the training of large language models or otherwise be ingested into large language models or generative AI offerings without OpenStax's permission.
Want to cite, share, or modify this book? This book uses the Creative Commons Attribution License and you must attribute OpenStax.
Access for free at https://openstax.org/books/university-physics-volume-1/pages/1-introduction
- Authors: William Moebs, Samuel J. Ling, Jeff Sanny
- Publisher/website: OpenStax
- Book title: University Physics Volume 1
- Publication date: Sep 19, 2016
- Location: Houston, Texas
- Book URL: https://openstax.org/books/university-physics-volume-1/pages/1-introduction
- Section URL: https://openstax.org/books/university-physics-volume-1/pages/1-3-unit-conversion
© Jul 23, 2024 OpenStax. Textbook content produced by OpenStax is licensed under a Creative Commons Attribution License . The OpenStax name, OpenStax logo, OpenStax book covers, OpenStax CNX name, and OpenStax CNX logo are not subject to the Creative Commons license and may not be reproduced without the prior and express written consent of Rice University.

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
Chapter 1: Algebra Review
1.6 Unit Conversion Word Problems
One application of rational expressions deals with converting units. Units of measure can be converted by multiplying several fractions together in a process known as dimensional analysis.
The trick is to decide what fractions to multiply. If an expression is multiplied by 1, its value does not change. The number 1 can be written as a fraction in many different ways, so long as the numerator and denominator are identical in value. Note that the numerator and denominator need not be identical in appearance, but rather only identical in value. Below are several fractions, each equal to 1, where the numerator and the denominator are identical in value. This is why, when doing dimensional analysis, it is very important to use units in the setup of the problem, so as to ensure that the conversion factor is set up correctly.
Example 1.6.1
If 1 pound = 16 ounces, how many pounds are in 435 ounces?
[latex]\begin{array}{rrll} 435\text{ oz}&=&435\cancel{\text{oz}}\times \dfrac{1\text{ lb}}{16\cancel{ \text{oz}}} \hspace{0.2in}& \text{This operation cancels the oz and leaves the lbs} \\ \\ &=&\dfrac{435\text{ lb}}{16} \hspace{0.2in}& \text{Which reduces to } \\ \\ &=&27\dfrac{3}{16}\text{ lb} \hspace{0.2in}& \text{Solution} \end{array}[/latex]
The same process can be used to convert problems with several units in them. Consider the following example.
Example 1.6.2
A student averaged 45 miles per hour on a trip. What was the student’s speed in feet per second?
[latex]\begin{array}{rrll} 45 \text{ mi/h}&=&\dfrac{45\cancel{\text{mi}}}{\cancel{\text{hr}}}\times \dfrac{5280 \text{ ft}}{1\cancel{ \text{mi}}}\times \dfrac{1\cancel{\text{hr}}}{3600\text{ s}}\hspace{0.2in}&\text{This will cancel the miles and hours} \\ \\ &=&45\times \dfrac{5280}{1}\times \dfrac{1}{3600} \text{ ft/s}\hspace{0.2in}&\text{This reduces to} \\ \\ &=&66\text{ ft/s}\hspace{0.2in}&\text{Solution} \end{array}[/latex]
Example 1.6.3
Convert 8 ft 3 to yd 3 .
[latex]\begin{array}{rrll} 8\text{ ft}^3&=&8\text{ ft}^3 \times \dfrac{(1\text{ yd})^3}{(3\text{ ft})^3}&\text{Cube the parentheses} \\ \\ &=&8\text{ }\cancel{\text{ft}^3}\times \dfrac{1\text{ yd}^3}{27\text{ }\cancel{\text{ft}^3}}&\text{This will cancel the ft}^3\text{ and replace them with yd}^3 \\ \\ &=&8\times \dfrac{1\text{ yd}^3}{27}&\text{Which reduces to} \\ \\ &=&\dfrac{8}{27}\text{ yd}^3\text{ or }0.296\text{ yd}^3&\text{Solution} \end{array}[/latex]
Example 1.6.4
A room is 10 ft by 12 ft. How many square yards are in the room? The area of the room is 120 ft 2 (area = length × width).
Converting the area yields:
[latex]\begin{array}{rrll} 120\text{ ft}^2&=&120\text{ }\cancel{\text{ft}^2}\times \dfrac{(1\text{ yd})^2}{(3\text{ }\cancel{\text{ft}})^2}&\text{Cancel ft}^2\text{ and replace with yd}^2 \\ \\ &=&\dfrac{120\text{ yd}^2}{9}&\text{This reduces to} \\ \\ &=&13\dfrac{1}{3}\text{ yd}^2&\text{Solution} \\ \\ \end{array}[/latex]
The process of dimensional analysis can be used to convert other types of units as well. Once relationships that represent the same value have been identified, a conversion factor can be determined.
Example 1.6.5
A child is prescribed a dosage of 12 mg of a certain drug per day and is allowed to refill his prescription twice. If there are 60 tablets in a prescription, and each tablet has 4 mg, how many doses are in the 3 prescriptions (original + 2 refills)?
[latex]\begin{array}{rrll} 3\text{ prescriptions}&=&3\cancel{\text{pres.}}\times \dfrac{60\cancel{\text{tablets}}}{1\cancel{\text{pres.}}}\times \dfrac{4\cancel{\text{mg}}}{1\cancel{\text{tablet}}}\times \dfrac{1\text{ dosage}}{12\cancel{\text{mg}}}&\text{This cancels all unwanted units} \\ \\ &=&\dfrac{3\times 60\times 4\times 1}{1\times 1\times 12}\text{ or }\dfrac{720}{12}\text{ dosages}&\text{Which reduces to} \\ \\ &=&60\text{ daily dosages}&\text{Solution} \\ \\ \end{array}[/latex]
Metric and Imperial (U.S.) Conversions
[latex]\begin{array}{rrlrrl} 12\text{ in}&=&1\text{ ft}\hspace{1in}&10\text{ mm}&=&1\text{ cm} \\ 3\text{ ft}&=&1\text{ yd}&100\text{ cm}&=&1\text{ m} \\ 1760\text{ yds}&=&1\text{ mi}&1000\text{ m}&=&1\text{ km} \\ 5280\text{ ft}&=&1\text{ mi}&&& \end{array}[/latex]
Imperial to metric conversions:
[latex]\begin{array}{rrl} 1\text{ inch}&=&2.54\text{ cm} \\ 1\text{ ft}&=&0.3048\text{ m} \\ 1\text{ mile}&=&1.61\text{ km} \end{array}[/latex]
[latex]\begin{array}{rrlrrl} 144\text{ in}^2&=&1\text{ ft}^2\hspace{1in}&10,000\text{ cm}^2&=&1\text{ m}^2 \\ 43,560\text{ ft}^2&=&1\text{ acre}&10,000\text{ m}^2&=&1\text{ hectare} \\ 640\text{ acres}&=&1\text{ mi}^2&100\text{ hectares}&=&1\text{ km}^2 \end{array}[/latex]
[latex]\begin{array}{rrl} 1\text{ in}^2&=&6.45\text{ cm}^2 \\ 1\text{ ft}^2&=&0.092903\text{ m}^2 \\ 1\text{ mi}^2&=&2.59\text{ km}^2 \end{array}[/latex]
[latex]\begin{array}{rrlrrl} 57.75\text{ in}^3&=&1\text{ qt}\hspace{1in}&1\text{ cm}^3&=&1\text{ ml} \\ 4\text{ qt}&=&1\text{ gal}&1000\text{ ml}&=&1\text{ litre} \\ 42\text{ gal (petroleum)}&=&1\text{ barrel}&1000\text{ litres}&=&1\text{ m}^3 \end{array}[/latex]
[latex]\begin{array}{rrl} 16.39\text{ cm}^3&=&1\text{ in}^3 \\ 1\text{ ft}^3&=&0.0283168\text{ m}^3 \\ 3.79\text{ litres}&=&1\text{ gal} \end{array}[/latex]
[latex]\begin{array}{rrlrrl} 437.5\text{ grains}&=&1\text{ oz}\hspace{1in}&1000\text{ mg}&=&1\text{ g} \\ 16\text{ oz}&=&1\text{ lb}&1000\text{ g}&=&1\text{ kg} \\ 2000\text{ lb}&=&1\text{ short ton}&1000\text{ kg}&=&1\text{ metric ton} \end{array}[/latex]
[latex]\begin{array}{rrl} 453\text{ g}&=&1\text{ lb} \\ 2.2\text{ lb}&=&1\text{ kg} \end{array}[/latex]
Temperature
Fahrenheit to Celsius conversions:
[latex]\begin{array}{rrl} ^{\circ}\text{C} &= &\dfrac{5}{9} (^{\circ}\text{F} - 32) \\ \\ ^{\circ}\text{F}& =& \left(\dfrac{9}{5}\right)(^{\circ}\text{C}) + 32 \end{array}[/latex]
| °F | −40°F | −22°F | −4°F | 14°F | 32°F | 50°F | 68°F | 86°F | 104°F | 122°F | 140°F | 158°F | 176°F | 194°F | 212°F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | −40°C | −30°C | −20°C | −10°C | 0°C | 10°C | 20°C | 30°C | 40°C | 50°C | 60°C | 70°C | 80°C | 90°C | 100°C |
For questions 1 to 18, use dimensional analysis to perform the indicated conversions.
- 7 miles to yards
- 234 oz to tons
- 11.2 mg to grams
- 1.35 km to centimetres
- 9,800,000 mm to miles
- 4.5 ft 2 to square yards
- 435,000 m 2 to square kilometres
- 8 km 2 to square feet
- 0.0065 km 3 to cubic metres
- 14.62 in. 2 to square centimetres
- 5500 cm 3 to cubic yards
- 3.5 mph (miles per hour) to feet per second
- 185 yd per min. to miles per hour
- 153 ft/s (feet per second) to miles per hour
- 248 mph to metres per second
- 186,000 mph to kilometres per year
- 7.50 tons/yd 2 to pounds per square inch
- 16 ft/s 2 to kilometres per hour squared
For questions 19 to 27, solve each conversion word problem.
- On a recent trip, Jan travelled 260 miles using 8 gallons of gas. What was the car’s miles per gallon for this trip? Kilometres per litre?
- A certain laser printer can print 12 pages per minute. Determine this printer’s output in pages per day.
- An average human heart beats 60 times per minute. If the average person lives to the age of 86, how many times does the average heart beat in a lifetime?
- Blood sugar levels are measured in milligrams of glucose per decilitre of blood volume. If a person’s blood sugar level measured 128 mg/dL, what is this in grams per litre?
- You are buying carpet to cover a room that measures 38 ft by 40 ft. The carpet cost $18 per square yard. How much will the carpet cost?
- A cargo container is 50 ft long, 10 ft wide, and 8 ft tall. Find its volume in cubic yards and cubic metres.
- A local zoning ordinance says that a house’s “footprint” (area of its ground floor) cannot occupy more than ¼ of the lot it is built on. Suppose you own a [latex]\frac{1}{3}[/latex]-acre lot (1 acre = 43,560 ft 2 ). What is the maximum allowed footprint for your house in square feet? In square metres?
- A car travels 23 km in 15 minutes. How fast is it going in kilometres per hour? In metres per second?
- The largest single rough diamond ever found, the Cullinan Diamond, weighed 3106 carats. One carat is equivalent to the mass of 0.20 grams. What is the mass of this diamond in milligrams? Weight in pounds?
Answer Key 1.6
Intermediate Algebra Copyright © 2020 by Terrance Berg is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book

Math Method
- Stat and Prob
- Patterns and Sequences
- Trigonometry
- Try RTL Mode

LESSON | Converting Units & Solving Problems Involving Conversion
The complete guide to converting units & solving problems involving conversion.

In this post, you'll learn how to solve problems that involve converting units and how to convert measurements from one unit to another in both the English and Metric systems. Since these systems are used by a lot of people, having a good understanding of them will help you solve these problems more accurately. in everyday life.
(toc) Table of Contents
CONVERSION OF MEASUREMENTS FROM ONE UNIT TO ANOTHER
| Units of Length in English System | Units of Length in the Metric System | System to System Conversions for Length |
|---|---|---|
| $ 1 foot( ft) = 12 inches (in)$ $1 yard (yd) = 3 feet (ft)$ $1 yard (yd) =36 inches (in)$ $1 mile (mi) = 5,280 feet (ft)$ | $1, 000 millimeter (mm) = 1 meter$ $100 centimeters (cm) = 1 meter$ $10 decimeter (dm) = 1 meter$ $1 dekameter (dam) = 10 meters$ $1 hectometer (hm) = 100 meters$ $1 kilometer (km) = 1000 meters$ | $1 in = 2.54 cm$ $1 meter ≈ 3. 28 ft$ $1 foot ≈ 0.30 m$ $1 yard ≈ 0.91 m$ $1 km ≈ 0.62 mi$ |
| Units of Mass in English System | Units of Mass in the Metric System | System to System Conversions for Mass |
|---|---|---|
| $1 ounces (oz) = 437.5 grains $ $ 1 pound (lb) = 16 ounces (oz)$ $ 1 ton (T) = 2, 000 lb$ | $1 gram (g) = 1, 000 milligram (mg)$ $1 gram (g) = 100 centigram (cg)$ $1 kilogram (kg)= 1000 grams (g)$ $1 metric ton (t) = 1, 000 kg$ | $1 oz ≈ 28.3 g$ $1 lb ≈0.45 kg$ |
| Units of Area in English System | Units of Area in the Metric System | System to System Conversions for Mass |
|---|---|---|
| $1 {ft^2} = 144 in^2$ $1 {yd^2} = 9 {ft^2}$ $1 acre = 43, 560 ft^2$ $1 {mi^2} = 640 acres$ | $1 cm^2 = 100 {mm^2}$ $1 {dm^2} = 100 {cm^2}$ $1 {m^2} = 100 {dm^2}$ $1 are (a) = 100 {m^2}$ $1 hectare (ha) = 100 a$ $100 hectares (ha) = 1 {km^2}$ | $1 in^2 ≈ 6.45 {cm^2}$ $1 m^2 ≈ 1.196 {yd^2}$ $1 ha ≈ 2.47 acres$ |
| Units of Volume in English System | Units of Volume in the Metric System | System to System Conversions for Volume |
|---|---|---|
| $1 ft^3 = 1, 728 in^3$ $1 yd^3 = 27 ft^3$ $1 cord = 128 ft^3$ | $1 cc = 1 cm^3$ $1 mL = 1 cm^3$ $1 L = 1, 000 mL$ $1 hL = 100 L$ $1 kL = 1, 000 L$ | $1 in^3 ≈ 16.39 mL$ $1 liter ≈ 1.06 qt$ $1 gallon ≈ 3.79 liters$ $1 m3 ≈ 35.31 ft^3$ $1 quart ≈ 0.95 L$ |
| Units of Fluid Volume in English System | Units of Time in the Both System | System to System Conversions for Temperature |
|---|---|---|
| $1 tablespoon (T) = 3 teaspoons (tsp)$ $1 fluid ounce (fl oz) = 2T 1$ $cup (c) = 8 fl oz$ $1 pint (pt) = 2 c$ $1 quart (qt) = 2 pt$ $1 gallon (gal) = 4 qt$ $1 gal = 128 fl oz 1$ $barrel = 42 gallon$ | $1 millisecond=1000 microseconds$ $1 second = 1000 millisecond$ $1 minute = 60 seconds$ $1 hour = 60 minutes$ $1 day ≈ 24 hours (hrs)$ $1 month ≈ 30 days$ $1 year ≈ 365 days$ $1 banking year = 360 days$ $1 decade = 10 years$ $1 score = 20 years$ $1 millennium = 1, 000 years$ | $°F \to °C$ $°C = {5 \over 9} (°F – 32)$ $°C \to °F$ $°F = {9 \over 5}°C + 32$ $°K \to °C$ $°K = °C + 273$ |
| Giga (G) | Mega (M) | Kilo (k) | Hecto (h) | Deka (da, D) | Gram(g) Meter(m) Liter(L) | Deci (d) | Centi (c) | Milli (m) | Micro (μ) | Nano (n) |
|---|---|---|---|---|---|---|---|---|---|---|
| $10^9$ | $10^6$ | $10^3$ | $10^2$ | $10^1$ | $1$ | $10^{-1}$ | $10^{-2}$ | $10^{-3}$ | $10^{-6}$ | $10^{-9}$ |
6 Steps on How to Convert A Unit of Measurement to Another Unit
1. Compare the two units.
2. Find the conversion factors that gives the appropriate ratio to the given unit.
3. Write the conversion as a fraction, where the denominator is in the same unit as the given unit.
4. Write a multiplication problem with the original number and the fraction.
5. Cancel out similar units that appears on the numerator and denominator.
Convert Lengths or Distance
Convert mass, convert area, convert volume, convert time, try this unit conversion calculator.
This unit converter is a free and easy to use tool for converting units of measurements.
It converts length, area, volume, weight, speed, density and temperature. It also converts between different units of measurement.
The converter is available in metric or imperial units. You can type in the unit you want to convert into the search bar or click on the links below to find your desired unit.
Conclusion: Use these Tips to Succeed at Unit Conversions with Ease
When changing from one unit of measurement to another, it is very important to know the table of conversion because this will be your guide.
There are some measurements in the table that can't be changed directly, so we should know which conversion factor is the easiest to use.
You need to know and be good at converting units before you can use the different problem-solving strategies to solve problems that involve converting units.
Unit conversions are a necessary skill in the workplace. Whether you are a student, engineer, or an accountant, you will need to know how to convert units of measurement.
Use these tips to succeed at unit conversions with ease:
- Use a calculator or an online conversion tool.
- Memorize the metric system conversion chart!
- Pay attention to units of measurements in context.
- Think about what units you want and what units you have before starting the conversion process.
- Never forget that there are always two values when converting from one unit to another - one from the original and one from the destination unit!
Post a Comment
Solving Problems Involving Conversion of Units of Measurement
Measuring is one of the human activities that we perform daily. A tailor measures the length of the dress; a butcher measures the weight of the meat; a surveyor measures the area of large land masses, and so on.
The ability to measure objects is always connected to the history of our civilization. During ancient times, our ancestors used their fingers, hands, and feet to determine the length of an object. In this era, humans had varying ways of measuring things.
Eventually, after the French Revolution in the late 18th century, a standardized way of measurement was developed (i.e., the metric system). Today, we have a convention of measurement units and advanced technological tools to measure objects.
In this module, you’ll learn what measurement is, the units of measurement (for length, weight, volume, time, and temperature), and how to convert units in the metric system.
Click below to go to the main reviewers:
Ultimate UPCAT Reviewer
Ultimate PNP Entrance Exam Reviewer
Ultimate NMAT Reviewer
Ultimate PMA Entrance Exam Reviewer
Ultimate LET Reviewer
Table of Contents
What is measurement.
Measurement is the provision of a numerical value to present and describe the magnitude or amount of a particular object.
We use measurement units to provide a more accurate description of the object’s measurement. Some examples of measurement units are meters, liters, grams, inches, Fahrenheit, and so on.
For instance, if we want to determine how long a piece of wood is, we measure its length. To do this, we use a particular SI unit of measurement (e.g., meters) and provide a number that describes the length of this wood (e.g., this wood is 3 meters long).
Since there are a lot of measurement units being used around the world, a standardized set of measurement units have been adopted by several countries. This is called the SI Units of Measurement , more commonly known as the Metric System (e.g., meter, gram, liter). On the other hand, there’s also the Imperial System or US Standard Units , which is also commonly used in the Philippines (e.g., feet, yards, pounds, etc.).
What Are the Different Ways To Measure Objects?
There are different ways to measure objects depending on the particular trait we want to describe or show.

This refers to the distance from one point to another. In other words, this describes how long or short an object is. Standard measurement units are meters, centimeters, inches, feet, etc.
The SI base unit for length is a meter.
This refers to the amount of space occupied by a two-dimensional figure. In other words, it tells us how much surface a plane figure covers . Commonly used measurement units are square meters, square kilometers, square yards, etc.
Metric units of the area have an exponent of 2 to indicate that we are measuring the amount of two-dimensional space occupied (e.g., the square meter is written as m 2 ).
The SI base unit for the area is square meters (m 2 ).
3. Volume or Capacity
The volume is the space occupied or enclosed by a three-dimensional figure . Commonly used measurement units are cubic meters, cubic kilometers, cubic yards, etc.
Metric volume units have an exponent of 3 to indicate that we are measuring the amount of three-dimensional space occupied (e.g., a cubic meter is written as m 3 ).

In Physics, mass and weight mean differently . Mass refers to the amount of matter an object has, while weight refers to the force that gravity exerts on an object.
The SI base units for mass and weight are different. The kilogram is the SI base unit for mass, while Newton is the SI base unit for weight.
However, outside a Physics classroom, these terms are often used interchangeably. Many people perceive mass and weight as the same thing, which refers to how heavy an object is. To avoid ambiguity and confusion in our discussion, we will strictly use in this article the word “mass” to refer to the heaviness of an object
This refers to the duration of the sequence of events. For instance, we measure time to determine how long you read this reviewer.
The SI base unit for time is seconds.
6. Temperature
Temperature tells us how hot or cold an object is. In Physics, the temperature is the average kinetic energy of the particles of an object . The primary way to measure temperature is through a thermometer .
SI base unit for temperature is Kelvin (K). However, Celsius (°C) and Fahrenheit (°F) are the more popular units.
This review will discuss the conversion of measurement units for length, area, volume, mass, time, and temperature. However, remember that these are not just the ways to measure objects. For instance, we can also measure their luminous intensity, electric current, amount of substance, and so on.
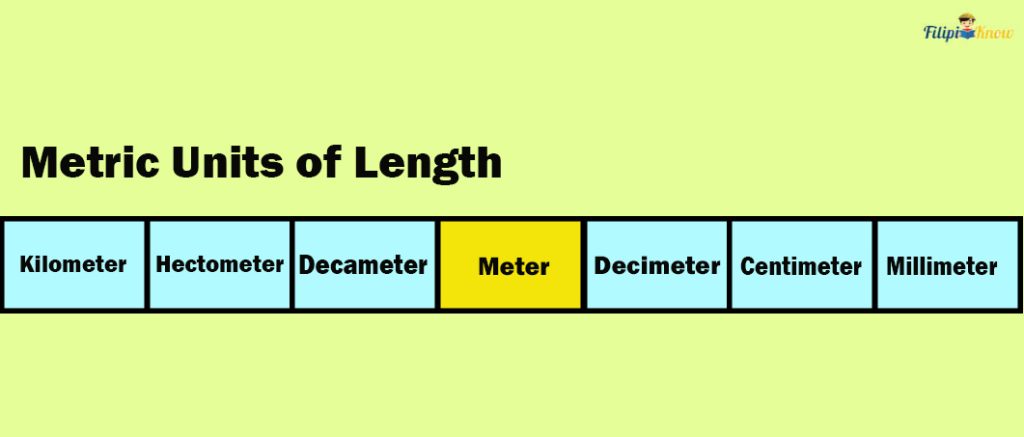
Metric System Units of Length
We learned earlier that meter (m) is the SI base unit for length. This means that other metric units for length are derived from the meter. For instance, a kilometer (km) means 1000 meters.
In the metric system, we use prefixes to indicate that a particular metric unit is a multiple of the base unit. For example, the prefix “kilo” means 1000 times the base unit. So, 1 km = 1000 meters.
Six prefixes are used in the metric system, and we list them below together with their equivalent value in the base unit.
| 1 kilometer (km) | 1000 meters |
| 1 hectometer (hm) | 100 meters |
| 1 decameter (dam) | 10 meters |
| 1 meter (m) | 1 meter |
| 1 decimeter (dm) | 0.1 meter |
| 1 centimeter (cm) | 0.01 meter |
| 1 millimeter (mm) | 0.001 meter |
An easier way to visualize these prefixes is by using a table:
Whether you want to memorize the conversion table above is up to you. However, it is advisable to remember the equivalent value of each prefix in terms of the base unit. These prefixes also apply to metric units for area and mass.
Converting Metric Units of Length
The easiest way to convert metric units is by simply moving decimal places.
For instance, let us convert 375 meters to kilometers by looking at the table of prefixes below.
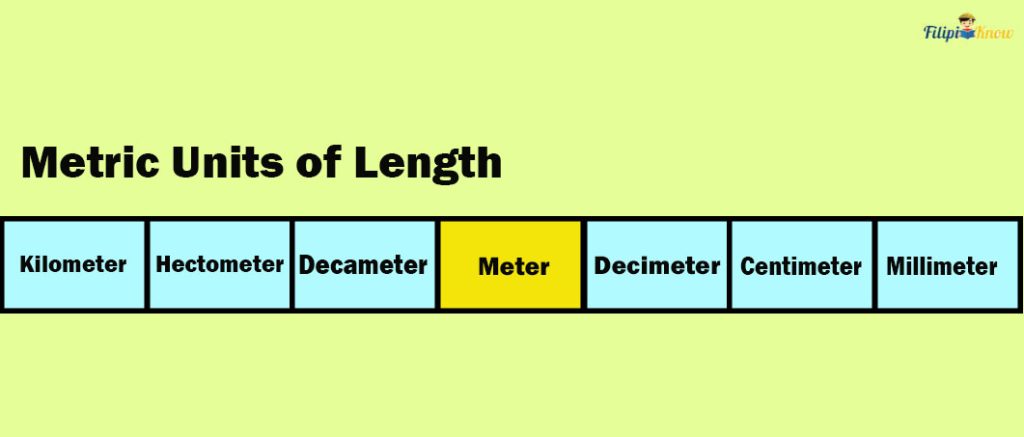
Note that the table above has three steps to the left from meter to kilometer. This means we must move three decimal places to the left in 375 meters to get its equivalent in kilometers.
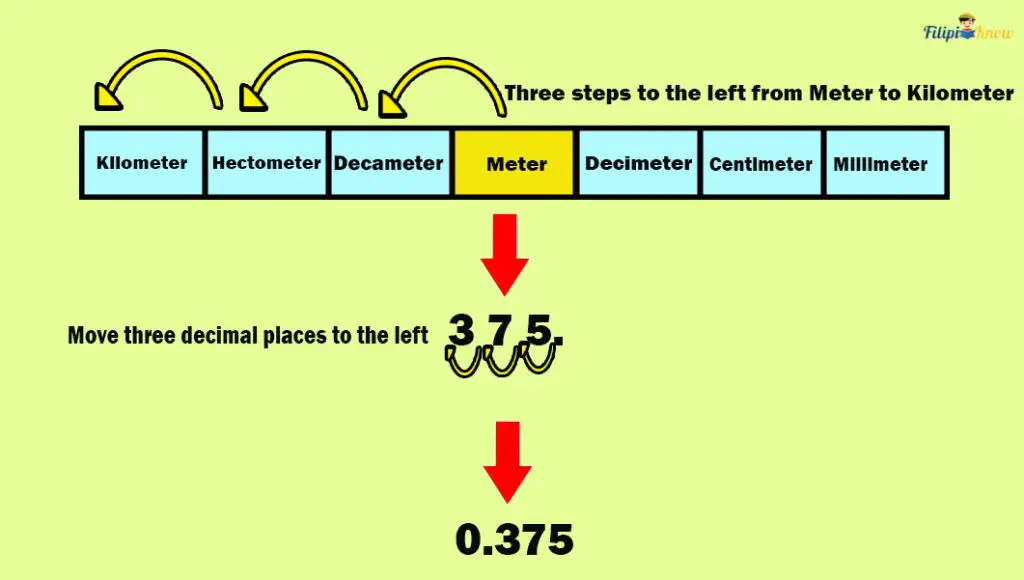
This means that 375 meters are equal to 0.375 kilometers.
Sample Problem 1: Convert 98.35 decameters to centimeters
Solution: Looking at the table of metric units of length, there are three steps to the right, from decameters to centimeters.
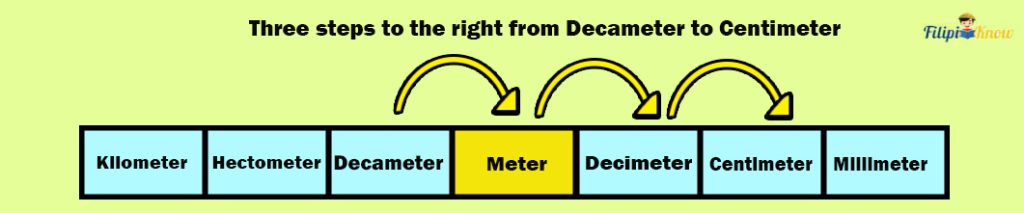
This implies that we must move three decimal places to the right to convert 98.35 decameters to centimeters.
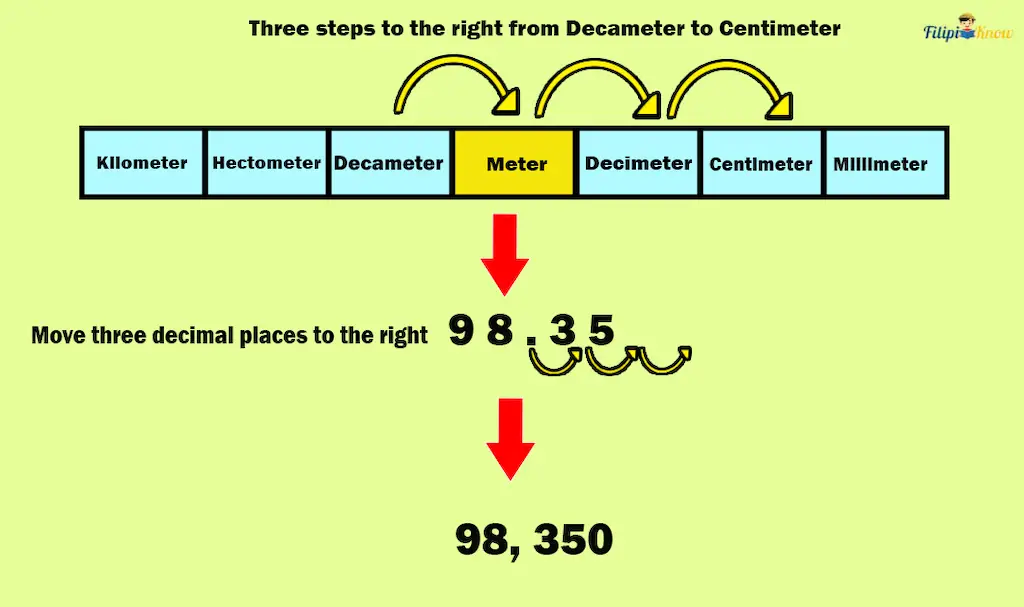
Hence, 98.35 decameters = 98,350 centimeters
Sample Problem 2: A ribbon was divided into two strips. The first strip measures 176.50 centimeters, while the second measures 89.56 centimeters. What is the length of the original ribbon in meters?
Solution: There are two steps to the left from centimeters to meters. Hence, we move two decimal places to the left to convert centimeters to meters.
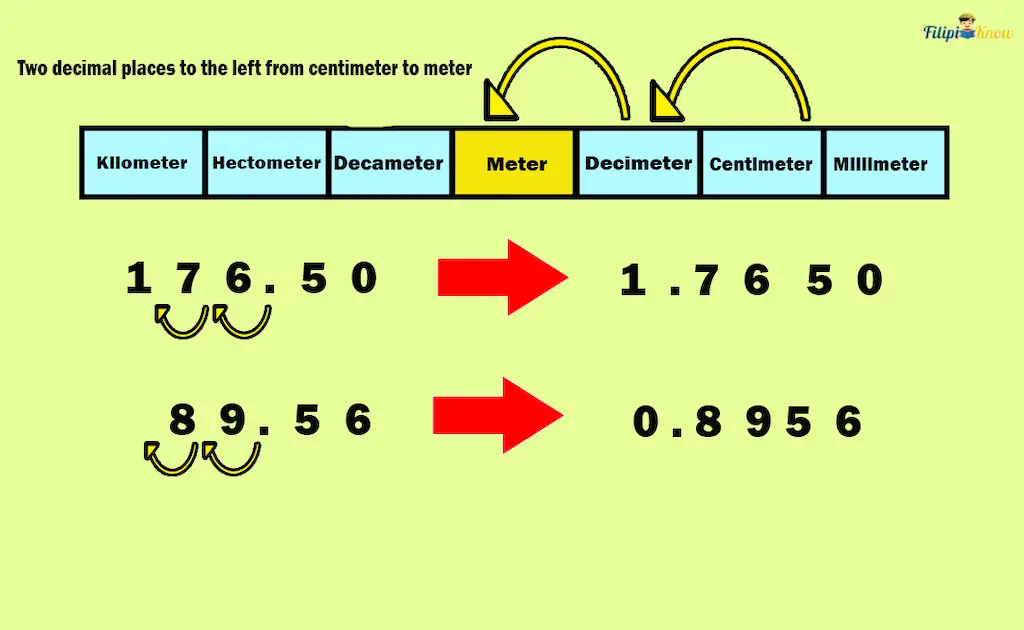
- Moving two decimal places in 176.50 centimeters results in 1.7650 meters.
- Moving two decimal places in 89.56 centimeters results in 0.8956 meters.
Adding the length of the strips converted to meters: 1.7650 m + 0.8956 m = 2.6606 m.
Hence, the length of the original ribbon is 2.6606 or 2.66 meters.
Metric System Units of Area
The SI base unit for the area is a square meter (m 2 ). Like the metric units of length, the metric units for the area are derived from the base unit (i.e., square meters).
The prefixes you have learned in the metric units for length also apply to metric units for the area. Again, these prefixes indicate that a particular metric unit is a multiple of the base unit.
Six prefixes are used in the metric system, and we list them below together with their equivalent value in the base unit. Note that all metric units for the area have a superscript of 2 to indicate that we are dealing with square units.
| 1 sq. kilometer (km ) | 1000 m |
| 1 sq. hectometer (hm ) | 100 m |
| 1 sq. decameter (dam ) | 10 m |
| 1 sq. meter (m ) | 1 m |
| 1 sq. decimeter (dm ) | 0.1 m |
| 1 sq. centimeter (cm ) | 0.01 m |
| 1 sq. millimeter (mm ) | 0.001 m |
Just like for length, it is easier to visualize these prefixes by using a table:
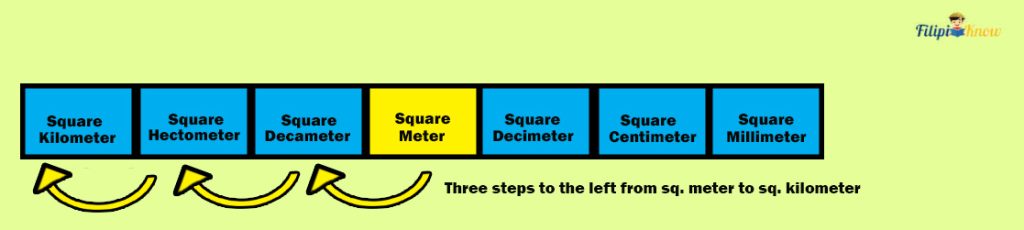
Converting Metric Units of Area
The method of converting metric units of the area is similar to the one we use to convert metric units of length. That is, by moving decimal places.
Let us convert 520 m 2 to km 2 . By looking at the table, there are three steps to the left, from square meters to square kilometers.
We move three decimal places to the left in 520 m 2 to obtain its equivalent in km 2 .
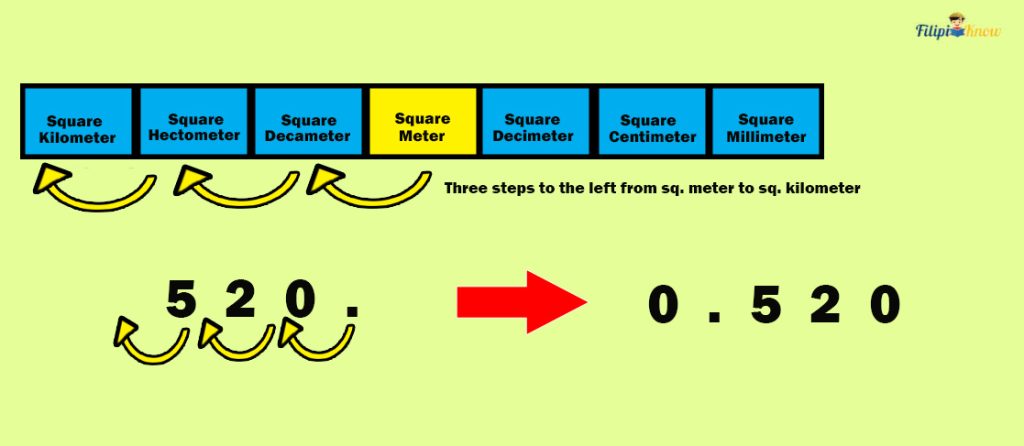
Thus, 520 m 2 is equal to 0.52 km 2 .
Since we always use the table of prefixes of metric units, I highly recommend memorizing the horizontal arrangement of these prefixes. They are not that hard to remember since there are only six prefixes. This is much easier than memorizing the conversion units.
Sample Problem: Every square meter of land in a province costs ₱4,000. Jennie plans to buy a 15-square decameter of land in this province. How much will Jennie have to pay to purchase the land?
Solution: Since the pricing of the land is expressed as ₱4,000 per square meter (m 2 ), we have to convert 15 square decameters (dam 2 ) to square meters (m 2 ) to calculate the land price accurately.
From square decameter to square meter, there’s one step to the right.
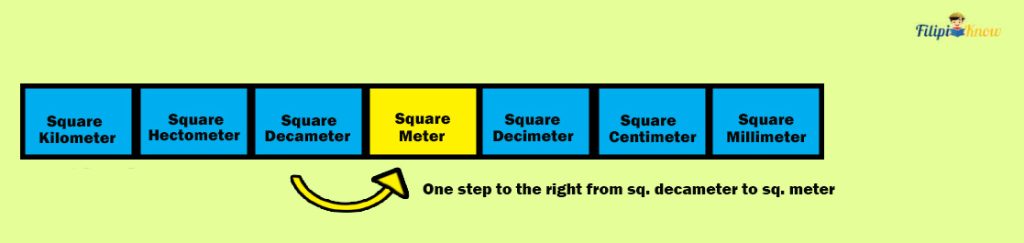
Hence, we move one decimal to the right in 15 dam 2 to convert it into m 2 :
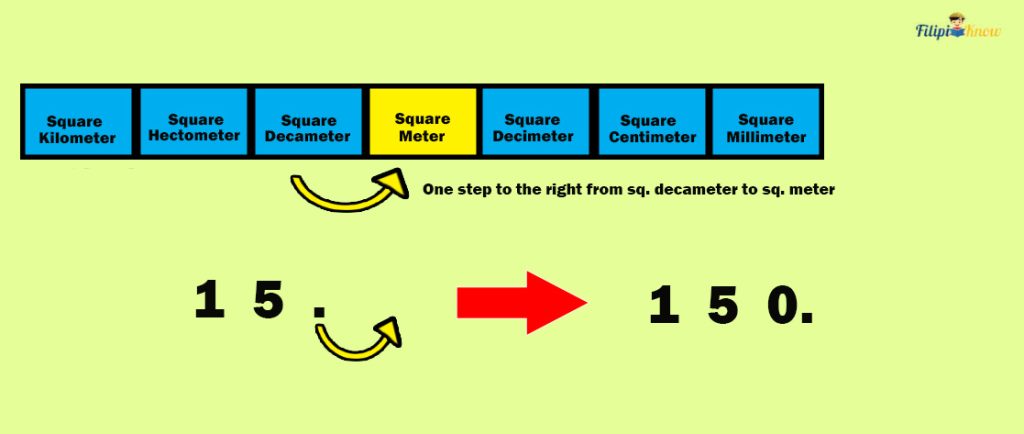
Thus, 15 dam 2 is equal to 150 m 2 .
Now, since the price of the land is ₱4000 per square meter (m 2 ), then a 150 m 2 of land will cost:
150 x 4000 = 600,000
The answer is ₱600,000.
Metric System Units of Volume
The SI base unit for volume is cubic meters (m 3 ). Like length, the metric units for volume or capacity are derived from cubic meters (m 3 ).
Six prefixes are used in the metric system, and we list them below together with their equivalent value in the base unit. Note that all metric units for volume have a superscript of 3 to indicate that we are dealing with cubic units.
| 1 cubic kilometer (km ) | 1000 m |
| 1 cubic hectometer (hm ) | 100 m |
| 1 cubic decameter (dam ) | 10 m |
| 1 cubic meter (m ) | 1 m |
| 1 cubic decimeter (dm ) | 0.1 m |
| 1 cubic centimeter (cm ) | 0.01 m |
| 1 cubic millimeter (mm ) | 0.001 m |
Just like for length and area, it is easier to visualize these prefixes by using a table:
The liter is another metric unit used for volume/capacity. The liter is a special name for cubic decimeter (dm 3 ). Thus, 1 liter equals 1 cubic decimeter (1 L = 1 dm 3 ).
Like any metric unit, prefixes are also used to derive other metric units for volume. For instance, the prefix “milli” in milliliter indicates that this unit is equal to thousandths (0.001) of a liter.
Although a liter is a metric unit for volume, there’s no need to put a superscript of 3 (which also applies to other metric units based on it).
Here are the other six prefixes associated with liter:
| 1 kiloliter (kL) | 1000 L |
| 1 hectoliter (hL) | 100 L |
| 1 decaliter (daL) | 10 L |
| 1 liter (L) | 1 L |
| 1 deciliter (dL) | 0.1 L |
| 1 centiliter (cL) | 0.01 L |
| 1 milliliter (mL) | 0.001 L |
Again, it’s easier to visualize these prefixes by using a table:
Converting Metric Units of Volume
Converting metric units of volume is similar to converting metric units for length and area.
Sample Problem 1 : A shoe box has a volume of 750 cm 3 . Determine its volume in m 3 .
Solution : There are two steps to the left from cm 3 to m 3 . Hence, we move two decimal places to the left in 750 cm 3 to transform it into m 3 .
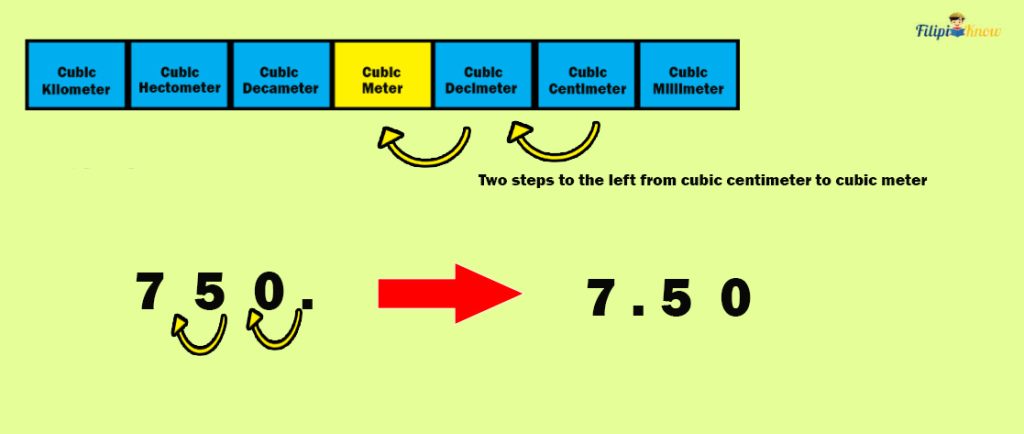
Therefore, 750 cm 3 is equivalent to 7.5 m 3 .
Sample Problem 2 : A large tank can be filled with 250 L of water. A water pipe puts 200 cL of water into the tank per minute. How long can the pipe fill the large tank?
Solution : To determine how long the pipe can fill the large tank, we divide 250L by 200 cL. However, we cannot perform this immediately since the given measurements differ in units.
First, let us convert 200 cL to L.
Looking at the table of prefixes, notice two steps to the left from cL to L. Hence, we move two decimal places to the left in 200 cL to convert it into L.
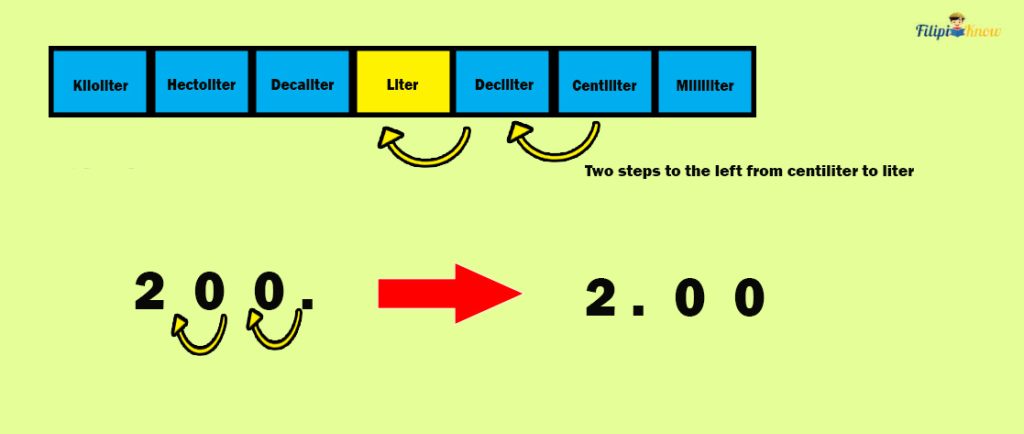
Hence, 200 cL = 2 L
We can now divide 250 L by 2 L. Dividing 250 L by 2 L, we’ll obtain
250 ÷ 2 = 125
This means that the pipe can fill the tank after 125 minutes.
Metric System Units of Mass
The SI base unit for mass is the kilogram (kg). However, note that “gram” is the primary basis for deriving other metric units for mass. “Gram” can be viewed as the “meter” in terms of mass.
Six prefixes are used in the metric system, and we list them here with their equivalent value in the base unit.
| 1 kilogram (kg) | 1000 g |
| 1 hectogram (hg) | 100 g |
| 1 decagram (dag) | 10 g |
| 1 gram (g) | 1 g |
| 1 decigram (dg) | 0.1 g |
| 1 centigram (cg) | 0.01 g |
| 1 milligram (mg) | 0.001 g |
It’s easier to visualize these prefixes by using a table:
Converting Metric Units of Mass
Sample Problem 1 : Myrna bought cough syrup with a mass of 50 grams. Determine its mass in milligrams.
Solution : Referring to the table of prefixes, there are three steps to the right from gram (g) to milligram (mg). Thus, we have to move three decimal places to the right to convert 50 g to mg:
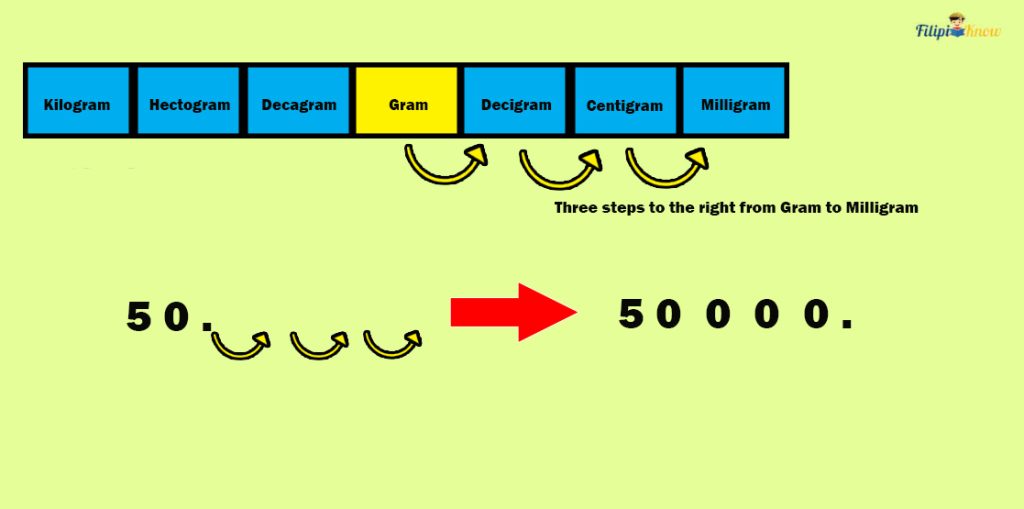
Therefore, the cough syrup’s mass is equal to 50,000 mg.
Sample Problem 2: Rosie bought 1250.50 grams of mangoes. What is the mass of the mangoes that Rosie bought in kilograms?
Solution : Let us convert 1250.50 grams to kilograms. There are three steps to the left, from grams (g) to kilograms (kg). Hence, we must move three decimal places to the left in 1250.50 g to convert it into kilograms (kg).
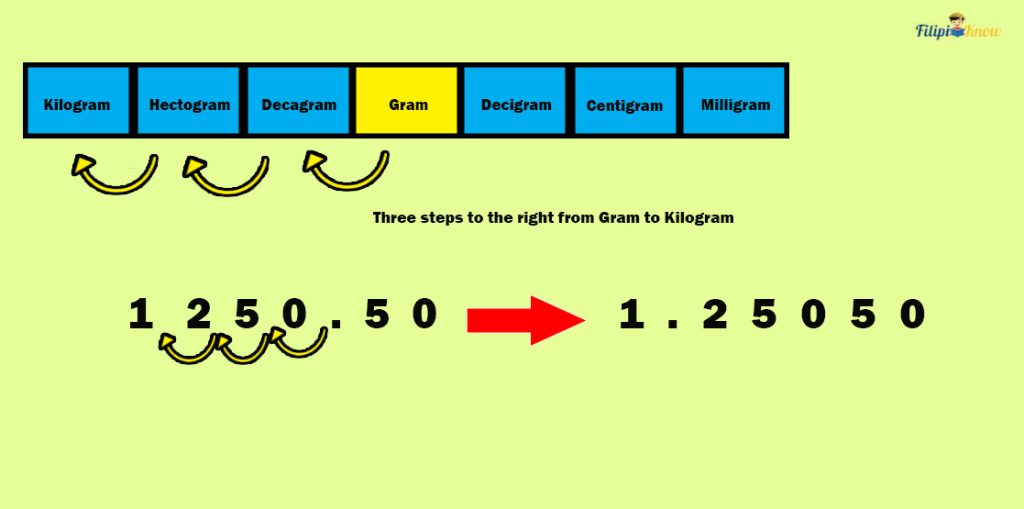
Hence, 1250.50 grams is equal to 1.25050 kilograms.
Converting Units of Time
Let’s now discuss how to convert units of time. Moving decimal places is not applicable for converting time units, unlike the metric units for length, area, volume, or mass. Instead, we have to refer to the conversion values for each unit.
Shown below is the conversion of time units:
| 1 minute | 60 seconds |
| 1 hour | 60 minutes |
| 1 day | 24 hours |
| 1 week | 7 days |
| 1 month | 4 weeks |
| 1 year | 12 months |
| 1 decade | 10 years |
| 1 century | 100 years |
| 1 millennium | 1000 years |
To convert one unit of time to another, follow these steps:
- Identify the given and to which unit we will convert it.
- Determine the relationship between the given units.
- Express the relationship between the given units as a conversion factor in a fractional form such that the denominator has a unit that is the same as the original unit.
- Multiply the given measurement by the conversion factor.
Let us apply the steps above to answer some examples.
Sample Problem 1 : How many hours are there in 5 days?
Step 1: Identify the given and to which unit we will convert it.
The problem is asking us to convert 5 days into hours.
Step 2: Determine the relationship between the given units.
There are 24 hours in one day. In other words, 1 day = 24 hours.
Step 3: Express the relationship between the given units as a conversion factor in a fractional form such that the denominator has a unit that is the same as the original unit.
In the previous step, we’re able to determine that 1 day is equivalent to 24 hours. Express this as a fraction with the unit that matches the original unit as the denominator. Since the original unit is “days,” we must express the conversion factor as 24 hours/1 day.
Step 4: Multiply the given measurement by the conversion factor.
Now, let us multiply 5 days by 24 hours/1 day:

Hence, there are 120 hours in 5 days.
Sample Problem 2 : Rhodora plans to go on a vacation to Lemongate Beach for 8 weeks. How many days will Rhodora be on vacation?
The problem is asking us to convert 8 weeks to days
Step 2 : Determine the relationship between the given units.
There are 7 days in one week or 1 week = 7 days.
In the previous step, we’re able to determine that 1 week is equivalent to 7 days. Express this as a fraction with the unit that matches the original unit as the denominator. Since the original unit is “week,” we must express the conversion factor as 7 days/1 week.
Now, let us multiply 8 weeks by 7 days/1 week:
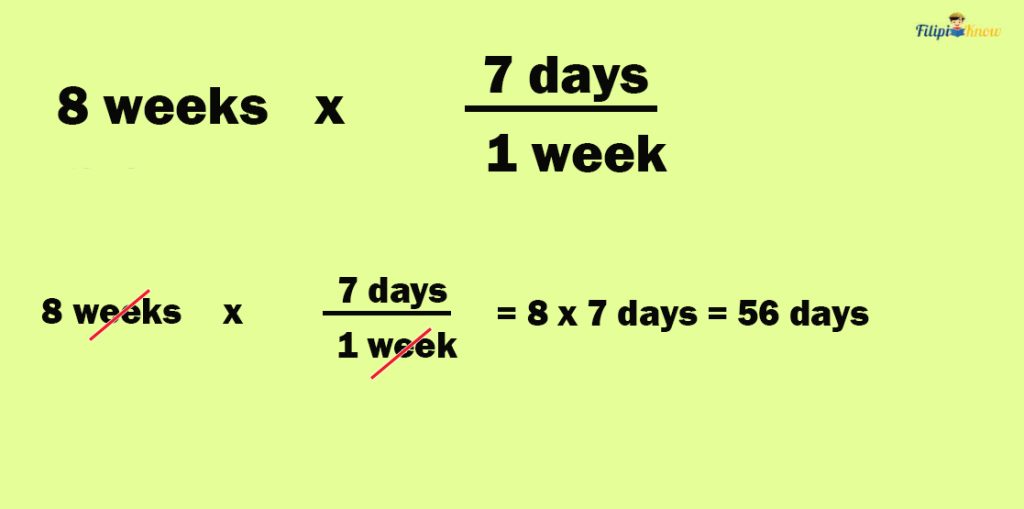
Hence, Rhodora will be on vacation for 56 days.
Sample Problem 3 : A worker is paid ₱0.5 per minute for his job. How much will the worker earn if he works for a total amount of time equivalent to 25 days?
Solution: The worker’s wage is expressed as ₱0.5 per minute. Therefore, we must convert 25 days to minutes first before determining the worker’s earnings.
The problem is asking us to convert 25 days to minutes.
Note that before converting days to minutes, we must first convert days to hours. Afterward, we will convert the result to minutes. This means that we will be dealing with two relationships in this problem.
- Relationship #1 (days to hours): 24 hours in 1 day or 1 day = 24 hours.
- Relationship #2 (hours to minutes): 60 minutes in 1 hour or 1 hour = 60 minutes.
Express the relationships we derived from Step 2 as conversion factors:
- For relationship #1 (days to hours), the original unit is days, so we have 24 hours/1 day
- For relationship #2 (hours to minutes), the original unit now is hours, so we have 60 minutes/1 hour
Multiply the 25 days by the two conversion factors.

Thus, there are 36,000 minutes in 25 days.
Remember, the worker’s wage is expressed as ₱0.5 per minute. If the worker renders 36,000 minutes of work, he will earn 36,000 x 0.5 = ₱18,000.
Sample Problem 4 : 504 hours is equivalent to how many weeks?
The problem is asking us to convert 504 hours to weeks.
To convert hours to weeks, we first need to convert hours to days. Afterward, we convert days to weeks. Thus, we will be dealing with two relationships of the unit of time in this problem:
- Relationship #1 (hours to days): 24 hours in 1 day or 1 day = 24 hours
- Relationship #2 (days to weeks): 7 days in 1 week or 1 week = 7 days
- For relationship #1 (hours to days), the original unit is hours, so we have 1 day/24 hours
- For relationship #2 (days to weeks), the original unit now is days, so we have 1 week/7 days
Multiply the 504 hours by the two conversion factors.
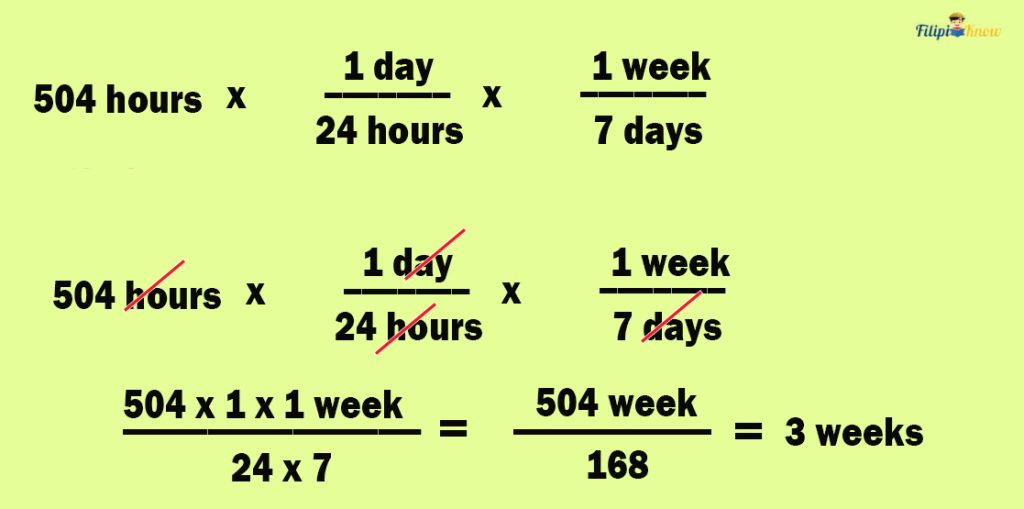
Thus, 504 hours is equal to 3 weeks.
Converting Units of Temperature
Kelvin, Celsius, and Fahrenheit are the units of measurement for temperature. This section will focus only on converting Celsius to Fahrenheit and vice versa.
To convert Celsius to Fahrenheit, use a conversion formula. We will discuss these formulas in this section.
How To Convert Celsius to Fahrenheit
Shown below is the formula to convert Celsius to Fahrenheit:
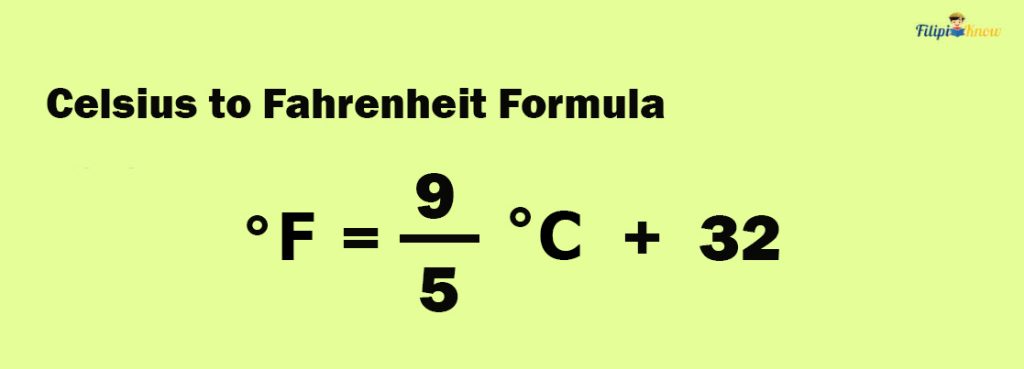
To use this formula, insert the given temperature expressed in Celsius into the formula and perform the calculation. The resulting value is the equivalent temperature in Fahrenheit.
Sample Problem 1: The freezing point of water is 0°C. What is the freezing point of water in Fahrenheit?
Solution : Applying the formula to convert Celsius to Fahrenheit:
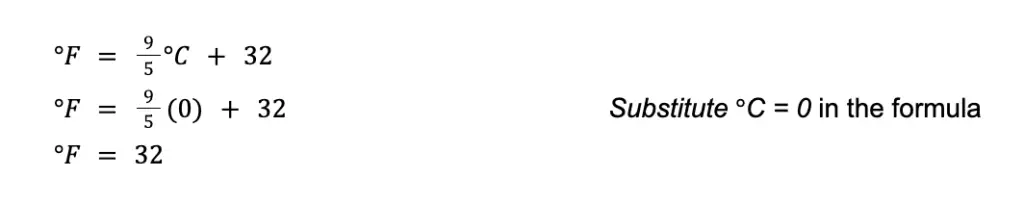
Hence, the freezing point of water in Fahrenheit is 32°F.
Sample Problem 2: The average body temperature is 37°C. What is the average body temperature in Fahrenheit?

Hence, the average body temperature in Fahrenheit is 98.6°F.
How To Convert Fahrenheit to Celsius
Shown below is the formula to convert Fahrenheit to Celsius:
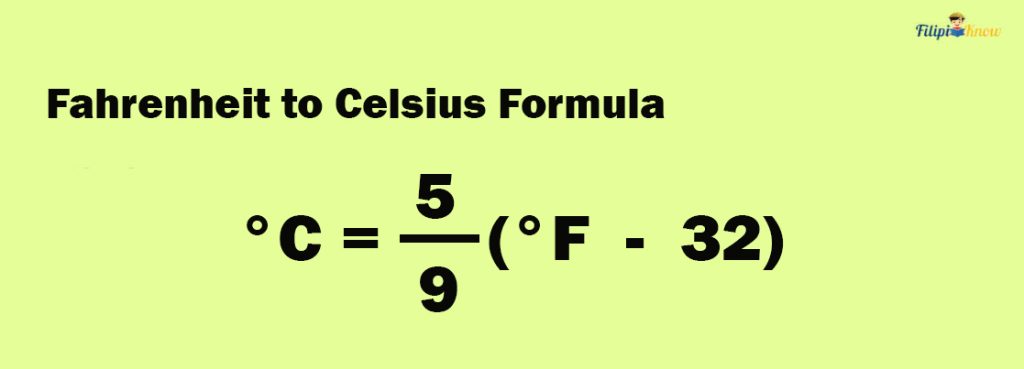
The formula above can be derived using the formula for converting Celsius to Fahrenheit. All you have to do is perform some basic algebra (in particular, solving a linear equation ).
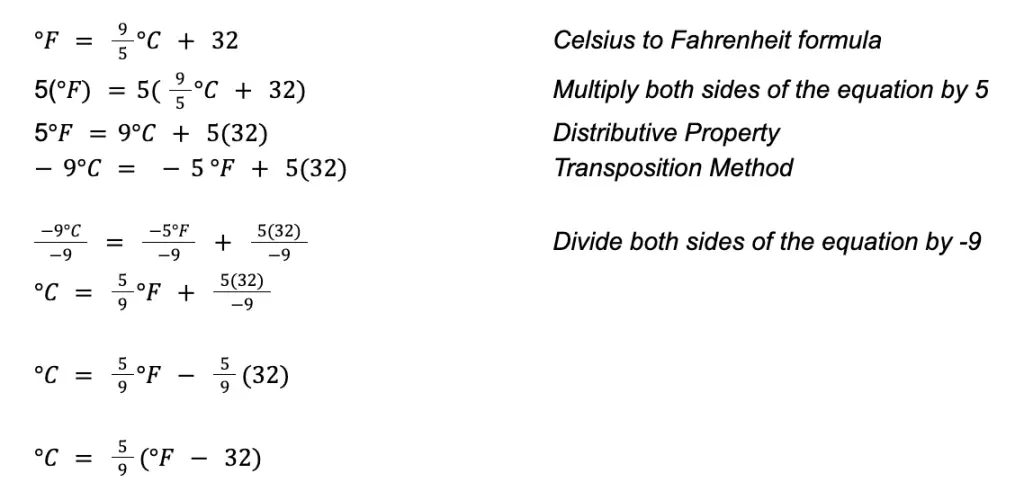
To use the formula, insert the given temperature expressed in Fahrenheit into the formula and perform the calculation. The resulting value is the equivalent temperature in Celsius.
Sample Problem 1 : Convert 32 °F to °C

Hence, 32 °F is equal to 0°C.
Sample Problem 2 : The required storage temperature of a particular drug is 59 °F. What is its equivalent in °C?
Solution :

Therefore, 59 °F is equal to 15 °C.
Next topic: Perimeter and Area of Plane Figures
Previous topic: Triangles: Classification and Theorems
Return to the main article: The Ultimate Basic Math Reviewer
Download Printable Summary/Review Notes
Download printable flashcards, test yourself, 1. practice questions [free pdf download], 2. answer key [free pdf download], 3. math mock exam + answer key.
Written by Jewel Kyle Fabula
in College Entrance Exam , LET , NAPOLCOM Exam , NMAT , PMA Entrance Exam , Reviewers , UPCAT
Jewel Kyle Fabula
Jewel Kyle Fabula is a Bachelor of Science in Economics student at the University of the Philippines Diliman. His passion for learning mathematics developed as he competed in some mathematics competitions during his Junior High School years. He loves cats, playing video games, and listening to music.
Browse all articles written by Jewel Kyle Fabula
Copyright Notice
All materials contained on this site are protected by the Republic of the Philippines copyright law and may not be reproduced, distributed, transmitted, displayed, published, or broadcast without the prior written permission of filipiknow.net or in the case of third party materials, the owner of that content. You may not alter or remove any trademark, copyright, or other notice from copies of the content. Be warned that we have already reported and helped terminate several websites and YouTube channels for blatantly stealing our content. If you wish to use filipiknow.net content for commercial purposes, such as for content syndication, etc., please contact us at legal(at)filipiknow(dot)net
Lesson 1: Converting Units and Problem Solving
Objective In this section we will answer the following questions: Recognize units used in the SI and English Systems. Covert units within the same system and convert units to another system. Apply the problem solving process. Lecture Units The measurement of any quantity is represented by a value and a particular unit . For example we can make a measurement of the length of an object and say that it is 10 ft . To say that something is 10 has no meaning. As a water/wastewater technician it is important to understand units in both the English and SI systems. It is also important to use a consistent set of units when making calculations. The most widely used system of measurement in the world is the Systéme International (French for International System), which is abbreviated SI. The base quantities are listed in Figure 1-1. The SI system is by far the easiest system to use when dealing with the laws and equations of water hydraulics. However, students typically find it difficult to visualize or conceptualize these units because they are unfamiliar with them. There will be problems throughout the semester that will use these units. Along with the base units comes the use of prefixes to define larger and smaller units in multiples of ten and those are listed in Figure 1-2. Quantity Unit Abbreviation Length Meter M Time Second S Mass Kilogram Kg Electric Current Ampere A Temperature Kelvin K Amount of Substance Mole Mol Luminous Intensisty candela cd Figure 1-1. SI Base Quantities Prefix Abbreviation Value Giga G Mega M Kilo k Hecto h Deka da Deci d Centi c Milli m micro μ nano n Figure 1-2. SI Prefixes In the United States, the most common system of measurement is the English system . This system consists of familiar units such as the pound, foot, inch, and so on. In this course, the English will be the primary system used. The English system is difficult to use because the list of units is extensive. There are, however, conversions we can use to aid us in problem solving. The most common base units are listed in Figure 1-3. Quantity Unit Abbreviation Force Pound lb Length Foot ft Time Second s Temperature Fahrenheit °F Figure 1-3. English Base Quantites In both systems, physical units can be placed into two categories: base units and derived units. The base units listed in Figures 1-1 and 1-3 are defined in terms of a standard. For example, the meter is defined as the length of path traveled by light in vacuum during a time interval of 1/299,792,458 m/s. English base units are defined in terms of their SI counterpart. Derived units are d efined in terms of base quantities. For example, pressure is defined as the force per unit area with force and length being the base quantities. Converting Units We know that any quantity measured is made up of a number and a unit. Many times we are given a quantity in one set of units, but we need to express it in another set of units. To do this we need a conversion factor . Suppose that we measure a length of rope to be 18 in. long, and we want to express this in centimeters. We will need to use the conversion factor 1 in. = 2.54 cm or 1 in. = 2.54 cm/in.
Since mathematics tells us that dividing any number by itself equals 1 and multiplying by 1 does not change anything, the length of our rope is
Note that the units of inches cancelled out and we were left with centimeters.
Example: How many yards are in the 100 m dash?
We know that …
1 yd = 3ft = 36 in. =
The conversion is
Next we set up the equation to solve.
Example: Express 55 miles per hour in meters/second and kilometers/hour.
Conversion factors
1 mi = 1609 m 1 hr = 60 min. = 3600s 12 in. = 1 ft
2.54 cm = 1 in. 100 cm = 1 m
- Convert 1 mile to meters
- Convert mi/hr to m/s
- Combine answers from parts (1) and (2) to solve. Note 1609 m = 1.609 km
Problem Solving Problem solving is a skill water/wastewater operators and maintenance personnel will use on a daily basis. The ability to understand a problem and arrive at a reasonable solution, makes one a valuable employee. The following is a systematic approach to solving problems encountered not only throughout the course but on the job as well. Lesson 1: Converting Units and Problem Solving Problem Solving Steps Read/reread the problem carefully. (Did I leave out a word or misunderstand?) Draw an accurate picture or diagram of the situation. (Where are the forces or direction of flow?) Write down all given data. (Are there quantities that are implied such as the acceleration due to gravity or atmospheric pressure.) Apply the physical principle to the problem. (What equation or relationship should I use?) Solve the problem. (How do I manipulate the equation algebraically to solve for the desired variable? Are there any unit conversions?) Report answer with correct units. Check answer to see if the answer is reasonabl e. Example: A rectangular holding tank 24.0 ft in length and 15.0 in width is used to store water for short periods of time in an industrial plant. If 2880 ft3 of water is pumped into the tank, what is the depth of the water? Sketch: Data: l = 24.0 ft w = 15.0 ft. V = 2880 ft3 h = ? Formula: V = lwh Working equation: h = V/lw Substitution: h = 2880 ft3/(24.0 ft)(15.0 ft) Answer: h = 8.00 ft Check: V = (8 ft)(15 ft)(24 ft) = 2880 ft3 Sources Giancoli, Douglas C. Physics for Scientists and Engineers . 3rd ed. Upper Saddle River, NJ: Prentice Hall, 2000. Print. Spellman, Frank R., and Joanne Drinan. Water Hydraulics . Lancaster, PA: Technomic Pub., 2001. Print. Assignment Please go to Canvas to complete the assignment for this lesson.
Solving problems involving converting between units of time
Switch to our new maths teaching resources.
Slide decks, worksheets, quizzes and lesson planning guidance designed for your classroom.
Play new resources video
Lesson details
Key learning points.
- In this lesson, we will solve word problems which compare different units of time.
This content is made available by Oak National Academy Limited and its partners and licensed under Oak’s terms & conditions (Collection 1), except where otherwise stated.
Starter quiz
5 questions, lesson appears in, unit maths / converting units of measure.

COMMENTS
Solution. To figure out how many kilometers he would run, you need to first add all of the lengths of the races together and then convert that measurement to kilometers. Use the factor label method and unit fractions to convert from meters to kilometers. Cancel, multiply, and solve. The runner would run 18 kilometers.
People measure the same thing in different ways and use different units. To communicate between scientists, there has to be a method of converting between the different measurement units. This is a collection of unit conversion example problems to help you learn the general method and mindset of converting units.
Problem UM1: Measurement. Identify the number of significant figures in a measurement and identify the quantity associated with a measurement based on its unit. Includes 8 problems. Problem Set UM2 - Unit Conversion 1. Use an understanding of metric prefixes to convert between metric units.
So, we multiply by 10 3 = 1000. 3 km = 3 × 1,000 = 3,000 m. Example: Convert 20 mm to cm. Solution: There is 1 "jump" to the left from millimeter to centimeter. So, we divide by 10. 20 mm = 20 ÷ 10 = 2 cm. How to convert to different metric units of measure for length, capacity, and mass?
1 Units and Measurement. Introduction; 1.1 The Scope and Scale of Physics; 1.2 Units and Standards; ... 6.1 Solving Problems with Newton's Laws; 6.2 Friction; 6.3 Centripetal Force; ... Check that the units of the final answer are the desired units. The problem asked us to solve for average speed in units of meters per second and, after the ...
1.6 Unit Conversion Word Problems. One application of rational expressions deals with converting units. Units of measure can be converted by multiplying several fractions together in a process known as dimensional analysis. The trick is to decide what fractions to multiply. If an expression is multiplied by 1, its value does not change.
6 Steps on How to Convert A Unit of Measurement to Another Unit 1. Compare the two units. 2. Find the conversion factors that gives the appropriate ratio to the given unit. 3. Write the conversion as a fraction, where the denominator is in the same unit as the given unit. 4. Write a multiplication problem with the original number and the ...
Sample Problem 1: Convert 98.35 decameters to centimeters. Solution: Looking at the table of metric units of length, there are three steps to the right, from decameters to centimeters. This implies that we must move three decimal places to the right to convert 98.35 decameters to centimeters.
In this concept, you will learn to use metric and customary units of measurement in problem solving. Measurement Conversions. Sometimes it is necessary to convert between customary and metric units of measurements. These measurement conversions will be estimates, because you cannot make an exact measurement when converting between systems of ...
Recognize units used in the SI and English Systems. Covert units within the same system and convert units to another system. Apply the problem solving process. Lecture. Units. The measurement of any quantity is represented by a value and a particular unit. For example we can make a measurement of the length of an object and say that it is 10 ft.
Metric conversions word problems; Units of measurement: Quiz 1; Convert units (metrics) Convert units word problems (metrics) Units of measurement: Quiz 2; Units of measurement: Unit test; Our mission is to provide a free, world-class education to anyone, anywhere. Khan Academy is a 501(c)(3) nonprofit organization.
Just as there's more than one way to measure something, there's more than one unit of measure! In this unit, you'll learn all about converting between different units of measure, time to metric and US customary measurements, so you can solve any word problem thrown your way.
Conversion of Measurement from Metric System unit to English System unit and vice versa. Solving Problems Involving Conversion of Units; After going through this module, you are expected to: 1. convert metric unit to another metric unit; 2. convert English system unit to another English system unit; 3. convert metric unit to English system unit ...
• Conversion of Measurement from Metric System unit to English System unit and vice versa • Solving Problems Involving Conversion of Units After going through this module, you are expected to: 1. convert metric unit to another metric unit (M7ME-IIb-1); 2. convert English system unit to another English system unit (M7ME-IIb-1); 3.
440 hours. Q4. From the list of people below, who has worked the most amount of hours? A works 8 hour shifts, five times a week. B works 12 hour shifts, three times a week. C works 9 hour shifts, four times a week. D works 6 hour shifts, six times a week. Q5. Anna has been training for a marathon.
"In this module, students build their competencies in measurement as they relate multiplication to the conversion of measurement units. Throughout the module, students explore multiple strategies for solving measurement problems involving unit conversion." ... Module 2: Unit conversions and problem solving with metric measurement. Unit 3 ...
If this problem persists, tell us. Our mission is to provide a free, world-class education to anyone, anywhere. Khan Academy is a 501(c)(3) nonprofit organization. Donate or volunteer today! Site Navigation. About. News; Impact; Our team; Our interns; Our content specialists; Our leadership; Our supporters; Our contributors; Our finances; Careers;