Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 23 June 2020

The high cost of prescription drugs: causes and solutions
- S. Vincent Rajkumar ORCID: orcid.org/0000-0002-5862-1833 1
Blood Cancer Journal volume 10 , Article number: 71 ( 2020 ) Cite this article
102k Accesses
71 Citations
279 Altmetric
Metrics details
- Cancer therapy
- Public health
Global spending on prescription drugs in 2020 is expected to be ~$1.3 trillion; the United States alone will spend ~$350 billion 1 . These high spending rates are expected to increase at a rate of 3–6% annually worldwide. The magnitude of increase is even more alarming for cancer treatments that account for a large proportion of prescription drug costs. In 2018, global spending on cancer treatments was approximately 150 billion, and has increased by >10% in each of the past 5 years 2 .
The high cost of prescription drugs threatens healthcare budgets, and limits funding available for other areas in which public investment is needed. In countries without universal healthcare, the high cost of prescription drugs poses an additional threat: unaffordable out-of-pocket costs for individual patients. Approximately 25% of Americans find it difficult to afford prescription drugs due to high out-of-pocket costs 3 . Drug companies cite high drug prices as being important for sustaining innovation. But the ability to charge high prices for every new drug possibly slows the pace of innovation. It is less risky to develop drugs that represent minor modifications of existing drugs (“me-too” drugs) and show incremental improvement in efficacy or safety, rather than investing in truly innovative drugs where there is a greater chance of failure.
Causes for the high cost of prescription drugs
The most important reason for the high cost of prescription drugs is the existence of monopoly 4 , 5 . For many new drugs, there are no other alternatives. In the case of cancer, even when there are multiple drugs to treat a specific malignancy, there is still no real competition based on price because most cancers are incurable, and each drug must be used in sequence for a given patient. Patients will need each effective drug at some point during the course of their disease. There is seldom a question of whether a new drug will be needed, but only when it will be needed. Even some old drugs can remain as virtual monopolies. For example, in the United States, three companies, NovoNordisk, Sanofi-Aventis, and Eli Lilly control most of the market for insulin, contributing to high prices and lack of competition 6 .
Ideally, monopolies will be temporary because eventually generic competition should emerge as patents expire. Unfortunately, in cancers and chronic life-threatening diseases, this often does not happen. By the time a drug runs out of patent life, it is already considered obsolete (planned obsolescence) and is no longer the standard of care 4 . A “new and improved version” with a fresh patent life and monopoly protection has already taken the stage. In the case of biologic drugs, cumbersome manufacturing and biosimilar approval processes are additional barriers that greatly limit the number of competitors that can enter the market.
Clearly, all monopolies need to be regulated in order to protect citizens, and therefore most of the developed world uses some form of regulations to cap the launch prices of new prescription drugs. Unregulated monopolies pose major problems. Unregulated monopoly over an essential product can lead to unaffordable prices that threaten the life of citizens. This is the case in the United States, where there are no regulations to control prescription drug prices and no enforceable mechanisms for value-based pricing.
Seriousness of the disease
High prescription drug prices are sustained by the fact that treatments for serious disease are not luxury items, but are needed by vulnerable patients who seek to improve the quality of life or to prolong life. A high price is not a barrier. For serious diseases, patients and their families are willing to pay any price in order to save or prolong life.
High cost of development
Drug development is a long and expensive endeavor: it takes about 12 years for a drug to move from preclinical testing to final approval. It is estimated that it costs approximately $3 billion to develop a new drug, taking into account the high failure rate, wherein only 10–20% of drugs tested are successful and reach the market 7 . Although the high cost of drug development is a major issue that needs to be addressed, some experts consider these estimates to be vastly inflated 8 , 9 . Further, the costs of development are inversely proportional to the incremental benefit provided by the new drug, since it takes trials with a larger sample size, and a greater number of trials to secure regulatory approval. More importantly, we cannot ignore the fact that a considerable amount of public funding goes into the science behind most new drugs, and the public therefore does have a legitimate right in making sure that life-saving drugs are priced fairly.
Lobbying power of pharmaceutical companies
Individual pharmaceutical companies and their trade organization spent approximately $220 million in lobbying in the United States in 2018 10 . Although nations recognize the major problems posed by high prescription drug prices, little has been accomplished in terms of regulatory or legislative reform because of the lobbying power of the pharmaceutical and healthcare industry.
Solutions: global policy changes
There are no easy solutions to the problem of high drug prices. The underlying reasons are complex; some are unique to the United States compared with the rest of the world (Table 1 ).
Patent reform
One of the main ways to limit the problem posed by monopoly is to limit the duration of patent protection. Current patent protections are too long, and companies apply for multiple new patents on the same drug in order to prolong monopoly. We need to reform the patent system to prevent overpatenting and patent abuse 11 . Stiff penalties are needed to prevent “pay-for-delay” schemes where generic competitors are paid money to delay market entry 12 . Patent life should be fixed, and not exceed 7–10 years from the date of first entry into the market (one-and-done approach) 13 . These measures will greatly stimulate generic and biosimilar competition.
Faster approval of generics and biosimilars
The approval process for generics and biosimilars must be simplified. A reciprocal regulatory approval process among Western European countries, the United States, Canada, and possibly other developed countries, can greatly reduce the redundancies 14 . In such a system, prescription drugs approved in one member country can automatically be granted regulatory approval in the others, greatly simplifying the regulatory process. This requires the type of trust, shared standards, and cooperation that we currently have with visa-free travel and trusted traveler programs 6 .
For complex biologic products, such as insulin, it is impossible to make the identical product 15 . The term “biosimilars” is used (instead of “generics”) for products that are almost identical in composition, pharmacologic properties, and clinical effects. Biosimilar approval process is more cumbersome, and unlike generics requires clinical trials prior to approval. Further impediments to the adoption of biosimilars include reluctance on the part of providers to trust a biosimilar, incentives offered by the manufacturer of the original biologic, and lawsuits to prevent market entry. It is important to educate providers on the safety of biosimilars. A comprehensive strategy to facilitate the timely entry of cost-effective biosimilars can also help lower cost. In the United States, the FDA has approved 23 biosimilars. Success is mixed due to payer arrangements, but when optimized, these can be very successful. For example, in the case of filgrastim, there is over 60% adoption of the biosimilar, with a cost discount of approximately 30–40% 16 .
Nonprofit generic companies
One way of lowering the cost of prescription drugs and to reduce drug shortages is nonprofit generic manufacturing. This can be set up and run by governments, or by nonprofit or philanthropic foundations. A recent example of such an endeavor is Civica Rx, a nonprofit generic company that has been set up in the United States.
Compulsory licensing
Developed countries should be more willing to use compulsory licensing to lower the cost of specific prescription drugs when negotiations with drug manufacturers on reasonable pricing fail or encounter unacceptable delays. This process permitted under the Doha declaration of 2001, allows countries to override patent protection and issue a license to manufacture and distribute a given prescription drug at low cost in the interest of public health.
Solutions: additional policy changes needed in the United States
The cost of prescription drugs in the United States is much higher than in other developed countries. The reasons for these are unique to the United States, and require specific policy changes.
Value-based pricing
Unlike other developed countries, the United States does not negotiate over the price of a new drug based on the value it provides. This is a fundamental problem that allows drugs to be priced at high levels, regardless of the value that they provide. Thus, almost every new cancer drug introduced in the last 3 years has been priced at more than $100,000 per year, with a median price of approximately $150,000 in 2018. The lack of value-based pricing in the United States also has a direct adverse effect on the ability of other countries to negotiate prices with manufacturers . It greatly reduces leverage that individual countries have. Manufacturers can walk away from such negotiations, knowing fully well that they can price the drugs in the United States to compensate. A governmental or a nongovernmental agency, such as the Institute for Clinical and Economic Review (ICER), must be authorized in the United States by law, to set ceiling prices for new drugs based on incremental value, and monitor and approve future price increases. Until this is possible, the alternative solution is to cap prices of lifesaving drugs to an international reference price.
Medicare negotiation
In addition to not having a system for value-based pricing, the United States has specific legislation that actually prohibits the biggest purchaser of oral prescription drugs (Medicare) from directly negotiating with manufacturers. One study found that if Medicare were to negotiate prices to those secured by the Veterans Administration (VA) hospital system, there would be savings of $14.4 billion on just the top 50 dispensed oral drugs 17 .
Cap on price increases
The United States also has a peculiar problem that is not seen in other countries: marked price increases on existing drugs. For example, between 2012 and 2017, the United States spent $6.8 billion solely due to price increases on the existing brand name cancer drugs; in the same period, the rest of the world spent $1.7 billion less due to decreases in the prices of similar drugs 18 . But nothing illustrates this problem better than the price of insulin 19 . One vial of Humalog (insulin lispro), that costs $21 in 1999, is now priced at over $300. On January 1, 2020, drugmakers increased prices on over 250 drugs by approximately 5% 20 . The United States clearly needs state and/or federal legislation to prevent such unjustified price increases 21 .
Remove incentive for more expensive therapy
Doctors in the United States receive a proportionally higher reimbursement for parenteral drugs, including intravenous chemotherapy, for more expensive drugs. This creates a financial incentive to choosing a more expensive drug when there is a choice for a cheaper alternative. We need to reform physician reimbursement to a model where the amount paid for drug administration is fixed, and not proportional to the cost of the drug.
Other reforms
We need transparency on arrangements between middlemen, such as pharmacy-benefit managers (PBMs) and drug manufacturers, and ensure that rebates on drug prices secured by PBMS do not serve as profits, but are rather passed on to patients. Drug approvals should encourage true innovation, and approval of marginally effective drugs with statistically “significant” but clinically unimportant benefits should be discouraged. Importation of prescription drugs for personal use should be legalized. Finally, we need to end direct-to-patient advertising.
Solutions that can be implemented by physicians and physician organizations
Most of the changes discussed above require changes to existing laws and regulations, and physicians and physician organizations should be advocating for these changes. It is disappointing that there is limited advocacy in this regard for changes that can truly have an impact. The close financial relationships of physician and patient organizations with pharmaceutical companies may be preventing us from effective advocacy. We also need to generate specific treatment guidelines that take cost into account. Current guidelines often present a list of acceptable treatment options for a given condition, without clear recommendation that guides patients and physicians to choose the most cost=effective option. Prices of common prescription drugs can vary markedly in the United States, and physicians can help patients by directing them to the pharmacy with the lowest prices using resources such as goodrx.com 22 . Physicians must become more educated on drug prices, and discuss affordability with patients 23 .
IQVIA. The global use of medicine in 2019 and outlook to 2023. https://www.iqvia.com/insights/the-iqvia-institute/reports/the-global-use-of-medicine-in-2019-and-outlook-to-2023 (Accessed December 27, 2019).
IQVIA. Global oncology trends 2019. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2019 (Accessed December 27, 2019).
Kamal, R., Cox, C. & McDermott, D. What are the recent and forecasted trends in prescription drug spending? https://www.healthsystemtracker.org/chart-collection/recent-forecasted-trends-prescription-drug-spending/#item-percent-of-total-rx-spending-by-oop-private-insurance-and-medicare_nhe-projections-2018-27 (Accessed December 31, 2019).
Siddiqui, M. & Rajkumar, S. V. The high cost of cancer drugs and what we can do about it. Mayo Clinic Proc. 87 , 935–943 (2012).
Article Google Scholar
Kantarjian, H. & Rajkumar, S. V. Why are cancer drugs so expensive in the United States, and what are the solutions? Mayo Clinic Proc. 90 , 500–504 (2015).
Rajkumar, S. V. The high cost of insulin in the united states: an urgent call to action. Mayo Clin. Proc. ; this issue (2020).
DiMasi, J. A., Grabowski, H. G. & Hansen, R. W. Innovation in the pharmaceutical industry: new estimates of R&D costs. J. Health Econ. 47 , 20–33 (2016).
Almashat, S. Pharmaceutical research costs: the myth of the $2.6 billion pill. https://www.citizen.org/news/pharmaceutical-research-costs-the-myth-of-the-2-6-billion-pill/ (Accessed December 31, 2019) (2017).
Prasad, V. & Mailankody, S. Research and development spending to bring a single cancer drug to market and revenues after approval. JAMA Intern. Med. 177 , 1569–1575 (2017).
Scutti, S. Big Pharma spends record millions on lobbying amid pressure to lower drug prices. https://www.cnn.com/2019/01/23/health/phrma-lobbying-costs-bn/index.html (Accessed December 31, 2019).
Amin, T. Patent abuse is driving up drug prices. https://www.statnews.com/2018/12/07/patent-abuse-rising-drug-prices-lantus/ (Accessed November 16, 2019).
Hancock, J. & Lupkin, S. Secretive ‘rebate trap’ keeps generic drugs for diabetes and other ills out of reach. https://khn.org/news/secretive-rebate-trap-keeps-generic-drugs-for-diabetes-and-other-ills-out-of-reach/ (Accessed November 16, 2019).
Feldman, R. ‘One-and-done’ for new drugs could cut patent thickets and boost generic competition. https://www.statnews.com/2019/02/11/drug-patent-protection-one-done/ (Accessed December 31, 2019).
Cohen, M. et al. Policy options for increasing generic drug competition through importation. Health Affairs Blog https://www.healthaffairs.org/do/10.1377/hblog20190103.333047/full/ (Accessed November 16, 2019).
Bennett, C. L. et al. Regulatory and clinical considerations for biosimilar oncology drugs. Lancet Oncol 15 , e594–e605 (2014).
Fein, A. J. We shouldn’t give up on biosimilars—and here are the data to prove it. https://www.drugchannels.net/2019/09/we-shouldnt-give-up-on-biosimilarsand.html (Accessed December 31, 2019).
Venker, B., Stephenson, K. B. & Gellad, W. F. Assessment of spending in medicare part D if medication prices from the department of veterans affairs were used. JAMA Intern. Med. 179 , 431–433 (2019).
IQVIA. Global oncology trends 2018. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncology-trends-2018 (Accessed January 2, 2018).
Prasad, R. The human cost of insulin in America. https://www.bbc.com/news/world-us-canada-47491964 (Accessed November 16, 2019).
Erman, M. More drugmakers hike U.S. prices as new year begins. https://www.reuters.com/article/us-usa-healthcare-drugpricing/more-drugmakers-hike-u-s-prices-as-new-year-begins-idUSKBN1Z01X9 (Accessed January 3, 2020).
Anderson, G. F. It’s time to limit drug price increases. Health Affairs Blog. https://www.healthaffairs.org/do/10.1377/hblog20190715.557473/full/ (Accessed November 16, 2019).
Gill, L. Shop around for lower drug prices. Consumer Reports 2018. https://www.consumerreports.org/drug-prices/shop-around-for-better-drug-prices/ (Accessed November 16, 2019).
Warsame, R. et al. Conversations about financial issues in routine oncology practices: a multicenter study. J. Oncol. Pract. 15 , e690–e703 (2019).
Download references
Author information
Authors and affiliations.
The Division of Hematology, Mayo Clinic, Rochester, MN, USA
S. Vincent Rajkumar
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to S. Vincent Rajkumar .
Ethics declarations
Conflict of interest.
The author declares that he has no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supported in part by grants CA 107476, CA 168762, and CA186781 from the National Cancer Institute, Rockville, MD, USA.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Vincent Rajkumar, S. The high cost of prescription drugs: causes and solutions. Blood Cancer J. 10 , 71 (2020). https://doi.org/10.1038/s41408-020-0338-x
Download citation
Received : 23 April 2020
Revised : 08 June 2020
Accepted : 10 June 2020
Published : 23 June 2020
DOI : https://doi.org/10.1038/s41408-020-0338-x
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Loading metrics
Open Access
Peer-reviewed
Research Article
Prescription opioid use and misuse among adolescents and young adults in the United States: A national survey study
Roles Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing
* E-mail: [email protected]
Affiliations Division of Emergency Medicine, Boston Children’s Hospital, Boston, Massachusetts, United States of America, Departments of Pediatrics and Emergency Medicine, Harvard Medical School, Boston, Massachusetts, United States of America
Roles Data curation, Formal analysis, Methodology, Software, Writing – review & editing
Affiliation Division of Emergency Medicine, Boston Children’s Hospital, Boston, Massachusetts, United States of America
Roles Data curation, Formal analysis, Investigation, Software, Writing – review & editing
Roles Conceptualization, Investigation, Supervision, Writing – review & editing
Affiliations Division of Emergency Medicine, Boston Children’s Hospital, Boston, Massachusetts, United States of America, Departments of Pediatrics and Emergency Medicine, Harvard Medical School, Boston, Massachusetts, United States of America, Pediatric Therapeutics and Regulatory Science Initiative, Computational Health Informatics Program, Boston Children’s Hospital, Boston, Massachusetts, United States of America
- Joel D. Hudgins,
- John J. Porter,
- Michael C. Monuteaux,
- Florence T. Bourgeois

- Published: November 5, 2019
- https://doi.org/10.1371/journal.pmed.1002922
- Reader Comments
Prescription opioid misuse has become a leading cause of unintentional injury and death among adolescents and young adults in the United States. However, there is limited information on how adolescents and young adults obtain prescription opioids. There are also inadequate recent data on the prevalence of additional drug abuse among those misusing prescription opioids. In this study, we evaluated past-year prevalence of prescription opioid use and misuse, sources of prescription opioids, and additional substance use among adolescents and young adults.
Methods and findings
This was a retrospective analysis of the National Survey on Drug Use and Health (NSDUH) for the years 2015 and 2016. Prevalence of opioid use, misuse, use disorder, and additional substance use were calculated with 95% confidence intervals (CIs), stratified by age group and other demographic variables. Sources of prescription opioids were determined for respondents reporting opioid misuse. We calculated past-year prevalence of opioid use and misuse with or without use disorder, sources of prescription opioids, and prevalence of additional substance use. We included 27,857 adolescents (12–17 years of age) and 28,213 young adults (18–25 years of age) in our analyses, corresponding to 119.3 million individuals in the extrapolated national population. There were 15,143 respondents (27.5% [95% CI 27.0–28.0], corresponding to 32.8 million individuals) who used prescription opioids in the previous year, including 21.0% (95% CI 20.4–21.6) of adolescents and 32.2% (95% CI 31.4–33.0) of young adults. Significantly more females than males reported using any prescription opioid (30.3% versus 24.8%, P < 0.001), and non-Hispanic whites and blacks were more likely to have had any opioid use compared to Hispanics (28.9%, 28.1%, and 25.8%, respectively; P < 0.001). Opioid misuse was reported by 1,050 adolescents (3.8%; 95% CI 3.5–4.0) and 2,207 young adults (7.8%; 95% CI 7.3–8.2; P < 0.001). Male respondents using opioids were more likely to have opioid misuse without use disorder compared with females (23.2% versus 15.8%, respectively; P < 0.001), with similar prevalence by race/ethnicity. Among those misusing opioids, 55.7% obtained them from friends or relatives, 25.4% from the healthcare system, and 18.9% through other means. Obtaining opioids free from friends or relatives was the most common source for both adolescents (33.5%) and young adults (41.4%). Those with opioid misuse reported high prevalence of prior cocaine (35.5%), hallucinogen (49.4%), heroin (8.7%), and inhalant (30.4%) use. In addition, at least half had used tobacco (55.5%), alcohol (66.9%), or cannabis (49.9%) in the past month. Potential limitations of the study are that we cannot exclude selection bias in the study design or socially desirable reporting among participants, and that longitudinal data are not available for long-term follow-up of individuals.
Conclusions
Results from this study suggest that the prevalence of prescription opioid use among adolescents and young adults in the US is high despite known risks for future opioid and other drug use disorders. Reported prescription opioid misuse is common among adolescents and young adults and often associated with additional substance abuse, underscoring the importance of drug and alcohol screening programs in this population. Prevention and treatment efforts should take into account that greater than half of youths misusing prescription opioids obtain these medications through friends and relatives.
Author summary
Why was this study done.
- Prescription opioid misuse is a leading cause of unintentional injury and death among adolescents and young adults.
- There is limited information on the source of prescription opioids among adolescents and young adults or whether those misusing prescription opioids engage in use of additional substances and drugs of abuse.
- Understanding these factors will inform strategies to ensure judicious opioid prescribing and effective treatment approaches.
What did the researchers do and find?
- Using the National Survey on Drug Use and Health for the years 2015 and 2016, we determined past-year prevalence of prescription opioid use, sources of prescription opioids, and additional substance use among adolescents and young adults ages 12–25.
- We found that 27.5% of respondents, corresponding to an estimated 32.8 million individuals, used prescription opioids in the previous year, including 21.0% of adolescents and 32.2% of young adults.
- The prevalence of opioid misuse was 3.8% among adolescents and 7.8% among young adults.
- Most individuals misusing prescription opioids obtained them for free from a friend or relative or from a single prescriber.
- Individuals with prescription opioid misuse reported high prevalence of use of other substances, including cocaine, hallucinogens, heroin, and inhalants.
What do these findings mean?
- The prevalence of prescription opioid use is high among adolescents and young adults in the United States despite known risks for progression to opioid and other substance use disorders in this population.
- Prevention and treatment efforts should take into account that adolescents and young adults misusing prescription opioids obtain these drugs most commonly from friends and relatives or from a single prescriber.
- Healthcare providers should consider screening all adolescents and young adults with opioid misuse for additional substance use and should have established intervention plans available to maximize the opportunity to provide substance use treatment to this population.
Citation: Hudgins JD, Porter JJ, Monuteaux MC, Bourgeois FT (2019) Prescription opioid use and misuse among adolescents and young adults in the United States: A national survey study. PLoS Med 16(11): e1002922. https://doi.org/10.1371/journal.pmed.1002922
Academic Editor: Margarita Alegria, Massachusetts General Hospital, UNITED STATES
Received: May 5, 2019; Accepted: September 25, 2019; Published: November 5, 2019
Copyright: © 2019 Hudgins et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Data Availability: The data underlying the results presented in the study are available from https://datafiles.samhsa.gov/study-dataset/national-survey-drug-use-and-health-2016-nsduh-2016-ds0001-nid17185 and https://datafiles.samhsa.gov/study-dataset/national-survey-drug-use-and-health-2015-nsduh-2015-ds0001-nid16894 .
Funding: FTB is supported by the Burroughs Wellcome Fund ( https://www.bwfund.org/ ), grant number 1017627. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing interests: The authors have declared that no competing interests exist.
Abbreviations: CI, confidence interval; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders , 4th edition; NSDUH, National Survey on Drug Use and Health; PMN, predictive mean neighborhood; SAMHSA, Substance Abuse and Mental Health Services Administration
Introduction
Over the past two decades, opioid misuse and poisonings have emerged as a public health crisis in the US. Since 1999, rates of deaths secondary to opioids have tripled, and in 2017 alone, over 72,000 Americans died from opioid overdoses [ 1 , 2 ]. Children and adolescents have not been spared, with substantial increases over the past two decades in opioid-related emergency department visits, hospital and intensive care unit admissions, and deaths [ 3 – 6 ]. Opioid exposures accounted for over 12% of all deaths in 2016 among 15- to 24-year-olds, which represents a 4-fold increase since 2001 [ 7 ]. According to the Centers for Disease Control’s 2018 National Vital Statistics report, unintentional injuries are now the leading cause of death among adolescents and young adults, with poisonings the most common unintentional injury [ 8 , 9 ].
Among adults in the US, roughly 1 in 3 is estimated to use prescription opioids, with 4.7%, or 11.5 million, misusing them [ 10 ]. Opioid misuse in adults has been linked to several risk factors, including mood and anxiety disorders, male gender, educational attainment, and age at first misuse [ 11 – 13 ]. Among adolescents and young adults, data are sparser and less consistent, although prevalence of opioid use disorder appears to be steadily increasing [ 14 , 15 ]. A recent meta-analysis examining past-year prevalence of prescription opioid misuse among adolescents and young adults reported estimates ranging from 0.7% to 16.3% [ 16 ]. Two of the largest adolescent drug surveys in the US are the Youth Risk Behavior Survey, which asks about misuse of “pain medications” broadly, and Monitoring the Future, which asks about misuse of “narcotics other than heroin” [ 17 , 18 ]. These studies report lifetime prevalence of misuse of 17.0% and 6.8%, respectively, among 12th grade students in 2017. Risk factors identified among adolescents include earlier onset of use, educational attainment, and chronic pain, although these links are less robust than in adult studies [ 19 – 21 ]. Importantly, legitimate use of opioids during adolescence appears to predispose to later opioid misuse [ 19 , 22 ].
There is limited information on how adolescents and young adults are obtaining prescription opioids. Studies suggest an indirect link between opioid prescriptions in adults and exposures in adolescents, suggesting that households and family members may be a contributing source [ 23 , 24 ]. In one survey study conducted from 2008 to 2011, parents and friends from school were identified as the most common sources of prescription opioids for adolescents [ 25 ].
In this study, we analyzed data from a large, nationally representative survey collecting information on prescription opioids to measure prevalence of opioid use and misuse among US adolescents and young adults. We also determined sources of prescription opioids and characterized opioid use and misuse according to additional use of other substances and drugs of abuse.
Survey methods
Data for this analysis were obtained from survey responses in the 2015 and 2016 National Survey on Drug Use and Health (NSDUH). The NSDUH is an annual cross-sectional survey that collects information on drug use, mental health, and other health-related issues in the US civilian, noninstitutionalized population aged 12 years and older. It is sponsored by the Substance Abuse and Mental Health Services Administration (SAMHSA) within the US Department of Health and Human Services. We used the publicly available version of the dataset, which was downloaded for use on February 16, 2018.
The survey includes interviews with approximately 70,000 individuals randomly selected annually, using a multistage area probability sample for each state and the District of Columbia. Certain populations, including younger age groups (i.e., 12 to 25 years), are oversampled to ensure robust estimates [ 26 ]. Interviews are conducted in the individual’s residence by a trained interviewer after verbal informed consent has been obtained. Data are collected using computer-assisted personal interviewing, in which the interviewer reads a question to the participant and enters the response into the computer. For questions on illicit drug use and other sensitive behaviors, a more private approach is used with audio computer-assisted self-interviewing, enabling respondents to read or listen to a question on headphones and enter the response into the computer themselves [ 26 ]. Respondents receive a $30 compensation for their participation. Additional information on the NSDUH sample design and survey methodology is available in the SAMHSA annual reports [ 26 ].
Our analysis was prospectively planned, including the definition of the study population, sociodemographic characteristics of interest, and outcome measures. Two modifications were made to the planned analysis. The first was our definition of young adult, which we initially defined as 18–23 years of age but modified to 18–25 years to match the NSDUH definition and data categorization. The second was the grouping of the different sources of prescription opioids, which was revised in response to peer-review comments. The study was deemed exempt from review by the Institutional Review Board at Boston Children’s Hospital.
Study population
We analyzed responses from adolescents 12 to 17 years of age and young adults 18 to 25 years of age. The NSDUH seeks to allocate 25% of its sample to adolescents aged 12 to 17 years, 25% to young adults aged 18 to 25 years, and the remaining 50% to adults 26 years and older [ 26 ]. The actual percentages of adolescents and young adults participating in 2015 were 23.1% and 24.6%, respectively, and in 2016, 23.3% and 23.9%, respectively [ 26 ]. The weighted interview response rates for adolescents and young adults were 77.7% and 74.4%, respectively, in 2015, and 77.0% and 72.3%, respectively, in 2016. By comparison, weighted interview response rates for adults were 67.4% in 2015 and 66.7% in 2016. All respondents were sampled independently. During the study period, less than 1% of respondents in our population were re-sampled in successive years. Each sampled observation is weighted within the context of the sampling frame for the given year, regardless of whether the observation represents a repeated record on the same individual.
Outcome measures
We examined three outcomes based on survey questions in the NSDUH: prescription opioid use, misuse, and use disorder, sources of prescription opioids for misuse, and use of other substances and drugs of abuse. NSDUH specifically identifies prescription opioids by presenting a list of prescription opioids and pictures of prescription opioid pills to respondents, and asks about past month, year or lifetime use of these opioids. For participants providing a positive response, follow-up questions determine classification into one of three groups: use without misuse, misuse without use disorder, or use disorder. Prescription opioid misuse is defined as “use in any way that a doctor did not direct you to use them” [ 27 ]. Specific examples constituting misuse are provided, including use without a prescription and using prescription opioids “more than you intended to” [ 28 ]. Opioid use disorder is classified as “recurrent use which causes clinically significant impairment, including health problems, disability, and failure to meet major responsibilities at work, school, or home,” based on Diagnostic and Statistical Manual of Mental Disorders , 4th edition (DSM-IV), criteria for substance use disorder [ 29 , 30 ].
For respondents reporting misuse of prescription opioids in the past year, NSDUH collects data on where the medications were obtained. Respondents are asked to select as many responses as apply from the following options: (a) obtained from one doctor, (b) obtained from more than one doctor, (c) stole from doctor office, clinic, hospital, or pharmacy, (d) obtained from friend or relative for free, (e) bought from friend or relative, (f) stole from friend or relative, (g) bought from drug dealer or stranger, and (h) got some other way.
The NSDUH also asks respondents about additional substance use and drug abuse. For our population, we examined responses to questions on tobacco, alcohol, cocaine, cannabis, heroin, inhalant, and hallucinogen use. Questions address use within the past month, past year, and lifetime. We combined use with and without use disorder for these substances and drugs.
Sociodemographic characteristics collected in the NSDUH include sex, race/ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and Asian/Native American), health insurance (private, Medicare, Medicaid, uninsured, or other), marital status, educational attainment, and past-year family income (<$20,000, $20,000–$49,999, $50,000–$74,999, and ≥$75,000).
The reliability of the NSDUH questionnaire related to substance use and misuse has been previously assessed, with measures showing good reproducibility over time [ 31 , 32 ]. Missing values in NSDUH are imputed by SAMHSA prior to release of the dataset using a predictive mean neighborhood (PMN) and a modified PMN as detailed in the NSDUH codebook [ 26 ]. PMN is a method of imputing missing or ambiguous values that is similar to predictive mean matching and has been used in the survey since 1999 [ 33 ]. The median percentage of values imputed for the initial demographic variables was 3.5% in 2015 and 3.7% in 2016. For the prescription drug variables, the median percentage of imputed values was 1.0% in 2015 and 0.65% in 2016 [ 26 , 34 ].
Statistical analysis
Data were analyzed using the person-level sample weight, which is the product of the corresponding sampling fractions at each stage in the sample design, and allows extrapolation to national population estimates. The sampling weights are adjusted using the generalized exponential model [ 35 ], which adjusts for survey nonresponse, post-stratification, and extreme weights, yielding an unbiased national estimate of occurrences and characteristics. When generating population estimates from survey data, we accounted for the survey design by specifying the primary sampling units and the degrees of freedom provided by NSDUH to ensure accurate estimates [ 34 ]. All analyses were performed in STATA Version 15 (StataCorp, College Station, TX) using the suite of estimation commands for survey data ( svyset and svy ).
We calculated descriptive statistics for adolescents and young adults with past-year use of prescription opioids, both overall and stratified by misuse and use disorder. We also assessed the sources of prescription opioids, overall and stratified by misuse and use disorder, and calculated the prevalence of additional use of other substances and drugs of abuse, stratified by prescription opioid use and misuse. Results were calculated and reported with 95% confidence intervals (CIs), and P values for comparisons were calculated using chi-squared test. The study conforms to the STROBE guideline ( S1 STROBE Checklist ).
The NSDUH included 27,857 adolescent and 28,213 young adult respondents during the survey years 2015 to 2016, corresponding to an estimated 119.3 million individuals in the extrapolated national population (49.8 million adolescents and 69.5 million young adults). Overall, 27.5% of these respondents, corresponding to an estimated 32.8 million individuals, reported using a prescription opioid in the previous year, including 21.0% (95% CI 20.4–21.6) of adolescents and 32.2% (95% CI 31.4–33.0) of young adults ( Table 1 ).
- PPT PowerPoint slide
- PNG larger image
- TIFF original image
https://doi.org/10.1371/journal.pmed.1002922.t001
Opioid misuse or use disorder in the past year was reported by 6.1% (95% CI 5.8–6.4) of respondents, corresponding to an estimated 1.9 million adolescents (3.8%; 95% CI 3.5–4.0) and 5.4 million young adults (7.8%; 95% CI 7.3–8.2; P < 0.001). Prevalence of opioid misuse without use disorder was higher in young adults compared with adolescents (21.1% [95% CI 19.8–22.3] versus 15.2% [95% CI 13.9–16.5], respectively; P < 0.001), with similar prevalence of opioid use disorder in the two groups. Considering both age groups together, female respondents were more likely to have had any prescription opioid use compared with males (30.3% [95% CI 29.7–30.9] versus 24.8% [95% CI 24.2–25.4], P < 0.001), but male respondents were more likely to have opioid misuse without use disorder compared with females (23.2% [95% CI 21.6–24.8] versus 15.8% [95% CI 14.7–16.9], respectively; P < 0.001). Non-Hispanic white and black respondents reported higher prevalence of past-year opioid use compared with Hispanic respondents ( P < 0.001), but prevalence of misuse without and with use disorder were similar between groups. Insurance status was not a significant determinant of opioid use, but uninsured respondents were more likely to report opioid misuse without use disorder compared with those with Medicaid.
Among those with prescription opioid misuse, 25.4% (95% CI 23.5–27.2) obtained them from the healthcare system, 55.7% (95% CI 53.7–57.6) from friends or relatives, and 18.9% (95% CI 17.4–20.5) through other means ( Table 2 ). Adolescents with opioid misuse obtained opioids most commonly for free from a friend or relative (33.5%; 95% CI 28.7–38.3) or by prescription from a single doctor (19.2%; 95% CI 16.4–22.1). Far fewer obtained them by stealing from a healthcare facility (1.7%; 95% CI 0.5–2.8), through prescriptions from multiple doctors (2.2%; 95% CI 1.3–3.2), or by buying them from a drug dealer or stranger (6.5%; 95% CI 4.4–8.6). Sources of opioids for young adults with opioid misuse were similar to adolescents, with free procurement from friends or relatives (41.4%; 95% CI 38.8–44.1) and prescription from a single doctor (24.0%; 95% CI 22.1–25.9) the most common sources.
https://doi.org/10.1371/journal.pmed.1002922.t002
Additional substance use and drug abuse associated with prescription opioid use is shown in Table 3 . Among adolescents and young adults with prescription opioid use without misuse, 50.5% (95% CI 49.2–51.7) had previously used tobacco, 70.5% (95% CI 69.3–71.7) had used alcohol, and 43.9% (95% CI 42.7–45.1) had used marijuana. These prevalence rates were significantly higher for respondents with opioid misuse, with 78.4% (95% CI 76.7–80.1; P < 0.001) reporting use of tobacco, 90.1% (95% CI 89.0–91.3; P < 0.001) use of alcohol, and 80.7% (95% CI 79.2–82.2; P < 0.001) use of marijuana. For cocaine, heroin, hallucinogen, and inhalant use, differences in prevalence of use between those with opioid use without misuse and those with opioid misuse were even more pronounced. Prevalence of cocaine use increased greater than 4-fold from 7.9% (95% CI 7.1–8.7) to 35.5% (95% CI 33.1–38.0; P < 0.001) for respondents with opioid use without misuse compared with those with opioid misuse. Similarly, the prevalence in these populations increased from 0.9% (95% CI 0.6–1.1) to 8.7% (95% CI 7.1–10.2; P < 0.001) for heroin use, 13.1% (95% CI 12.3–13.9) to 49.4% (95% CI 46.9–51.8; P < 0.001) for hallucinogen use, and 12.1% (95% CI 11.3–12.8) to 30.4% (95% CI 28.1–32.8; P < 0.001) for inhalant use.
https://doi.org/10.1371/journal.pmed.1002922.t003
Among adolescents and young adults with opioid misuse, prevalence of additional substance use was significantly higher among young adults for all substances except inhalant use ( Table 4 ). These differences were seen both for past month use and for any prior use of substances. Among adolescents, past month use of tobacco, alcohol, and cannabis was 31.2% (95% CI 28.4–34.1), 36.7% (95% CI 32.9–40.5), and 35.3% (95% CI 32.5–38.0), respectively. These prevalence rates increased to 63.9% (95% CI 61.3–66.5; P < 0.001), 77.4% (95% CI 75.3–79.5; P < 0.001), and 55.0 (95% CI 52.4–57.5; P < 0.001), respectively, among young adults. For cocaine and hallucinogens, lifetime prevalence of using the substance more than doubled between adolescents (11.5% [95% CI 9.3–13.7] and 25.4% [95% CI 22.2–28.6], respectively) and young adults (43.9% [95% CI 40.8–47.0], P < 0.001; and 57.7% [95% CI, 54.7–60.7], P < 0.001, respectively) with opioid misuse.
https://doi.org/10.1371/journal.pmed.1002922.t004
In this national sample of adolescents and young adults, 27.5% reported using a prescription opioid in the past year, with 3.8% of adolescents and 7.8% of young adults engaged in opioid misuse or having a use disorder. Respondents stated that opioids were obtained most frequently from friends or relatives or from a single prescriber, and much less often through drug dealers or from multiple prescribers. Adolescents and young adults with any type of opioid misuse were significantly more likely to use additional substances and drugs of abuse, with a third or more reporting prior use of cocaine, hallucinogens, and inhalants and half or more reporting past month use of tobacco, alcohol, or cannabis. Overall, young adults engaging in opioid misuse have higher rates of additional substance use and drug abuse compared with adolescents.
These findings are consistent with prior research indicating that the opioid epidemic is taking a significant toll on adolescents and young adults. In one large longitudinal study of youths 13 to 25 years of age, new diagnoses of opioid use disorder increased 6-fold between 2001 and 2014 [ 14 ]. Exposures to opioids have also been accompanied by rising rates of opioid poisonings and overdoses among adolescents and young adults. For adolescents 15 to 19 years of age, the annual rate of hospitalizations for opioid poisonings has increased by greater than 170%, while opioid-related deaths have increased by roughly 250% since the late 1990s [ 5 , 15 ]. Overall, among adolescents and young adults, overdose deaths reached an all-time high of 12.6 per 100,000 in 2017, with the majority of these overdoses linked to opioids [ 36 ].
The risk of progression to opioid use disorder and other substance abuse is well-documented for youths exposed to prescription opioids [ 22 , 37 – 39 ]. High school seniors receiving a first-time medical prescription for an opioid have been shown to have a 33% increased risk of future opioid misuse after high school [ 19 ]. This risk increases for adolescents engaging in nonmedical use of prescription opioids. In one survey study, among adolescents engaging in even occasional (3–9 lifetime uses) nonmedical prescription opioid use, greater than 50% met criteria for a substance use disorder by age 35 [ 38 ]. In addition, prescription opioid use among adolescents and young adults is linked to progression to heroin use. An analysis of NSDUH data from 2004 to 2011 showed that the hazard of heroin initiation was 13 times higher among adolescents and young adults 12 to 21 years of age with a history of nonmedical prescription opioid use compared with those without such prior use [ 40 ]. Overall, 76% of respondents who reported a history of heroin use had previously engaged in nonmedical use of prescription opioids. This risk of progression from nonmedical prescription opioid use to heroin use appears to be significantly greater for young adults compared with persons 25 years and older [ 41 ].
Sources of prescription opioids have not been well defined among adolescents and young adults misusing these drugs. Adults misusing prescription opioids have been found to obtain opioids both from prescribers and through friends and relatives [ 10 ]. Previous work among middle and high school students indicates that diversion of opioids, defined as selling, trading, giving away, or loaning prescription opioids, plays an important role in opioid misuse, with as many as 22% of students with medical use of prescription opioids approached to divert their opioid medication [ 42 , 43 ]. Our findings confirm these reports and quantify sources of prescription opioids, indicating that nearly half of adolescents and 58% of young adults misusing opioids receive them from friends and relatives. In addition, 22% of adolescents and 25% of young adults obtain opioid medications from prescribers, pointing to another area for targeted intervention for reducing opioid misuse. However, it should be noted that very few respondents received prescriptions from multiple prescribers, indicating that prescription drug monitoring programs—which are designed to monitor opioid prescriptions across providers and settings—alone may be insufficient as a policy approach to reduce opioid misuse in youths.
Adolescents and young adults misusing opioids are at high risk of abusing other substances. In addition to our findings of high prevalence of recent substance abuse, prior studies have demonstrated high prevalence of explicit co-ingestion of drugs with prescription opioids. Among high school seniors, approximately 70% report co-ingesting another drug while engaging in prescription opioid misuse, with greater than half reporting concurrent use of marijuana or alcohol, and 10% concurrent use of cocaine, tranquilizers, or amphetamines [ 44 ]. These patterns highlight the importance of screening adolescents and young adults with opioid misuse for use of other substances. In the emergency department setting, screening followed by brief interventions have been shown to be well received and effective at reducing alcohol and marijuana use among adolescents and young adults [ 45 – 47 ]. Providers should consider screening all adolescents and young adults presenting to the emergency department or other healthcare settings with opioid misuse for additional substance use. Healthcare facilities should also have established intervention plans or referral options available to maximize the opportunity to provide substance use treatment to this population.
Our study has several limitations. While the NSDUH had a high response rate of approximately 75% during the years analyzed, we cannot preclude self-selection bias among participants. In addition, despite sophisticated interviewing techniques, we cannot exclude socially desirable reporting among participants. The NSDUH also does not survey homeless people, active military personnel, or people in prison, which might impact findings reported for young adults in the study, especially because high rates of opioid misuse and use disorder have been identified in these populations [ 48 , 49 ]. Because the study does not employ a longitudinal design, we were unable to evaluate patterns of opioid use or progression to other substance use and drug abuse over time for individuals.
Our findings suggest that the prevalence of prescription opioid use among adolescents and young adults in the US is high despite known risks for future opioid and other drug use disorders. Prevention and treatment efforts should take into account that greater than half of adolescents and young adults misusing prescription opioids obtain these drugs from friends and relatives. Both adolescents and young adults misusing opioids demonstrate high prevalence of additional substance use and drug abuse, underscoring the importance of drug and alcohol screening programs in this population.
Supporting information
S1 strobe checklist. strobe statement—a checklist of items that should be included in reports of observational studies..
https://doi.org/10.1371/journal.pmed.1002922.s001
- View Article
- PubMed/NCBI
- Google Scholar
- 2. National Institute on Drug Abuse (NIDA). Overdose Death Rates [cited 2018 Aug 13]. Available from: https://www.drugabuse.gov/related-topics/trends-statistics/overdose-death-rates
- 3. Curtin SC, Tejada-Vera B, Warner M. Drug Overdose Deaths Among Adolescents Aged 15–19 in the United States: 1999–2015 Key findings. 1999 [cited 2018 Sep 4]. Available from: https://www.cdc.gov/nchs/data/databriefs/db282.pdf .
- 8. Curtin SC, Heron M, Miniño AM, Warner M. National Vital Statistics Reports Volume 67, Number 4 June 01, 2018 Deaths: Recent Increases in Injury Mortality Among Children and Adolescents Aged 10–19 Years in the United States: 1999–2016. 2018;67 [cited 2018 Nov 10]. Available from: https://www.cdc.gov/nchs/data/nvsr/nvsr67/nvsr67_04.pdf .
- 17. Centers for Disease Control and Prevention: National Center for Health Statistics. Youth Risk Behavior Survey Questionnaire. 2015 [cited 2018 Aug 31]. Available from: www.cdc.gov/yrbs .
- 18. Johnston LD, Miech RA, O’Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future national survey results on drug use: 1975–2017: Overview, key findings on adolescent drug use. 2018 [cited 2018 Sep 1]. Available from: http://www.monitoringthefuture.org/pubs/monographs/mtf-overview2017.pdf .
- 26. Center for Behavioral Health Statistics and Quality. 2016 National Survey on Drug Use and Health: Methodological summary and definitions. 2017 [cited 2018 Oct 3]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-MethodSummDefs-2016/NSDUH-MethodSummDefs-2016.htm#seca .
- 27. Center for Behavioral Health Statistics and Quality. Results from the 2015 National Survey on Drug Use and Health: Detailed Tables, Prevalence Estimates, Standard Errors, P Values, and Sample Sizes [cited 2018 Oct 4]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf .
- 28. Center for Behavioral Health Statistics and Quality. 2016 National Survey on Drug Use and Health (NSDUH): Final Approved CAI Specifications for Programming [cited 2018 Oct 10]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUHmrbCAIquex2016v2.pdf .
- 29. American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, D.C.: American Psychiatric Association, Washington, DC; 2000.
- 30. Hedden SL, Kennet J, Lipari R, Medley G, Tice P, Copello EA, et al. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health [cited 2018 Dec 8]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-FRR1-2014/NSDUH-FRR1-2014.pdf .
- 31. Substance Abuse and Mental Health Services Administration. Reliability of Key Measures in the National Survey on Drug Use and Health. 2010 [cited 2018 Dec 8]. Available from: https://www.samhsa.gov/data/sites/default/files/2k6ReliabilityP/2k6ReliabilityP.pdf .
- 32. Kennet J, Painter D, Hunter SR, Granger RA, Bowman KR. Assessing the reliability of key measures in the National Survey on Drug Use and Health using a test-retest methodology [cited 2018 Nov 3]. Available from: https://pdfs.semanticscholar.org/7218/49d3fb6340708b3914ebe512b7b6c8caf654.pdf .
- 33. Singh AC, Grau EA, Folsom RE. Predictive Mean Neighborhood Imputation with Application to the Person-Pair Data of the National Household Survey on Drug Abuse [cited 2018 Jan 8]. Available from: http://www.asasrms.org/Proceedings/y2001/Proceed/00460.pdf .
- 34. Center for Behavioral Health Statistics and Quality. 2015 National Survey on Drug Use and Health Methodological Resource Book Section 11: Person-Level Sampling Weight Calibration. 2017 [cited 2018 Nov 5]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUHmrbSamplingWgt2015.pdf .
- 35. Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health. 2015 [cited 2018 Nov 5]. Available from: https://www.samhsa.gov/data/sites/default/files/NSDUH-RedesignChanges-2015.pdf .
- 36. Hedegaard H, Miniño AM, Warner M. Drug overdose deaths in the United States, 1999–2017. NCHS Data Brief, 329. 2018 [cited 2018 Jan 5]. Available from: https://www.cdc.gov/nchs/data/databriefs/db329_tables-508.pdf#3 .
- 41. Muhuri PK, Gfroerer JC, Davies MC. CBHSQ Data Review: Associations of Nonmedical Pain Reliever Use and Initiation of Heroin Use in the United States [cited 2018 Dec 10]. Available from: http://www.samhsa.gov/data/ .
Misuse of Prescription Drugs Research Report
About this resource:.
Source: National Institute on Drug Abuse
The last reviewed date indicates when the evidence for this resource last underwent a comprehensive review.
Workgroups: Older Adults Workgroup , Substance Use Workgroup
This report defines misuse of prescription drugs and describes the extent of prescription drug misuse in the United States. It also provides information about the safety of using prescription drugs in combination with other medicines, describes ways to prevent and treat prescription drug misuse and addiction, and lists resources that provide more information. In addition, the report includes information about prescription drug misuse in specific populations, like adolescents and older adults.
Objectives related to this resource (2)
Suggested citation.
National Institutes of Health, National Institute on Drug Abuse. (2020). Misuse of Prescription Drugs Research Report. Retrieved from https://www.drugabuse.gov/publications/research-reports/misuse-prescription-drugs
The Office of Disease Prevention and Health Promotion (ODPHP) cannot attest to the accuracy of a non-federal website.
Linking to a non-federal website does not constitute an endorsement by ODPHP or any of its employees of the sponsors or the information and products presented on the website.
You will be subject to the destination website's privacy policy when you follow the link.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
The High Cost of Prescription Drugs in the United States: Origins and Prospects for Reform
Affiliation.
- 1 Program On Regulation, Therapeutics, And Law (PORTAL), Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, Massachusetts.
- PMID: 27552619
- DOI: 10.1001/jama.2016.11237
Importance: The increasing cost of prescription drugs in the United States has become a source of concern for patients, prescribers, payers, and policy makers.
Objectives: To review the origins and effects of high drug prices in the US market and to consider policy options that could contain the cost of prescription drugs.
Evidence: We reviewed the peer-reviewed medical and health policy literature from January 2005 to July 2016 for articles addressing the sources of drug prices in the United States, the justifications and consequences of high prices, and possible solutions.
Findings: Per capita prescription drug spending in the United States exceeds that in all other countries, largely driven by brand-name drug prices that have been increasing in recent years at rates far beyond the consumer price index. In 2013, per capita spending on prescription drugs was $858 compared with an average of $400 for 19 other industrialized nations. In the United States, prescription medications now comprise an estimated 17% of overall personal health care services. The most important factor that allows manufacturers to set high drug prices is market exclusivity, protected by monopoly rights awarded upon Food and Drug Administration approval and by patents. The availability of generic drugs after this exclusivity period is the main means of reducing prices in the United States, but access to them may be delayed by numerous business and legal strategies. The primary counterweight against excessive pricing during market exclusivity is the negotiating power of the payer, which is currently constrained by several factors, including the requirement that most government drug payment plans cover nearly all products. Another key contributor to drug spending is physician prescribing choices when comparable alternatives are available at different costs. Although prices are often justified by the high cost of drug development, there is no evidence of an association between research and development costs and prices; rather, prescription drugs are priced in the United States primarily on the basis of what the market will bear.
Conclusions and relevance: High drug prices are the result of the approach the United States has taken to granting government-protected monopolies to drug manufacturers, combined with coverage requirements imposed on government-funded drug benefits. The most realistic short-term strategies to address high prices include enforcing more stringent requirements for the award and extension of exclusivity rights; enhancing competition by ensuring timely generic drug availability; providing greater opportunities for meaningful price negotiation by governmental payers; generating more evidence about comparative cost-effectiveness of therapeutic alternatives; and more effectively educating patients, prescribers, payers, and policy makers about these choices.
PubMed Disclaimer
- Drug prices are kept high in US by protection and price negotiations, study finds. McCarthy M. McCarthy M. BMJ. 2016 Aug 23;354:i4640. doi: 10.1136/bmj.i4640. BMJ. 2016. PMID: 27553038 No abstract available.
- Factors Influencing Prescription Drug Costs in the United States. Arbiser JL. Arbiser JL. JAMA. 2016 Dec 13;316(22):2430-2431. doi: 10.1001/jama.2016.17290. JAMA. 2016. PMID: 27959990 No abstract available.
- Factors Influencing Prescription Drug Costs in the United States. Roy V, Hawksbee L, King L. Roy V, et al. JAMA. 2016 Dec 13;316(22):2431. doi: 10.1001/jama.2016.17293. JAMA. 2016. PMID: 27959991 No abstract available.
- Cost-related medication underuse: Strategies to improve medication adherence at care transitions. Miranda AC, Serag-Bolos ES, Cooper JB. Miranda AC, et al. Am J Health Syst Pharm. 2019 Apr 8;76(8):560-565. doi: 10.1093/ajhp/zxz010. Am J Health Syst Pharm. 2019. PMID: 31361859 No abstract available.
Similar articles
- Determinants of Market Exclusivity for Prescription Drugs in the United States. Kesselheim AS, Sinha MS, Avorn J. Kesselheim AS, et al. JAMA Intern Med. 2017 Nov 1;177(11):1658-1664. doi: 10.1001/jamainternmed.2017.4329. JAMA Intern Med. 2017. PMID: 28892528 Review.
- How to Reduce Prescription Drug Prices: First, Do No Harm. Atlas S. Atlas S. Mo Med. 2020 Jan-Feb;117(1):14-15. Mo Med. 2020. PMID: 32158034 Free PMC article. No abstract available.
- Trends in Prices of Popular Brand-Name Prescription Drugs in the United States. Wineinger NE, Zhang Y, Topol EJ. Wineinger NE, et al. JAMA Netw Open. 2019 May 3;2(5):e194791. doi: 10.1001/jamanetworkopen.2019.4791. JAMA Netw Open. 2019. PMID: 31150077 Free PMC article.
- Bib Pharma Monopoly: Why Consumers Keep Landing on "Park Place" and How the Game is Rigged. Levy MS. Levy MS. Am Univ Law Rev. 2016;66(1):247-303. Am Univ Law Rev. 2016. PMID: 28225582
- Points to consider about prescription drug prices: an overview of federal policy and pricing studies. Kucukarslan S, Hakim Z, Sullivan D, Taylor S, Grauer D, Haugtvedt C, Zgarrick D. Kucukarslan S, et al. Clin Ther. 1993 Jul-Aug;15(4):726-38. Clin Ther. 1993. PMID: 8221823 Review.
- Patent Portfolios Protecting 10 Top-Selling Prescription Drugs. Horrow C, Gabriele SME, Tu SS, Sarpatwari A, Kesselheim AS. Horrow C, et al. JAMA Intern Med. 2024 Jul 1;184(7):810-817. doi: 10.1001/jamainternmed.2024.0836. JAMA Intern Med. 2024. PMID: 38739386
- The Economic Impact of Atopic Dermatitis. Adamson AS. Adamson AS. Adv Exp Med Biol. 2024;1447:91-104. doi: 10.1007/978-3-031-54513-9_9. Adv Exp Med Biol. 2024. PMID: 38724787 Review.
- Lessons From Insulin: Policy Prescriptions for Affordable Diabetes and Obesity Medications. Nagel KE, Ramachandran R, Lipska KJ. Nagel KE, et al. Diabetes Care. 2024 Aug 1;47(8):1246-1256. doi: 10.2337/dci23-0042. Diabetes Care. 2024. PMID: 38536964 Review.
- Insurance-based Disparities in Outcomes and Extracorporeal Membrane Oxygenation Utilization for Hospitalized COVID-19 Patients. Glance LG, Joynt Maddox KE, Mazzeffi M, Shippey E, Wood KL, Yoko Furuya E, Stone PW, Shang J, Wu IY, Gosev I, Lustik SJ, Lander HL, Wyrobek JA, Laserna A, Dick AW. Glance LG, et al. Anesthesiology. 2024 Jul 1;141(1):116-130. doi: 10.1097/ALN.0000000000004985. Anesthesiology. 2024. PMID: 38526387
- Delayed treatment initiation of oral anticoagulants among Medicare patients with atrial fibrillation. Luo X, Chaves J, Dhamane AD, Dai F, Latremouille-Viau D, Wang A. Luo X, et al. Am Heart J Plus. 2024 Feb 2;39:100369. doi: 10.1016/j.ahjo.2024.100369. eCollection 2024 Mar. Am Heart J Plus. 2024. PMID: 38510996 Free PMC article.
Publication types
- Search in MeSH
Related information
- Cited in Books
LinkOut - more resources
Full text sources.
- Ovid Technologies, Inc.
- Silverchair Information Systems
Other Literature Sources
- scite Smart Citations
- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
- Open access
- Published: 03 March 2022
A systematic umbrella review of the association of prescription drug insurance and cost-sharing with drug use, health services use, and health
- G. Emmanuel Guindon 1 , 2 , 3 ,
- Tooba Fatima 1 ,
- Sophiya Garasia 1 , 2 &
- Kimia Khoee 1
BMC Health Services Research volume 22 , Article number: 297 ( 2022 ) Cite this article
3145 Accesses
10 Citations
18 Altmetric
Metrics details
Increasing spending and use of prescription drugs pose an important challenge to governments that seek to expand health insurance coverage to improve population health while controlling public expenditures. Patient cost-sharing such as deductibles and coinsurance is widely used with aim to control healthcare expenditures without adversely affecting health.
We conducted a systematic umbrella review with a quality assessment of included studies to examine the association of prescription drug insurance and cost-sharing with drug use, health services use, and health. We searched five electronic bibliographic databases, hand-searched eight specialty journals and two working paper repositories, and examined references of relevant reviews. At least two reviewers independently screened the articles, extracted the characteristics, methods, and main results, and assessed the quality of each included study.
We identified 38 reviews. We found consistent evidence that having drug insurance and lower cost-sharing among the insured were associated with increased drug use while the lack or loss of drug insurance and higher drug cost-sharing were associated with decreased drug use. We also found consistent evidence that the poor, the chronically ill, seniors and children were similarly responsive to changes in insurance and cost-sharing. We found that drug insurance and lower drug cost-sharing were associated with lower healthcare services utilization including emergency room visits, hospitalizations, and outpatient visits. We did not find consistent evidence of an association between drug insurance or cost-sharing and health. Lastly, we did not find any evidence that the association between drug insurance or cost-sharing and drug use, health services use or health differed by socioeconomic status, health status, age or sex.
Conclusions
Given that the poor or near-poor often report substantially lower drug insurance coverage, universal pharmacare would likely increase drug use among lower-income populations relative to higher-income populations. On net, it is probable that health services use could decrease with universal pharmacare among those who gain drug insurance. Such cross-price effects of extending drug coverage should be included in costing simulations.
Peer Review reports
As the US strives to reduce its uninsurance rate, it faces an intensifying challenge of increasing out-of-pocket costs in employer-sponsored health insurance [ 1 , 2 ]. All the while Canada is debating how best to provide drug insurance to all its residents [ 3 ]. Canada is often cited as the only high-income country with universal health insurance coverage lacking universal coverage for prescription drugs [ 4 ]. Increasing spending and use of prescription drugs pose an important challenge to governments that seek to expand health insurance coverage to improve population health while controlling public expenditures. Patient cost-sharing such as deductibles and coinsurance is widely used with aim to control healthcare expenditures without adversely affecting health [ 5 ].
Since the seminal RAND Health Insurance Experiment [ 6 ], numerous studies have examined, at various times and across diverse settings, the impact of health insurance generally, and drug insurance in particular, on utilization and health outcomes. For example, in the US, the introduction of Medicare Part D in 2003 and the Affordable Care Act in 2010 have generated a wealth of new research [ 7 , 8 ]. Likewise in Canada, the prospect of universal pharmacare and important changes to provincial drug programs such as the 1997 public/private prescription drug program that covered all Québec residents and British Columbia’s adoption of income-based Pharmacare in 2003 in place of an age-based drug benefits program have resulted in an abundance of new analyses [ 3 , 9 , 10 ]. Countless reviews have examined the impact of prescription drug insurance and drug cost-sharing on an array of outcomes such as drug use, health services use, and health, in varied settings and among heterogenous populations. To our knowledge, there has not been an attempt to assess the quality and synthesize evidence from existing reviews. In addition to identifying the strength/credibility of combined associations from reviews to present an objective and comprehensive synthesis of the evidence, such a review of reviews can identify knowledge gaps in the literature, provide useful guidance for future reviews, and have greater implications for policy and practice.
We conducted a systematic umbrella review in order to provide a closer examination of what policy introductions of prescription drug coverage (with and without cost-sharing) would mean for both individuals and governments financing this coverage. We examined reviews that studied the association between having prescription drug coverage (primary and supplementary), as well as varying types and levels of cost-sharing, and:
the utilization of prescription drugs (i.e., own-price effects on drug use);
the utilization of healthcare services (i.e., cross-price effects on the use of health services such as physician, emergency department, and inpatient services);
health outcomes (i.e., own-price effects on health outcomes);
We also examined the degree to which the associations identified in 1–3 above differed across levels of socioeconomic status (SES, e.g., income, education), populations of differing health status such as the chronically ill, age, and sex.
A review protocol was prepared in advance and registered with PROSPERO (CRD42017052018). We searched five electronic bibliographic databases: MEDLINE, Embase, Scopus, EconLit, and Health Systems Evidence. Grey literature was searched via the New York Academy of Medicine Grey Literature Report, Open Grey, Google, and Google Scholar. Eight specialty journals (BMC Health Services Research, Health Affairs, Healthcare Policy, Health Economics, Journal of Health Economics, Health Economics, Policy and Law, Health Services Research, and Medical Care Research and Review) and two working paper repositories (RePEc, Research Papers in Economics and the National Bureau of Economic Research working paper series) were ‘hand-searched.’ We examined references of included reviews and of reviews that cited key studies using Web of Science and Google Scholar. The database search was last updated on September 15, 2020. At least two reviewers, using distillerSR, screened titles and abstracts of citations to determine relevance, then full text if relevance was unclear.
Inclusion and exclusion criteria
Types of studies: all reviews (e.g., narrative, rapid, scoping, systematic, meta-analysis, meta-regression). Types of interventions: (1) insurance: all studies that examined the expansion of prescription drug insurance, irrespective of the insurance provider (e.g., government, employers, professional associations) and studies that examined partial or full-delisting of prescription drugs from insurance coverage; (2) cost-sharing: all studies that examined any form of direct patient payment for prescription drugs including, but not limited to, fixed copayment, coinsurance, ceilings, and caps. Types of outcomes: all reviews that included as an outcome any of drug utilization, health services utilization, or health outcomes. Time period: all reviews published since January 2000. Languages: we included only studies written in English and French. We excluded reviews that focused solely on low- and middle-income countries.
Quality assessment and data extraction
We used the Assessment of Multiple Systematic Reviews (AMSTAR) measurement tool as a methodological guide [ 11 ]. Although AMSTAR’s focus is primarily on the reporting quality of reviews, we paid particular attention to the quality assessment conducted in each review. At least two reviewers independently extracted detailed study characteristics for each included review using a standardized form, including all AMSTAR 2 items (see Additional file 1 ). The following study characteristics were extracted, where possible: citation, type of review, population investigated, research question, outcomes studied, whether there was an ‘a priori design’ and duplicate study selection and data extraction, the comprehensiveness of the search including if grey literature was searched, year/month of last search, whether the keywords/search strategy were reported, total number of studies included, total number of studies included that focused on drug insurance and/or cost-sharing, whether a list of included and excluded studies were provided, whether the characteristics of the included studies were provided, whether the scientific quality of the included studies was assessed, documented, and used appropriately in formulating conclusions, whether the methods used to combine the findings of studies were appropriate, whether the likelihood of publication bias was assessed, whether funding and competing of interests were clearly reported, key results for each of drug use, healthcare services utilization, and health, and reviews’ conclusion (as stated by the authors). In assessing the quality of the included studies, we paid particular attention to the following components: ‘a priori’ design; duplicate study selection and data extraction; systematic search strategy; presentation of characteristics of included studies and list of excluded studies and reasons for exclusion; quality assessment of included studies; and the generalizability of the findings. We did not compute total scores as empirical evidence does not support their use [ 12 , 13 , 14 ]. We created summary tables, organized by outcome and subgroup, using our completed standardized forms. For each study, we highlighted the direction and magnitude of the associations. In our descriptive table, we present the study citations, research question, outcomes studied, study selection and extraction process, quality assessment, and limitations/risk of bias. Lastly, given the current policy debate surrounding universal pharmacare in Canada, we also reported the total number of Canadian studies included that focused on drug insurance and/or cost-sharing [ 3 ].
The database search produced 5567 records after the removal of duplicate citations, from which 5261 were excluded based on the title/abstract screen and 268 were subsequently removed after a full-text screen, yielding 38 reviews that met all inclusion criteria (Fig. 1 ). Selected study characteristics and our assessment of study’s limitations are presented in Table 1 . Detailed characteristics of included studies are presented in the Additional file 1 . Of 38 reviews, 16 focused on the general population of which eight also commented on subgroups (e.g., seniors, the poor, and chronically ill), nine focused on seniors (most often on the US Medicare population), and 11 focused on the poor and/or chronically ill. A further two reviews examined drug insurance and cost-sharing among Canadians and one review examined publicly insured populations. Most included reviews were narrative reviews. We included six meta-analyses and one meta-regression. A list of excluded studies and reasons for exclusion is provided in the Additional file 1 . We present a synthesis of results in Table 2 and more detailed findings for each reviews in Tables 3 , 4 , 5 , 6 , 7 , and 8 .
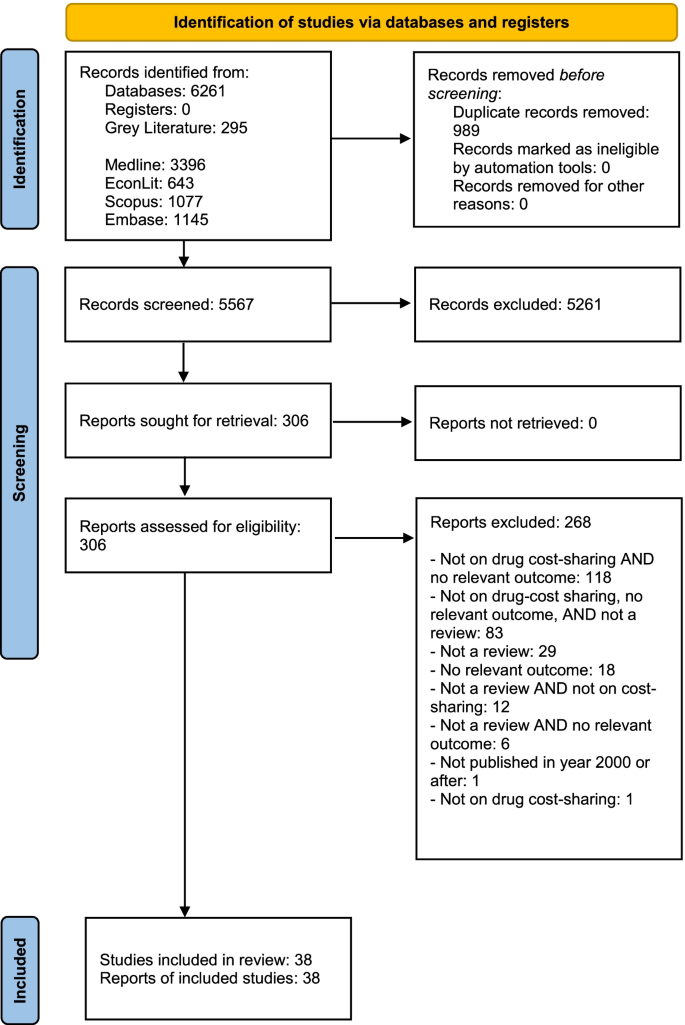
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only
Associations of prescription drug insurance and cost-sharing with drug utilization
Examining the 20 reviews that investigated, with a focus on the general population, the association between having prescription drug coverage or varying levels of cost-sharing on drug use, there was a clear inverse association, but the magnitude of the association was unclear (Tables 2 and 3 ). Across the literature, the outcomes were reported in elasticities, changes in drug use, and changes in medication adherence, with reviews published between 2004 and 2019. Reviews assessing medication adherence generally found that the absence of prescription drug insurance, or having copayments, reduced medication adherence [ 21 , 32 , 36 , 41 , 46 ] with specific estimates ranging as low as a 0.4% decrease in adherence for each dollar increase in copays, and an average reduction of 3% after 1 year of copayment reductions [ 32 ]. Another review reported that publicly insured patients who were required to pay copays for their prescription medicines had 11% higher odds of reporting nonadherence relative to those who faced no copayments [ 36 ]. However, not all associations were statistically significant, with variations in adherence reported depending on the drug class. Reviews reporting on drug use also generally found consistent results, reporting that increasing cost-sharing or not having drug insurance decreased drug use, but with varying impacts by drug class or type, and some not reporting clear effect sizes or very small to moderate impacts [ 16 , 26 , 27 , 37 , 39 , 40 , 51 ]. Own-price elasticities reported in seven older reviews, published in 2011 or earlier, generally found that the demand for prescription drugs was inelastic, with most estimates ranging from − 0.2 to − 0.6 depending on the drug class and essentiality, suggesting that a 10% increase in price resulted in a 2 to 6% decrease in use [ 19 , 22 , 23 , 24 , 25 , 29 , 30 ].
Differences between subgroups: SES, health status, age, and sex/gender
These results varied when assessing vulnerable population subgroups including the elderly, children, the poor, and the chronically ill (Tables 2 and 4 ). We identified two reviews that focused specifically on low-income groups [ 17 , 44 ] and six that generally commented on low-SES populations [ 23 , 24 , 25 , 27 , 29 , 37 ]. One older review focusing on low-income populations reported price elasticities ranging from − 0.3 and − 0.5 and argued there was unequivocal evidence that increasing cost-sharing decreased drug use among the poor [ 17 ]. This conclusion was supported by all other reviews except one [ 25 ]. However, when comparing price responsiveness between the poor and non-poor, four reviews provided mixed or no evidence that individuals with lower income were more price sensitive than those with higher income [ 23 , 24 , 25 , 27 ]. Although one review concluded that higher copayments led to a greater reduction in drug use in vulnerable populations (low socioeconomic status measured by income, education, or social status) than the non-vulnerable population) [ 37 ].
With respect to the chronically ill, reviews generally concluded that higher copayments or the absence of drug insurance were associated with increased medication nonadherence for a range of illnesses and drug classes [ 17 , 23 , 25 , 27 , 33 , 34 , 38 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 ]. However, the magnitude of effects was often unclear and difficult to synthesize given the diverse outcome measures employed in each review. A recent meta-analysis focusing on individuals with chronic cardiovascular diseases found that access to insurance or other programs that assisted with medication costs resulted in a 37% decrease in the risk of medication nonadherence [ 48 ]. Another recent review examined the association between cost-sharing on specialty drugs for rheumatoid arthritis, multiple sclerosis, and cancer, and the use of specialty drugs and nondrug medical services, and health outcomes [ 43 ]. Although no research was found that pertained to the use of nondrug medical services and health outcomes, the review found that higher cost-sharing was associated with higher prescription abandonment and discontinuation/persistence, and lower initiation and adherence. Findings varied by diseases but generally indicated stronger effects for noninitiation or abandonment of a prescription at the pharmacy and somewhat smaller effects for refill behaviour once patients initiated therapy [ 43 ]. Lastly, we are unable to comment on how the impact of cost-sharing or drug insurance on drug use compared to drug use by the healthy population as such comparisons were not drawn in the literature.
The elderly was the most studied group apart from the chronically ill, with 14 reviews focusing on this particular population. Of the 14 reviews, 11 concluded that seniors were sensitive to price changes [ 7 , 15 , 18 , 21 , 27 , 28 , 29 , 30 , 31 , 47 , 51 ]; drug use decreased with increasing cost-sharing or the lack of drug insurance, with the others finding mixed or no evidence for price responsiveness among older adults [ 20 , 24 , 35 ]. Again, the magnitude of effect was difficult to evaluate, and we are unable to comment on age differences (elderly vs non-elderly) in price responsiveness.
Only two reviews mentioned potential sex/gender differences in responsiveness to changes in cost-sharing or insurance. One review reported that one study had found that a drug policy change had not reduced the use of essential cardiac medications among Québec elderly who experienced acute myocardial infarction and that this finding did not vary by sex [ 23 ]. Another review reported that one study had found that low-income single elderly women were much less price responsive to drug fees than low-income single elderly men in British Columbia [ 17 ].
Associations of prescription drug insurance and cost-sharing with health services use
On the whole, most reviews concluded that increasing prescription drug cost-sharing or limiting drug insurance were associated with higher healthcare services utilization, such as emergency room visits, hospitalizations, and outpatient visits in the general population, although the magnitudes of associations were unclear (Tables 2 and 5 ) [ 24 , 25 , 30 , 32 , 37 , 39 , 50 ]. Two older reviews (published in 2005 and 2007) found no evidence of associations between prescription drug cost-sharing and changes in the use of healthcare services such as outpatient visits or hospitalizations [ 19 , 23 ] while three relatively more recent reviews from 2010, 2015, and 2018 concluded the evidence was mixed or uncertain [ 26 , 40 , 46 ].
When assessing results by subgroups, the findings were generally the same as those reported in the general population (Tables 2 and 6 ). Three reviews that focused on both the poor and chronically ill found that, in most studies reviewed, drug cost-sharing was associated with increased emergency department visits, hospitalizations, and nursing home admissions [ 17 , 29 , 44 ]. The magnitude of these associations was, however, unclear. A more recent review of cost-related nonadherence to prescription medications in Canada provides further support and reported that, among the elderly and individuals on social assistance, the introduction of cost-sharing was associated with increased rates of emergency department and physician visits [ 46 ]. It was unclear, however, if any of these associations differed in magnitude when compared to healthier or higher-income populations.
Five reviews specifically discussed the association between prescription drug cost-sharing and healthcare services utilization in the chronically ill [ 23 , 25 , 27 , 45 , 46 ]. Four of these reviews found evidence that prescription drug cost-sharing was associated with increased use of health services including greater hospitalizations, emergency room visits, and nursing home admissions [ 23 , 25 , 27 , 46 ]. The magnitude of these associations was, however, unclear. One review concluded that there was ‘no strong’ evidence showing a direct association between drug cost-sharing and healthcare services use among patients with diabetes mellitus, although there was limited evidence that higher drug copayments were associated with an increased risk of hospitalization among patients with heart failure [ 45 ]. Nonetheless, it was unclear how chronically ill patients compared to the healthier population in terms of the association between drug-cost sharing and healthcare services use.
Five reviews examined the association between drug cost-sharing or drug insurance and healthcare services utilization in older adults [ 15 , 18 , 20 , 29 , 46 ]. Four of these reviews concluded that there was some evidence that higher drug cost-sharing and lack of insurance were associated with greater hospitalizations or nursing home admissions in seniors, although the magnitude was unclear, whereas one older review reported inconclusive findings [ 18 ]. It was also unclear how seniors compared to non-seniors with respect to healthcare service utilization when faced with drug cost-sharing. Lastly, one recent review reporting on the association between drug cost-sharing and health services use found that government insurance plans with high-cost sharing on generic drugs were associated with lower use of health services among children. Again, the magnitude of effect was unclear and no comparison was drawn with older individuals [ 51 ].
Only one review mentioned potential sex/gender differences in responsiveness to changes in cost-sharing or insurance. One review reported that one study had found that a drug policy change had not reduced the use of medical services among Québec elderly who experienced acute myocardial infarction and that this finding did not vary by sex [ 23 ].
Associations of prescription drug insurance and cost-sharing with health
A total of 21 reviews reported on the association between prescription drug insurance or cost-sharing and health outcomes (Tables 2 and 7 ). Eleven of these reviews explored the association in the general population [ 19 , 23 , 24 , 25 , 26 , 29 , 30 , 32 , 37 , 39 , 50 ] of which two focused specifically on the Canadian population [ 16 , 46 ]. Six reviews examined health generally [ 23 , 25 , 26 , 29 , 30 , 46 ], five all-cause mortality [ 16 , 24 , 37 , 39 , 50 ], four self-reported health [ 19 , 32 , 39 , 50 ] and one review investigated cardiovascular-related mortality [ 50 ], adverse events [ 32 ] and vascular events [ 50 ].
Overall, there was limited evidence of a clear relationship between prescription drug insurance or cost-sharing and health outcomes. With one exception [ 32 ], several older reviews reported that very few empirical studies had examined the association between drug insurance/cost-sharing and health, and concluded that, on the whole, existing studies provided mixed or unclear evidence [ 19 , 23 , 25 , 26 , 29 , 30 , 37 ]. More recent reviews (published in 2015 and 2019) tended to conclude that drug insurance and lower cost-sharing were associated with better health. One review found that individuals with drug insurance had better health outcomes than those without, that drug insurance restrictions led to a decline in health status, and that extending drug coverage yielded mixed results [ 39 ]. Another review found that in all included studies, there was an inverse association between higher drug cost-sharing and health outcomes such as self-assessed health, major vascular events, cardiovascular-related mortality and all-cause mortality [ 50 ]. The above conclusions were, however, all based on very few primary studies.
Differences between subgroups: SES, health status, age and sex/gender
Reviews highlighted a paucity of studies that examined the associations of prescription drug insurance and cost-sharing with health among the poor and the chronically ill (Tables 2 and 8 ). Two older reviews found some evidence that drug cost-sharing was associated with adverse health outcomes in lower-income populations and another suggested that low-income individuals were at greater risk of poor health outcomes due to increased cost-sharing than higher-income individuals [ 17 , 23 , 29 ]. Three of four reviews that specifically discussed the chronically ill found that cost-sharing was associated with adverse health outcomes in patients with heart disease, hypertension, lipid disorders, and diabetes [ 30 , 34 , 45 ]. Two of the three reviews, however, discussed the association between health insurance generally (i.e., including but not limited to drug insurance) and health outcomes [ 30 , 34 ]. One review found no evidence of an association between drug cost-sharing and clinical outcomes among patients with cardiovascular-related chronic disease [ 38 ]. Four older reviews specifically focused on the association between insurance and cost-sharing and health among seniors [ 15 , 18 , 20 , 35 ]. Two reviews reported mixed findings [ 20 , 35 ] while two reviews reported that higher cost-sharing was associated with worse health outcomes, including higher mortality and morbidity among seniors [ 15 , 18 ]. One review pointed out that this association did not remain when there were generous provisions in place to protect vulnerable populations from incurring undue financial risk as a result of cost-sharing [ 18 ]. However, similar to previous reported outcomes, no comparisons were drawn between the poor and non-poor, the chronically ill and non-chronically ill, and the elderly and non-elderly and how health outcomes may have differed between them. We did not identify a single review that discussed potential differences between sex/gender in the association of prescription drug insurance and cost-sharing with health.
Risk of bias assessment
In our umbrella review, we found that the most common limitations were the lack of an a priori study design and issues with clarity in reporting search strategies and results. Reviews often did not clearly report data screening and extraction procedures including exclusion and inclusion criteria, had poorly described search strategies or non-systematic search strategies, failed to provide or clearly synthesize study characteristics and, most often than not, did not provide a list of excluded studies. The most important limitation was, however, the lack of attention given to quality assessments. About half of the included reviews did not conduct any formal quality assessments and many that did often failed to appropriately describe and justify their quality assessment.
Main findings
We found consistent evidence that changes in drug cost-sharing and/or drug insurance were associated with drug use. Lower cost-sharing and having drug insurance were associated with increased drug use while higher drug cost-sharing and the lack or loss of drug insurance were associated with decreased drug use. We also found consistent evidence that the poor, the chronically ill, seniors and children were similarly responsive to changes in insurance and cost-sharing. Although the direction of the associations between changes in drug insurance and cost-sharing was clear, the magnitude of these associations was difficult to ascertain. The demand for prescription drugs is most certainly inelastic (i.e., a percentage change in price is associated with a smaller percentage change in demand) with an own-price elasticity ranging from about − 0.2 to − 0.6, depending on drug class, intervention, disease, and population studied. We found that lower drug cost-sharing and drug insurance were associated with lower healthcare services utilization including emergency room visits, hospitalizations, and outpatient visits. Similar results were found in all population subgroups aside from children, although the literature on the poor and children was very limited. We did not find consistent evidence of an association between cost-sharing and insurance and health. While several reviews reported mixed or no evidence, more recent reviews tended to conclude that there was some evidence that increased cost-sharing led to poorer health outcomes because of reduced drug adherence. Again, the magnitude of effect was unclear and evidence on the elderly, chronically, ill, and poor was limited and mixed. Lastly, we did not find any evidence that the association between drug insurance or cost-sharing and drug use, health services use, or health differed by SES, health status, age or sex.
We found two reviews that specifically studied the Canadian population. An older review examined Canadian evidence of the effects of cost-sharing mechanisms of provincial drug benefit programs on drug utilization and health [ 16 ]. A more recent scoping review examined the extent, determinants, and consequences of cost-related nonadherence to prescription medications in Canada [ 46 ]. The two reviews generally found that higher drug cost-sharing reduced drug use. There was, however, little discussion of the magnitude of associations or subgroup differences in price responsiveness [ 16 , 46 ]. The review of cost-related nonadherence to prescription medications found limited and mixed evidence that cost-sharing increased health services use [ 46 ]. A more recent review examined the prevalence, predictors, and clinical impact of cost-related medication nonadherence in Canada [ 52 ]. Along with lower income, younger age, and poorer health, high out-of-pocket spending and drug insurance were found to be associated with medication cost-related nonadherence [ 52 ].
Limitations
Our review has some inherent limitations. Although we identified 38 relevant reviews, this does not equate to 38 independent reviews because there was considerable overlap between the studies that were included in the reviews. Although we are confident about the direction of the associations we examined, we had difficulties commenting on the precise magnitude of associations as these were often not clearly identified and reported in the reviews themselves, and could not be easily extracted and synthesized. Lastly, our review did not examine reviews that focused specifically on an alternative cost-sharing design called “value-based cost-sharing” or more generally “value-based insurance design.” The key feature of value-based insurance design is to link the amount of cost-sharing across services with the documented effectiveness and cost-effectiveness of a service, drug or device. A list of reviews that focused specifically on value-based designs is provided in the Additional file 1 .
Implications for research
Our umbrella review highlights a paucity of research focused on children and youth. We identified no reviews that specifically focused on children and youth. The reviews we included generally sparingly discussed the potential impact of drug insurance and cost-sharing among youth. In our search, we identified a single review that focused specifically on children, which we excluded because it focused primarily on access and not on drug use. Unger and Ariely, identified two studies that compared insured and uninsured paediatric populations which showed increased access to healthcare services and medications for insured children [ 53 ]. The review noted that access to prescription drugs frequently differed by the type of health insurance provider and the type of cost-sharing arrangement and that more research was needed. The lack of discussion of potential sex/gender differences in the associations of prescription drug insurance and cost-sharing with drug use, health services use, and health is of concern. Only two reviews discussed this issue and reported on just two primary studies. It is unclear if the lack of discussion of potential sex/gender differences is due to reviews or primary studies not investigating it.
Future reviews need to give more consideration to appropriately synthesizing and discussing magnitudes of effect for given associations as solely presenting the direction or significance of a relationship provides minimal information. A stronger emphasis also needs to be placed on improving the methodological rigour of reviews by employing systematic and transparent methods to develop and execute search strategies as well as conducting quality assessment that is applicable to the literature being reviewed and ensuring that it is adequately discussed. Lastly, our umbrella review highlights the importance of searching systematically both peer-reviewed and grey literature, and not to overly rely on a single repository of research evidence. For example, only 11 reviews are included in Health Systems Evidence, which is perhaps the most comprehensive repository of reviews relevant to health systems.
Implications for health equity
Socioeconomic, racial and ethnic inequities in health care and drug coverage are well documented in the US and Canada [ 1 , 54 , 55 ]. For example, in 2015–16 in Canada, relative to adults in the lowest income decile, those in the 10th decile had odds of reporting drug insurance coverage that were more than five times higher [ 54 ]. In the US, Black and Latinx/Hispanic adults have historically reported substantially higher uninsured rates than white adults. In 2019, while the uninsured rate among white adults was only 9%, the uninsured rates among Black and Latinx/Hispanic adults stood at 14 and 26%, respectively. Consequently, universal pharmacare would likely increase drug use among lower-income populations relative to higher-income populations, and potentially reduce health inequities.
Implications for policy
Although cost-sharing can be used as a mechanism to reduce pharmaceutical expenditures, the associated impacts on health service use may offset those benefits. These cross-price effects of extending drug coverage are, however, often ignored in costing simulation, [ 56 , 57 ] and need to be taken into consideration by policymakers. Lastly, current Canadian universal pharmacare proposed designs most often include cost-sharing for all but the most vulnerable despite evidence that cost-sharing reduces drug use and treatment adherence, and likely results in increases in health services use [ 3 , 58 ].
Availability of data and materials
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Abbreviations
Angiotensin-converting enzyme inhibitors
Assessment of Multiple Systematic Reviews
Angiotensin-receptor blockers
Confidence Intervals
Emergency Department
Effective Public Health Practice Project
Effective Practice and Organisation of Care
Grading of Recommendations, Assessment, Development and Evaluations
Hazard Ratio
Interrupted Time Series
National Institute of Health
Randomized Controlled Trial
Research Papers in Economics
Repeated Measures
Relative Risk
Socioeconomic Status
Strengthening the Reporting of Observational Studies in Epidemiology
United States
Sommers BD. Health insurance coverage: what comes after the ACA? Health Aff (Millwood). 2020;39(3):502–8.
Article Google Scholar
Rosenthal MB. The growing problem of out-of-pocket costs and affordability in employer-sponsored insurance. JAMA. 2021;326(4):305–6.
Article PubMed Google Scholar
Health Canada. A prescription for Canada: achieving pharmacare for all. Final report of the Advisory Council on the Implementation of National Pharmacare. Ottawa: Government of Canada; 2019.
Google Scholar
Marchildon GP. Canada: health system review. Copenhagen: WHO Regional Office for Europe on behalf of the European Observatory on Health Systems and Policies; 2013.
OECD. Health at a glance 2019: OECD indicators. Paris: Organization for Economic Cooperation and Development; 2019.
Book Google Scholar
Newhouse JP, The Insurance Experiment G. Free for all? Lessons from the RAND health insurance experiment: a RAND study. Cambridge and London: Harvard University Press; 1993.
Park YJ, Martin EG. Medicare part D’s effects on drug utilization and out-of-pocket costs: a systematic review. Health Serv Res. 2017;52(5):1685–728.
Glied SA, Collins SR, Lin S. Did the ACA lower Americans’ financial barriers to health care? Health Aff (Millwood). 2020;39(3):379–86.
Morgan S, Coombes M. Income-based drug coverage in British Columbia: towards an understanding of the policy. Healthc Policy. 2006;2(2):92–108.
PubMed PubMed Central Google Scholar
Morgan SG, Gagnon M-A, Charbonneau M, Vadeboncoeur A. Evaluating the effects of Quebec’s private-public drug insurance system. CMAJ. 2017;189(40):E1259–E63.
Article PubMed PubMed Central Google Scholar
Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008.
Greenland S, O'Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2(4):463–71.
Article CAS PubMed Google Scholar
Lundh A, Gotzsche PC. Recommendations by Cochrane review groups for assessment of the risk of bias in studies. BMC Med Res Methodol. 2008;8:22.
Higgins J, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of Interventions version 6.0 (updated July 2019): Cochrane; 2019. Available from www.training.cochrane.org/handbook
Adams AS, Soumerai SB, Ross-Degnan D. The case for a medicare drug coverage benefit: a critical review of the empirical evidence. Annu Rev Public Health. 2001;22:49–61.
Harten C, Ballantyne P. The impact of cost-sharing within Canadian provincial drug benefit programs: a review. J Pharm Finance Econ Policy. 2004;13(1):35–53.
Lexchin J, Grootendorst P. Effects of prescription drug user fees on drug and health services use and on health status in vulnerable populations: a systematic review of the evidence. Int J Health Serv. 2004;34(1):101–22.
Rice T, Matsuoka KY. The impact of cost-sharing on appropriate utilization and health status: a review of the literature on seniors. Med Care Res Rev. 2004;61(4):415–52.
Gibson TB, Ozminkowski RJ, Goetzel RZ. The effects of prescription drug cost sharing: a review of the evidence. Am J Manag Care. 2005;11(11):730–40.
PubMed Google Scholar
Maio V, Pizzi L, Roumm AR, et al. Pharmacy utilization and the Medicare modernization act. Milbank Q. 2005;83(1):101–30.
Briesacher BA, Gurwitz JH, Soumerai SB. Patients at-risk for cost-related medication nonadherence: a review of the literature. J Gen Intern Med. 2007;22(6):864–71.
Gemmill MC, Costa-Font J, McGuire A. In search of a corrected prescription drug elasticity estimate: a meta-regression approach. Health Econ. 2007;16(6):627–43.
Goldman DP, Joyce GF, Zheng Y. Prescription drug cost sharing: associations with medication and medical utilization and spending and health. JAMA. 2007;298(1):61–9.
Article CAS PubMed PubMed Central Google Scholar
Gemmill MC, Thomson S, Mossialos E. What impact do prescription drug charges have on efficiency and equity? Evidence from high-income countries. Int J Equity Health. 2008;7:12.
Remler DK, Greene J. Cost-sharing: a blunt instrument. Annu Rev Public Health. 2009;30:293–311.
Green CJ, Maclure M, Fortin PM, Ramsay CR, Aaserud M, Bardal S. Pharmaceutical policies: effects of restrictions on reimbursement. Cochrane Database Syst Rev. 2010;(8):CD008654. https://doi.org/10.1002/14651858.CD008654 .
Holst J. Patient cost sharing: reforms without evidence, Theoretical considerations and empirical findings from industrialized countries, WZB discussion paper, no. SP I 2010–303. Berlin: Wissenschaftszentrum Berlin für Sozialforschung (WZB); 2010.
Polinski JM, Kilabuk E, Schneeweiss S, Brennan T, Shrank WH. Changes in drug use and out-of-pocket costs associated with Medicare part D implementation: a systematic review. J Am Geriatr Soc. 2010;58(9):1764–79.
Swartz K. Cost-sharing: effects on spending and outcomes. The synthesis project. Research synthesis report no. 20. Princeton: Robert Wood Johnson Foundation; 2010.
Baicker K, Goldman D. Patient cost-sharing and healthcare spending growth. J Econ Perspect. 2011;25(2):47–68.
Polinski JM, Donohue JM, Kilabuk E, Shrank WH. Medicare part D’s effect on the under- and overuse of medications: a systematic review. J Am Geriatr Soc. 2011;59(10):1922–33.
Eaddy MT, Cook CL, O'Day K, Burch SP, Cantrell CR. How patient cost-sharing trends affect adherence and outcomes: a literature review. P T. 2012;37(1):45–55.
Lemstra M, Blackburn D, Crawley A, Fung R. Proportion and risk indicators of nonadherence to statin therapy: a meta-analysis. Can J Cardiol. 2012;28(5):574–80.
Maimaris W, Paty J, Perel P, et al. The influence of health systems on hypertension awareness, treatment, and control: a systematic literature review. PLoS Med. 2013;10(7):e1001490.
Pimentel CB, Lapane KL, Briesacher BA. Medicare part D and long-term care: a systematic review of quantitative and qualitative evidence. Drugs Aging. 2013;30(9):701–20.
Sinnott S-J, Buckley C, O'Riordan D, Bradley C, Whelton H. The effect of copayments for prescriptions on adherence to prescription medicines in publicly insured populations; a systematic review and meta-analysis. PLoS One. 2013;8(5):e64914.
Kiil A, Houlberg K. How does copayment for health care services affect demand, health and redistribution? A systematic review of the empirical evidence from 1990 to 2011. Eur J Health Econ. 2014;15(8):813–28.
Mann BS, Barnieh L, Tang K, et al. Association between drug insurance cost sharing strategies and outcomes in patients with chronic diseases: a systematic review. PLoS One. 2014;9(3):e89168.
Kesselheim AS, Huybrechts KF, Choudhry NK, et al. Prescription drug insurance coverage and patient health outcomes: a systematic review. Am J Public Health. 2015;105(2):E17–30.
Luiza VL, Chaves LA, Silva RM, et al. Pharmaceutical policies: effects of cap and co-payment on rational use of medicines. Cochrane Database Syst Rev. 2015;5:CD007017.
Aziz H, Hatah E, Makmor Bakry M, Islahudin F. How payment scheme affects patients’ adherence to medications? A systematic review. Patient Prefer Adherence. 2016;10:837–50.
Banerjee A, Khandelwal S, Nambiar L, et al. Health system barriers and facilitators to medication adherence for the secondary prevention of cardiovascular disease: a systematic review. Open Heart. 2016;3(2):e000438.
Doshi JA, Li P, Ladage VP, Pettit AR, Taylor EA. Impact of cost sharing on specialty drug utilization and outcomes: a review of the evidence and future directions. Am J Manag Care. 2016;22(3):188–97.
Powell V, Saloner B, Sabik LM. Cost sharing in Medicaid: assumptions, evidence, and future directions. Med Care Res Rev. 2016;73(4):383–409.
Gourzoulidis G, Kourlaba G, Stafylas P, Giamouzis G, Parissis J, Maniadakis N. Association between copayment, medication adherence and outcomes in the management of patients with diabetes and heart failure. Health Policy. 2017;121(4):363–77.
Gupta S, McColl MA, Guilcher SJ, Smith K. Cost-related nonadherence to prescription medications in Canada: a scoping review. Patient Prefer Adherence. 2018;12:1699–715.
Ofori-Asenso R, Jakhu A, Curtis AJ, et al. A systematic review and meta-analysis of the factors associated with nonadherence and discontinuation of statins among people aged ≥65 years. J Gerontol A Biol Sci Med Sci. 2018;73(6):798–805.
Schneider APH, Gaedke MA, Garcez A, Barcellos NT, Paniz VMV. Effect of characteristics of pharmacotherapy on non-adherence in chronic cardiovascular disease: a systematic review and meta-analysis of observational studies. Int J Clin Pract. 2018;72(1). https://doi.org/10.1111/ijcp.13044 .
Cheen MHH, Tan YZ, Oh LF, Wee HL, Thumboo J. Prevalence of and factors associated with primary medication non-adherence in chronic disease: a systematic review and meta-analysis. Int J Clin Pract. 2019;73(6):e13350.
Kolasa K, Kowalczyk M. The effects of payments for pharmaceuticals: a systematic literature review. Health Econ Policy Law. 2019;14(3):337–54.
Mishuk AU, Fasina I, Qian J. Impact of U.S. federal and state generic drug policies on drug use, spending, and patient outcomes - a systematic review. Res Soc Adm Pharm. 2020;16(6):736-45. https://doi.org/10.1016/j.sapharm.2019.08.031 .
Holbrook AM, Wang M, Lee M, et al. Cost-related medication nonadherence in Canada: a systematic review of prevalence, predictors, and clinical impact. Syst Rev. 2021;10(1):11.
Ungar WJ, Ariely R. Health insurance, access to prescription medicines and health outcomes in children. Expert Rev Pharmacoecon Outcomes Res. 2005;5(2):215–25.
Guo EX, Sweetman A, Guindon GE. Socioeconomic differences in prescription drug supplemental coverage in Canada: a repeated cross-sectional study. Health Policy. 2020;124(3):252–60.
Baumgartner JC, Collins SR, Radley DC. Racial and ethnic inequities in health care coverage and access, 2013–2019. Data brief, June 2021. New York: Commonwealth Fund; 2021.
Morgan SG, Law M, Daw JR, Abraham L, Martin D. Estimated cost of universal public coverage of prescription drugs in Canada. CMAJ. 2015;187(7):491–7.
Office of the Parliamentary Budget Officer. Federal cost of a national pharmacare program. Ottawa: Parliamentary Budget Officer (PBO); 2017.
Yeung K, Morgan SG. Should national pharmacare apply a value-based insurance design? CMAJ. 2019;191(29):E811–E5.
Download references
Acknowledgements
We thank our collaborator Carley Hay from the Ontario Ministry of Health, Umaima Abbas, Bria Barton, Gioia Buckley, Selene Miller, Erica Stone and Riya Trivedi for their research assistance, and Arthur Sweetman and Jeremiah Hurley for their comments and discussion.
Canadian Institutes for Health Research (grant # 378730). GEG holds the Centre for Health Economics and Policy Analysis (CHEPA)/Ontario Ministry of Health and Long-Term Care (MOHLTC) Chair in Health Equity, an endowed Chair funded in part by the MOHLTC. The funders had no role in the study design, analysis, interpretation, writing of the report, or in the decision to submit this article for publication.
Author information
Authors and affiliations.
Centre for Health Economics and Policy Analysis, McMaster University, Room 229, 1280 Main Street West, Hamilton, ON, L8S 4K1, Canada
G. Emmanuel Guindon, Tooba Fatima, Sophiya Garasia & Kimia Khoee
Department of Health Research Methods, Evidence, and Impact, McMaster University, Hamilton, ON, Canada
G. Emmanuel Guindon & Sophiya Garasia
Department of Economics, McMaster University, Hamilton, ON, Canada
G. Emmanuel Guindon
You can also search for this author in PubMed Google Scholar
Contributions
GEG conceptualized the study. SG and GEG designed the search strategy. GEG, TF, SG, and KK assessed studies for inclusion, extracted detailed study characteristics, identified study limitations, and contributed to the interpretation of the findings. GEG and TF led the writing of the article. SG and KK revised the article critically for important intellectual content. GEG obtained funding. The authors read and approved the final manuscript.
Corresponding author
Correspondence to G. Emmanuel Guindon .
Ethics declarations
Ethics approval and consent to participate.
Institutional review board approval was not needed because we reviewed and synthesized existing research.
Consent for publication
We confirm that all contributors agreed to submit this paper for publication.
Competing interests
Additional information, publisher’s note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: appendix a..
Search strategy. Appendix B. Quality Assessment / Risk of Bias tools. Appendix C. Characteristics of included studies. Appendix D. Excluded studies. Appendix E. List of Canadian studies included in reviews. Appendix F. List of reviews that focused specifically on value-based cost-sharing/insurance design.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/ . The Creative Commons Public Domain Dedication waiver ( http://creativecommons.org/publicdomain/zero/1.0/ ) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
Reprints and permissions
About this article
Cite this article.
Guindon, G.E., Fatima, T., Garasia, S. et al. A systematic umbrella review of the association of prescription drug insurance and cost-sharing with drug use, health services use, and health. BMC Health Serv Res 22 , 297 (2022). https://doi.org/10.1186/s12913-022-07554-w
Download citation
Received : 30 August 2021
Accepted : 27 January 2022
Published : 03 March 2022
DOI : https://doi.org/10.1186/s12913-022-07554-w
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Cost-sharing
- Prescription drug
BMC Health Services Research
ISSN: 1472-6963
- General enquiries: [email protected]
Prescription Opioid Misuse, Abuse, and Treatment in the United States: An Update
Information & authors, metrics & citations, view options, conclusions:, definitions.
| DSM-5 Criteria for Dependence | Medical Use of Opioids for Chronic Pain |
|---|---|
| Tolerance | Expected with prolonged use |
| Withdrawal | Expected with prolonged use |
| Using larger amounts for longer time | Pain may last longer than expected |
| Wants to quit, not able | Pain can interfere with dose tapering |
| Lots of time getting, using, recovering from substance use | Criterion can be applied: if opioids are prescribed, should not be spending excessive time procuring |
| Cravings or urges to use | Distinguish from pain-related urges |
| Not managing at work, etc. | Distinguish problems related to pain from problems related to opioid use |
| Continuing to use despite problems in relationships | Criterion can be applied: if pain is adequately treated, relationships should improve |
| Giving up important activities | Criterion can be applied: if pain is adequately treated, ability to engage in activities should improve |
| Continuing to use despite danger | Criterion can be applied: if opioids are used as prescribed, should be minimal danger |
| Using despite physical or psychological problems | Must distinguish from problems related to pain |
| Clearly Problematic | Potentially Problematic |
|---|---|
| Selling | Hoarding |
| Forging prescriptions | Specific type of drug requested |
| Stealing drugs from others | |
| Using by nonprescribed route (e.g., injecting or crushing and snorting) | |
| Doctor shopping | |
| Repeated losing, running out early | Single loss, running out early |
| Multiple dosage increases | Single dosage increase |
Scope of the Problem
Prescription opioid overdose, prescription practices, efforts to prevent opioid misuse and abuse, screening and assessment.
| Measure | Format/Purpose |
|---|---|
| Screener and Opioid Assessment for Patients With Pain ( ) | Self-report; used to facilitate assessment and planning for patients being considered for long-term opioid treatment |
| Opioid Risk Tool ( ) | Self-report; used to assess risk of opioid abuse in primary care settings for patients being considered for opioid treatment |
| Alcohol Use Disorders Identification Test ( ) | Self-report; used to identify hazardous and harmful patterns of alcohol consumption |
| Alcohol, Smoking, and Substance Involvement Screening Test ( ) | Self-report; used to detect substance use and related problems in primary and general medical care settings |
| Drug Abuse Screening Test ( ) | Self-report; used to help identify individuals who are abusing drugs |
| Alcohol Use Disorders and Associated Disabilities Interview Schedule ( ) | Clinician administered; diagnostic interview |
| Composite International Diagnostic Interview ( ) | Clinician administered; diagnostic interview |
| Structured Clinical Interview for DSM-IV ( ) | Clinician administered; diagnostic interview |
| Mini-International Neuropsychiatric Interview ( ) | Clinician administered; diagnostic interview |
| Current Opioid Misuse Measure ( ) | Self-report; used to identify whether a patient currently on long-term opioid treatment is exhibiting aberrant behaviors associated with misuse |
| Addiction Severity Index ( ) | Clinician administered; used to assess areas of functioning often affected by substance abuse (e.g., medical, legal, psychiatric, alcohol, drug, social) |
| Timeline follow-back ( ) | Clinician administered; used to monitor amount and frequency of substance use |
Treatment of Prescription Opioid Use Disorders
Treatment outcomes specific to prescription opioid use disorders, critical evidence gaps in treatment of prescription opioid use disorders, treatment of pain in substance-dependent individuals, conclusions and recommendations, supplementary material, information, published in.

Competing Interests
Funding information, export citations.
If you have the appropriate software installed, you can download article citation data to the citation manager of your choice. Simply select your manager software from the list below and click Download. For more information or tips please see 'Downloading to a citation manager' in the Help menu .
| Format | |
|---|---|
| Citation style | |
| Style | |
To download the citation to this article, select your reference manager software.
There are no citations for this item
View options
Login options.
Already a subscriber? Access your subscription through your login credentials or your institution for full access to this article.
Purchase Options
Purchase this article to access the full text.
PPV Articles - American Journal of Psychiatry

Not a subscriber?
Subscribe Now / Learn More
PsychiatryOnline subscription options offer access to the DSM-5-TR ® library, books, journals, CME, and patient resources. This all-in-one virtual library provides psychiatrists and mental health professionals with key resources for diagnosis, treatment, research, and professional development.
Need more help? PsychiatryOnline Customer Service may be reached by emailing [email protected] or by calling 800-368-5777 (in the U.S.) or 703-907-7322 (outside the U.S.).
Share article link
Copying failed.
PREVIOUS ARTICLE
Next article, request username.
Can't sign in? Forgot your username? Enter your email address below and we will send you your username
If the address matches an existing account you will receive an email with instructions to retrieve your username
Create a new account
Change password, password changed successfully.
Your password has been changed
Reset password
Can't sign in? Forgot your password?
Enter your email address below and we will send you the reset instructions
If the address matches an existing account you will receive an email with instructions to reset your password.
Your Phone has been verified
As described within the American Psychiatric Association (APA)'s Privacy Policy and Terms of Use , this website utilizes cookies, including for the purpose of offering an optimal online experience and services tailored to your preferences. Please read the entire Privacy Policy and Terms of Use. By closing this message, browsing this website, continuing the navigation, or otherwise continuing to use the APA's websites, you confirm that you understand and accept the terms of the Privacy Policy and Terms of Use, including the utilization of cookies.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Research Reports
Common comorbidities with substance use disorders research report.

Heroin Research Report

Inhalants Research Report

Medications to Treat Opioid Use Disorder Research Report

Methamphetamine Research Report

Misuse of Prescription Drugs Research Report

Prescription Opioids and Heroin Research Report

Substance Use in Women Research Report

Tobacco, Nicotine, and E-Cigarettes Research Report
- Academic Medicine
- Acid Base, Electrolytes, Fluids
- Allergy and Clinical Immunology
- American Indian or Alaska Natives
- Anesthesiology
- Anticoagulation
- Art and Images in Psychiatry
- Artificial Intelligence
- Assisted Reproduction
- Bleeding and Transfusion
- Caring for the Critically Ill Patient
- Challenges in Clinical Electrocardiography
- Climate and Health
- Climate Change
- Clinical Challenge
- Clinical Decision Support
- Clinical Implications of Basic Neuroscience
- Clinical Pharmacy and Pharmacology
- Complementary and Alternative Medicine
- Consensus Statements
- Coronavirus (COVID-19)
- Critical Care Medicine
- Cultural Competency
- Dental Medicine
- Dermatology
- Diabetes and Endocrinology
- Diagnostic Test Interpretation
- Drug Development
- Electronic Health Records
- Emergency Medicine
- End of Life, Hospice, Palliative Care
- Environmental Health
- Equity, Diversity, and Inclusion
- Facial Plastic Surgery
- Gastroenterology and Hepatology
- Genetics and Genomics
- Genomics and Precision Health
- Global Health
- Guide to Statistics and Methods
- Hair Disorders
- Health Care Delivery Models
- Health Care Economics, Insurance, Payment
- Health Care Quality
- Health Care Reform
- Health Care Safety
- Health Care Workforce
- Health Disparities
- Health Inequities
- Health Policy
- Health Systems Science
- History of Medicine
- Hypertension
- Images in Neurology
- Implementation Science
- Infectious Diseases
- Innovations in Health Care Delivery
- JAMA Infographic
- Law and Medicine
- Leading Change
- Less is More
- LGBTQIA Medicine
- Lifestyle Behaviors
- Medical Coding
- Medical Devices and Equipment
- Medical Education
- Medical Education and Training
- Medical Journals and Publishing
- Mobile Health and Telemedicine
- Narrative Medicine
- Neuroscience and Psychiatry
- Notable Notes
- Nutrition, Obesity, Exercise
- Obstetrics and Gynecology
- Occupational Health
- Ophthalmology
- Orthopedics
- Otolaryngology
- Pain Medicine
- Palliative Care
- Pathology and Laboratory Medicine
- Patient Care
- Patient Information
- Performance Improvement
- Performance Measures
- Perioperative Care and Consultation
- Pharmacoeconomics
- Pharmacoepidemiology
- Pharmacogenetics
- Pharmacy and Clinical Pharmacology
- Physical Medicine and Rehabilitation
- Physical Therapy
- Physician Leadership
- Population Health
- Primary Care
- Professional Well-being
- Professionalism
- Psychiatry and Behavioral Health
- Public Health
- Pulmonary Medicine
- Regulatory Agencies
- Reproductive Health
- Research, Methods, Statistics
- Resuscitation
- Rheumatology
- Risk Management
- Scientific Discovery and the Future of Medicine
- Shared Decision Making and Communication
- Sleep Medicine
- Sports Medicine
- Stem Cell Transplantation
- Substance Use and Addiction Medicine
- Surgical Innovation
- Surgical Pearls
- Teachable Moment
- Technology and Finance
- The Art of JAMA
- The Arts and Medicine
- The Rational Clinical Examination
- Tobacco and e-Cigarettes
- Translational Medicine
- Trauma and Injury
- Treatment Adherence
- Ultrasonography
- Users' Guide to the Medical Literature
- Vaccination
- Venous Thromboembolism
- Veterans Health
- Women's Health
- Workflow and Process
- Wound Care, Infection, Healing
- Download PDF
- Share X Facebook Email LinkedIn
- Permissions
Improving Prescription Drug Affordability Through Regulatory Action
- 1 Office of the Assistant Secretary for Planning and Evaluation, US Department of Health and Human Services, Washington, DC
- Viewpoint New Reforms to Prescription Drug Pricing in the US: Opportunities and Challenges Thomas J. Hwang, MD; Aaron S. Kesselheim, MD, JD, MPH; Benjamin N. Rome, MD, MPH JAMA
- Original Investigation Prescription Drug Spending in Fee-for-Service Medicare, 2008-2019 Stacie B. Dusetzina, PhD; Haiden A. Huskamp, PhD; Xuanzi Qin, PhD; Nancy L. Keating, MD, MPH JAMA
Prescription drug prices in the US are more than 2.5 times as high as those in other similar high-income nations and are a leading health care concern among US residents. Given these factors, and in response to President Biden’s executive order promoting competition, the US Department of Health and Human Services (HHS) released a comprehensive plan to address drug prices in September 2021. 1 The plan highlighted 3 priorities: (1) making drug prices more affordable and equitable for all consumers and throughout the health care system, (2) improving competition throughout the prescription drug industry, and (3) fostering scientific innovation to promote better health care and improve health.
To support this agenda, the Office of the Assistant Secretary for Planning and Evaluation (ASPE) conducts research on drug pricing, utilization, access, and innovation. For instance, a 2021 ASPE report found that more than 5 million Medicare beneficiaries reported difficulty affording medicines in 2019. 2 A recent journal article projected that savings from biosimilars from 2021-2025 would total at least $38 billion and as much as $124 billion—or approximately 6% to 19% of total spending on biosimilars. 3 A recent report on US and international generic drug utilization showed that US brand-name drug prices are 344% of prices in similar high-income countries, whereas US prices for generic drugs are actually lower than in comparator countries (84% of foreign prices). 4 A series of projects are also examining the drug supply chain, including an overview of stakeholders and relationships. In addition, the HHS plans to publicly track large price increases in prescription drugs to improve drug price transparency.
Using evidence and research to guide this work, the HHS plan outlined actions that the Biden administration is taking to reduce high drug prices. One key agency in this work is the Centers for Medicare & Medicaid Services (CMS), which is addressing prescription drug costs in Medicare, Medicaid, and the health insurance marketplace. In Medicare, a rule finalized in April established that price concessions that Medicare Part D plans receive from pharmacies must get passed along to beneficiaries at the pharmacy counter, saving an estimated $26.5 billion between 2024 and 2032. 5 For physician-administered drugs, paid for by Medicare Part B, the CMS implemented a provision from 2021 legislation that requires drug manufacturers without Medicaid drug rebate agreements to submit average sales price information for their Part B drugs, which will enable payment to be pegged to a lower rate than was previously in place for these drugs (including, for instance, common treatments such as hyaluronic acid injections, where payment may decline by ≥50% under this new rule). This change is projected to reduce Medicare costs by $3.5 billion over 10 years and produce significant savings to beneficiaries. In addition, Medicare is working with the US Food and Drug Administration (FDA) to share educational materials with clinicians about biosimilar and interchangeable biological products.
The HHS is also taking actions related to private insurance and Medicaid. In May 2022, the department finalized regulations designed to prevent health insurance marketplace plans from discriminating against beneficiaries with certain health conditions through the use of selectively designed drug formularies. The rule contained examples of how drug formularies might be discriminatory and how issuers can correct them, building on prior research that found that some insurers were putting all drugs from certain classes (for instance, antiretrovirals for HIV) on the highest cost-sharing tier. In Medicaid, a new regulation effective in July 2022 incentivizes manufacturers to offer states the same value-based purchasing arrangements for high-cost drugs that they offer to other insurers, and the CMS will also be launching a new learning collaborative in October 2022 to support state efforts on drug cost management and transparency. Medicaid is also working to encourage uptake of generic and biosimilar drugs through education to state drug utilization review boards.
Meanwhile, the FDA has established policies that support a competitive marketplace for generic drugs and biosimilar products, which can lower prices . Through its Drug Competition Action Plan and Biosimilars Action Plan , the FDA is working to help remove barriers to generic and biosimilar market entry, including through workshops and guidance to facilitate product development; initiatives to improve the quality of applications and enhance the efficiency of review by the FDA; and efforts to reduce “gaming” that delays competition and extends monopolies beyond what Congress intended. The agency has issued policy documents designed to ensure timelier generic drug approvals and earlier patient access by reducing the number of assessment cycles , facilitating prompt labeling updates , and providing advice on how to avoid common application deficiencies that lead to approval delays.
The FDA approved the first interchangeable biosimilar insulin product last summer, Semglee (insulin glargine-yfgn), which is biosimilar to its reference product, a long-acting insulin analog called Lantus (insulin glargine). As noted in the research discussed earlier, biosimilar and interchangeable products can reduce drug costs significantly, given that biosimilar prices average 15% to 35% less than their reference products.
Legislation remains a critical potential tool in reducing drug prices and costs, and the HHS plan released last fall highlights priorities such as drug price negotiation in Medicare, caps on out-of-pocket costs, and policies to slow price increases over time on existing drugs. While Congress continues to explore legislative options in this area, the Biden administration has been tackling these issues through its available regulatory tools. None is a silver bullet, but collectively they offer the potential for substantial improvements in drug affordability for millions of people in the US. Future research to assess these policies will be critical in efforts to address high drug prices to protect consumers and health care programs more broadly.
Published: August 4, 2022. doi:10.1001/jamahealthforum.2022.3180
Open Access: This is an open access article distributed under the terms of the CC-BY License . © 2022 Bush L et al. JAMA Health Forum .
Corresponding Author: Benjamin D. Sommers, MD, PhD, Office of the Assistant Secretary for Planning and Evaluation, US Department of Health and Human Services, 200 Independence Ave SW, Washington, DC 20201 ( [email protected] ).
Conflict of Interest Disclosures: Dr Sommers reported receiving personal fees from the Health Research and Educational Trust, the Urban Institute, AcademyHealth, the American Economics Journal , and the Illinois Department of Healthcare and Family Services and receiving grants from Baylor Scott & White Health, the Commonwealth Fund, and the Robert Wood Johnson Foundation. No other disclosures were reported.
See More About
Bush L , Sommers BD. Improving Prescription Drug Affordability Through Regulatory Action. JAMA Health Forum. 2022;3(8):e223180. doi:10.1001/jamahealthforum.2022.3180
Manage citations:
© 2024
Artificial Intelligence Resource Center
Best of JAMA Network 2022
Browse and subscribe to JAMA Network podcasts!
Others Also Liked
Misuse of prescription and over-the-counter drugs to obtain illicit highs: how pharmacists can prevent abuse
There have been increasing reports of misuse of a range of prescription and over-the-counter (otc) drugs for recreational purposes. the use of psychoactive pharmaceuticals and ‘pharming’ are new, widespread phenomena involving the non-medical use of prescription and otc drugs, which are recreationally used to achieve psychoactive effects either on their own or in combination with other substances., this article provides an overview of the topic, focusing on a range of medicines (e.g. prescription medicines such as quetiapine, gabapentinoids, z-drugs, bupropion, venlafaxine and over-the-counter medicines such as loperamide, dextromethorphan, benzydamine, promethazine, chlorphenamine, diphenhydramine and hyoscine butylbromide) that have emerged as misused and diverted, or are already described through the literature, as well as recorded by drug users’ online websites reporting new trends and experimentations of drug abuse., this rapidly changing drug scenario represents a challenge for pharmacy, psychiatry, public health and drug control policies. moreover, possibly resulting from the covid-19 pandemic, drug use habits and availability have changed, causing a shift in behaviours relating to both prescription and otc medicines. healthcare professionals should be aware of potential prescription drugs diversion, recognise misuse cases, consider the possibility of polydrug misuse, and prevent it where possible. pharmacists can prevent and reduce drug abuse, and should be involved in evidence-based actions to detect, understand and prevent drug diversion activities and the adverse effects of drug misuse., keywords: drug abuse; prescription drug misuse; over-the-counter drug abuse; novel psychoactive substances (nps); pharmacovigilance, originally submitted: 30 march 2020; revised submitted : 17 august 2020; accepted for publication : 20 august 2020; published online: 17 november 2020; doi: 10.1211/pj.2020.20208538.
- The use of ‘psychoactive pharmaceuticals’ and ‘pharming’ are increasingly reported phenomena involving the non-medical use of prescription (e.g. pain relievers, tranquilisers, stimulants, sedatives) and OTC drugs (e.g. loperamide, promethazine, antitussive cough syrups), either on their own or in combination with other licit or illicit substances, for recreational purposes.
- A range of medications have emerged as misused and diverted, or are known anecdotally to be used, or already described through the literature or pharmacovigilance datasets, as well as recorded by drug users’ online websites reporting new trends and experimentations of drug abuse.
- Drug use habits and availability may have changed as a result of the COVID-19 pandemic, causing a shift in behaviours relating to both prescription and OTC medicines.
- Pharmacists should play a key role in preventing and reducing drug abuse and be involved in evidence-based actions to detect, understand and prevent drug diversion activities and drug misuse adverse effects.
Source: Shutterstock.com
Pharmacists have an important role to play in preventing and reducing abuse of prescription and over-the-counter medicines
Introduction
In recent years, drug abuse scenarios have evolved owing to the appearance of novel psychoactive substances (NPSs) and the recreational use of pharmaceuticals [1] , [2] , [3] , [4] . Misuse of prescription drugs is a growing health problem, involving not only specific drug-related risks, but also the context in which they are consumed (e.g. the concomitant abuse of other substances with synergistic effects, psychiatric diagnoses and social circumstances) [4] , [5] , [6] , [7] , [8] .
Therefore, side effects, drug interactions and individual variation in responses (owing to existing comorbidities [such as a mental disorder, renal or hepatic dysfunction, systemic diseases] and previous substance abuse or dependence) might be associated with a range of severe adverse drug reactions (ADRs), including seizures, arrhythmias, respiratory arrest and fatalities [2] , [9] , [10] , [11] . In this context, ‘pharming’ is a new, worldwide phenomenon involving the non-medical use of prescription (e.g. pain relievers, tranquilisers, stimulants, sedatives) and over-the-counter (OTC) drugs (e.g. those containing dextromethorphan and promethazine) [2] , [7] , [12] , [13] , [14] , [15] .
According to data from the United Nations Office on Drugs and Crime (UNODC), the prevalence of prescription drug misuse and related fatalities is increasing worldwide [16] , [17] , [18] . Vulnerable groups at higher risk of misusing medications are adolescents and young adults; women; older adults; and healthcare professionals [19] . Other at-risk groups include people with mental illnesses, inmates and individuals suffering from acute or chronic pain, who might abuse opiate medications [17] . A range of factors are thought to contribute to the non-medical use of prescription/OTC drugs, such as:
- The perception of prescription drugs as more socially acceptable;
- Less stigmatising;
- Safer than the intake of illicit substances, as well as their likely lack of detection in standard drug screens [13] .
This article aims to undertake a comprehensive review of the relevant literature describing the drugs primarily associated with potential diversion, typical patterns of their misuse and harms associated with medicine abuse; report factors that might influence and exacerbate diversion in the current COVID-19 crisis; and consider how pharmacists can reduce and prevent substance abuse.
Material and methods
A literature search was performed on PubMed, Medline and Web-of-Science in May 2020 and covered the past 20 years. We used combinations of the following search terms [Title/Abstract]: ‘prescription drug’, ‘non-prescription drug’, ‘over-the-counter drug’, ‘misuse’, ‘abuse’, ‘non-medical use’, ‘addiction’ and ‘dependence’. Additional searches were then undertaken based on identified medicines, and these included ‘gabapentinoid’, ‘antidepressants’, ‘antipsychotics’, ‘Z-drugs’, ‘dextromethorphan’, ‘antihistamine’, ‘loperamide’, ‘benzydamine’, ‘pseudoephedrine’ and ‘scopolamine’.
Finally, authors performed further secondary searches by using the reference listing of all eligible papers. All titles/abstracts were examined, and full texts of potentially relevant papers obtained. Relevant works were chosen to obtain a full representation of the available literature on the selected topic. Experimental and observational studies; post-marketing surveillance reports; case reports; case series; and fatalities reports were included. The exclusion criteria included: non-original researches (e.g. review, commentary, editorial, book chapter, letter to the editor); non-full-text articles (e.g. meeting abstract); works in a language other than English; animal/ in vitro studies; and articles that did not cover the abuse/misuse/dependence of the selected drugs.
Opioids and benzodiazepines are traditionally misused and, even though mostly controlled through regulation, are still diverted and associated with risky behaviours and higher overdose risk [18] . The emergence of potent new, ‘designer’ benzodiazepines or new, synthetic opioids on the drug market is a reason for continued concern [20] , [21] . These medicines were excluded from this review as these categories require additional insight that is beyond the scope of this work. Image and performance-enhancing drugs were excluded from consideration in this review because their typical pattern of use is quite different from a typical recreational value (e.g. aesthetic use, competitive bodybuilding) [22] , [23] . Similarly, cognitive enhancers were not considered in this review because their primary use is to maintain wakefulness, improve recall and enhance executive functions [22] , [24] .
Definitions
‘Abuse’ was specifically defined as the intentional, non-therapeutic use by a patient or consumer of a product, OTC or prescription medicine, for a perceived reward or desired non-therapeutic effect including, but not limited to, getting ‘high’ (euphoria) [25] .
‘Misuse’ was defined as the intentional use, by a patient or consumer of a product, OTC or prescription medicine, for a therapeutic purpose other than as prescribed or not in accordance with the authorised product information [25] . ‘Drug misuse’ is used to distinguish improper or unhealthy use from the use of medicine as prescribed or alcohol in moderation [19] . These include the use of drugs to produce pleasure, alleviate stress, and/or alter or avoid reality [19] .
‘Addiction’ refers to a chronic, relapsing disorder characterised by compulsive drug seeking, continued use despite harmful consequences, and long-lasting changes in the brain [19] .
‘Dependence’ refers to a maladaptive pattern of substance use leading to clinically significant impairment or distress [26] . It is manifested by three or more of the following criteria, occurring at any time in the same 12-month period:
i) Tolerance;
ii) Withdrawal;
iii) Taking the substance often in larger amounts or over a longer period than was intended;
iv) Having a persistent desire or unsuccessful efforts to cut down or control substance use [26] .
Physical dependence developed as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Psychological dependence refers to a state in which individuals have impaired control over drug use based on the rewarding properties of the drug (ability to produce positive sensations that increase the likelihood of drug use) or the psychological distress produced in the absence of the drug [26] . In 2013, the American Psychiatric Association (APA) updated the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), replacing the categories of substance abuse and substance dependence with the unique category of substance use disorder (SUD) [27] .
Publications and findings
A total of n=314 publications were identified; after removing duplicates (n=9), and applying the exclusion criteria, a total of n=74 papers were retrieved and analysed. The findings are described in detail, organised in relation to the specific drug/group of drugs (see Table and supplement [1] , [7] , [9] , [12] , [28] , [29] , [30] , [31] , [32] , [33] , [34] , [35] , [36] , [37] , [38] , [39] , [40] , [41] , [42] , [43] , [44] , [45] , [46] , [47] , [48] ).
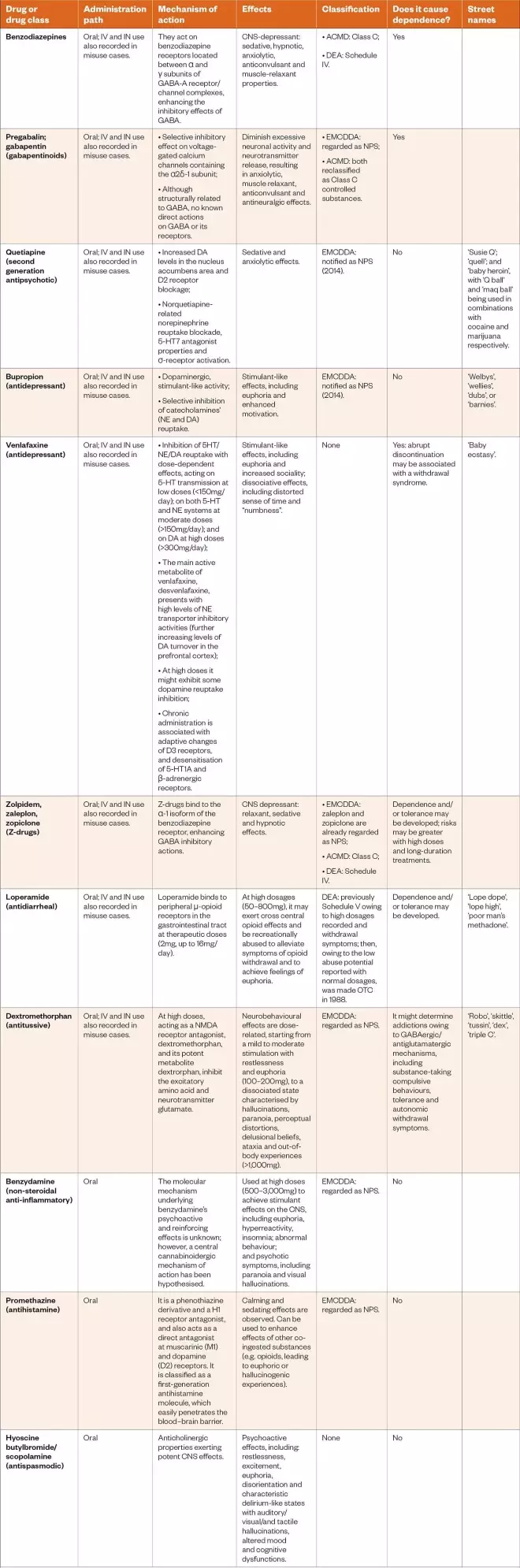
Table: Drug classification and the main characteristics relevant to pharmacists and other healthcare professionals
ACMD: Advisory Council on the Misuse of Drugs; DEA: Drug Enforcement Administration; DA: dopamine; EMCDDA: European Monitoring Centre for Drugs and Drug Addiction; GABA: gamma-amino-butyric acid; H: histamine; IN: intranasal; IV: intravenous; CNS: central nervous system; NE: norepinephrine; NPS: new psychoactive substance; OTC: over-the-counter; 5-HT: serotonin
Sources: Curr Opin Pediatr [7] , Psychol Med [9] , Subst Abuse Rehabil [ 12] , Addict Behav [28] , Eur Neuropsychopharmacol [29] , J Clin Psychopharmacol [30] , Subst Abuse Rehabil [31] , Hum Psychopharmacol [32] , Subst Use Misuse [33] , Basic Clin Pharmacol [34] , Eur J Pediatr [35] , Basic Clin Pharmacol Toxicol [36] , J Psychoactive Drugs [37] , Braz J Psychiatry [38] , Addiction [39] , South Med J [40] , PLoS One [41] , CNS Neurosci Ther [42] , Riv Psichiatr [43] , Cambridge University Press [44] , Am J Addict [45] , J Addict Med [46] , Subst Use Misuse [47] , Health Policy [48]
Download the full PDF version of the table here .
Prescription drugs misuse.
Prescription drug abuse has become a concerning modern-day epidemic [49] , [50] , [51] . This is especially true among young adults and adolescents, where their use has surpassed all use of illicit drugs, with the exception of marijuana [52] , [53] . Traditionally, concern has centered on opioids, benzodiazepines and stimulants, but other widely prescribed drugs may be misused, abused or diverted for non-medical purposes [54] . Young people take prescription drugs for recreational purposes (e.g. to get ‘high’); to relieve anxiety or relax; or to improve academic performance [5] , [7] , [8] , [55] . Drugs might be acquired from friends or relatives, directly prescribed by a doctor, obtained from a drug dealer or via the internet [2] , [56] , [57] . Although there is little nationwide data on the prevalence of prescription drug abuse among young people in the UK, there are an increasing number of reports to suggest the problem is growing [16] .
According to the literature, quetiapine appears to be the most documented second-generation antipsychotic being abused because of its sedative, relaxant and anxiolytic characteristics [58] , [59] . High rates of quetiapine-related ambulance attendances/emergency department visits have been reported: data from the Drug Abuse Warning Network (DAWN) for prevalence of emergency department (ED) visits among the US general population involving quetiapine showed an increase between 2005 and 2011, from 35,581 ED visits to 67,497 [60] . Similar data regarding increasing quetiapine rates of ambulance attendances have been recorded in Australia, and associated with concurrent heroin and opioid replacement therapy toxicity, history of heroin and alcohol misuse, and mood disorders [61] .
Moreover, drug-seeking behaviours, such as an illicit drug provision, and an increase in quetiapine availability on the black market, have been registered [62] . Prison inmates, psychiatric outpatients, users with a history of drug misuse and opioid addicts represent the most at-risk of misusing populations [30] , [47] . Finally, intranasal and intravenous routes of consumption have been described [63] .
Gabapentinoids
Gabapentin and pregabalin are approved treatments for epilepsy and neuropathic pain disorders [44] , [64] , [65] . Both have increasingly been reported for their misuse potential; however, pregabalin is considered to have a higher abuse potential owing to its rapid absorption, faster onset of action and higher potency [29] , [42] , [66] , [67] . Death, physical dependence, and the propensity to cause depression of the central nervous system (CNS), especially when used in combination with opioids and sedatives, are harms identified for both gabapentinoids [42] . The principal population at risk for addiction are those with other current or past SUD, mostly opioid and polydrug users [29] , [42] , [66] , [67] . Opioid users often misuse pregabalin to self-treat physical pain, to achieve a desired psychoactive effect (e.g. potentiate the effects of heroin/cocaine), and combat opioid withdrawal symptoms [42] , [66] , [68] , [69] , [70] .
Moreover, rates of pregabalin misuse-related ambulance attendances have increased markedly over the past ten years (e.g. in Australia from 0.28 cases per 100,000 population in the first half of 2012 to 3.32 cases per 100,000 in the second half of 2017) [71] . Therefore, pregabalin and gabapentin were found to have the potential for misuse, addiction and overdose [29] , [42] , [66] , [67] . In 2018, after safety warnings following an increase in deaths related to their use, the Advisory Council on the Misuse of Drugs recommended that both are controlled under the Misuse of Drugs Act 1971 as Class C substances, and scheduled under the Misuse of Drugs Regulations 2001 as Schedule 3, so as not to preclude legitimate use on prescription (see Table) [72] .
The Z-drugs (zolpidem, zaleplon, zopiclone), so-called hypnotic drugs, were introduced in the 1980s for the short-term treatment of insomnia [44] . It was believed that they possessed a favourable and safer profile compared with benzodiazepines, because of their receptor selectivity and improved pharmacokinetic properties [73] . Their significant hypnotic effects involve both a sleep latency reduction and a sleep quality improvement through an increased γ-aminobutyric acid (GABA) transmission at the same GABA-type A receptor as benzodiazepines.
In recent years, concern has grown for their safety because of abuse and dependence issues [9] , [36] , drug-assisted sexual assaults and dangerous sleep behaviours (e.g. sleep eating, sleep driving and sleepwalking). Problematic use of hypnotic drugs has been described in male and young recreational users of high-dose drugs. They are often abused with other licit/illicit drugs via intranasal/intravenous administration [74] .
A second abusing population was studied, and comprised long-term users, including patients with comorbidities of mood/neurotic disorders and SUDs, and older people using Z-drug hypnotics to treat insomnia, who were then unable to cut down the dosages needed to manage withdrawal symptoms [75] , [76] . Zolpidem and zopiclone presented with the same dependence risk, but zopiclone was most indicated in being sold on the black market and in overdose adverse drug reactions [9] , [77] . Similarly to benzodiazepines, since 2013, Z-drugs have been controlled as Class C and Schedule 4 substances under the Misuse of Drugs Act and Regulations, respectively [78] , [79] .
Among antidepressants, the dopaminergic, stimulant-like activities of bupropion may explain its possible recreational value [4] . It is a second-generation antidepressant acting as a selective inhibitor of catecholamine (i.e. noradrenaline and dopamine) reuptake [44] . In the UK, it is licensed as smoking cessation treatment only [80] . A 14-year retrospective review showed that 975 single substance bupropion cases were reported to the National Poison Data System (NPDS), with “intentional abuse” as the coded reason for exposure in individuals aged 13 and older [46] .
The prevalence of abuse increased by 75% from 2000 to 2012, and mostly involved adolescents and young adults, who reported clinical effects of tachycardia, seizures and agitation/irritability [46] . Its recreational use by oral/nasal/intravenous routes has been reported, with people misusing the drug to get a ‘high’ similar to that obtained through other stimulants, such as cocaine (see Table) [31] , [81] , [82] , [83] , [84] .
Venlafaxine
Venlafaxine is an antidepressant in the serotonin-norepinephrine reuptake inhibitor class [44] . Its recreational use is related to its reuptake inhibition, with dose-dependent effects on selective serotonin (5-HT) transmission at low doses (<150mg/day); on both 5-HT and norepinephrine systems at moderate doses (>150mg/day); and on dopamine at high doses (>300mg/day) [41] . Large venlafaxine dosages might be consumed to produce amphetamine/ecstasy-like effects [31] , [32] . Euphoria and increased sociality, as well as dissociative effects, including distorted sense of time and “numbness”, have been described [31] , [32] . Patients with prior SUD (e.g. opioid abuse/dependence) appeared to be more vulnerable to venlafaxine misuse [41] , [85] .
Fatalities have been reported relating to numerous overdose cases, with associated symptoms of tachycardia, seizures, coma and serotonin syndrome; moreover, dependence issues following long-term use have been described [4] , [41] , [86] . A retrospective review of venlafaxine exposures reported to the NPDS from 2000 to 2016 described 752 intentional-abuse venlafaxine exposures of the total of 85,621, with prevalence decreasing from 107/10,000 venlafaxine exposures in 2000 to 59.3/10,000 in 2016 [87] . The median age was 23 years and 50% were female [87] . Primary route was ingestion (90.8%), with 4.7% using venlafaxine via inhalation/intranasal administration. The most frequent clinical effects reported were tachycardia (33.9%), drowsiness (20.7%) and agitation (11.5%) [87] . The decrease in intentional abuse exposures in the study was explained by authors through several reasons, including underreporting or a possible decrease in the prevalence of venlafaxine abuse as patients shift to other agents, but changes in prescribing patterns for venlafaxine were excluded [87] .
Over-the-counter drugs misuse
The potential for misuse of OTC medications that have not previously been deemed to have a diversion potential has been reported worldwide [41] , [88] , [89] . Their abuse appears facilitated by their accessibility, low cost, decreased perception of the potential for harm and growing social acceptability [2] , [7] , [12] , [15] , [90] . In contrast to prescribed and illicit drugs, medications available for individuals to purchase legally without prescription have been perceived to be relatively safe [2] , [7] , [12] , [90] .
They are typically purchased not only from pharmacies, but also from non-medical outlets (e.g. online illicit websites and the darknet). The internet can be used to obtain them without restrictions (e.g. prescription drugs might be obtained without a prescription) [90] . Apart from OTC products that can be misused, such as some codeine or dextromethorphan-containing cough syrups, and decongestants (e.g. pseudoephedrine), other medicines have been found to be misused [2] , [7] , [12] , [90] . Alcohol and illicit drug use are highly associated with the abuse of OTC medications [2] , [7] .
Loperamide is a common anti-diarrhoeal drug, that binds to µ-opioid receptors in the gastrointestinal tract, decreasing peristalsis and increasing sphincter tone [41] . At therapeutic doses (e.g. 2mg, with a maximum dosage of 16mg), loperamide does not exert cross central opioid effects; however, at high dosages (e.g. 50–800mg), it might be recreationally abused to achieve a euphoric state, which is informally referred to as “lope high” [40] . It might be used to manage and cope with opioid withdrawal symptoms [7] , [40] , [91] , [92] .
Loperamide toxicity involves gastrointestinal (e.g. nausea, vomiting, constipation), CNS (e.g. respiratory depression, altered mental status, miosis) and cardiovascular effects (e.g. ventricular dysrhythmias and electrocardiogram alterations, such as prolonged QT, QRS widening and torsades de pointes ), which might be fatal [41] , [93] , [94] , [95] , [96] , [97] , [98] , [99] , [100] , [101] . Consistently, loperamide exposures reported to the NPDS indicated intentional misuse and abuse. There was a 91% increase in reported exposures from 2010 to 2015, with a total of 201 and 383 exposures in 2010 and 2015, respectively, and a rate of around 38 cases per year [102] , most of them involving single-agent loperamide abuse and cardiotoxicity [102] , [103] .
Since September 2019, the Food and Drug Administration (FDA) has limited loperamide package sizes to reduce inappropriate use [104] . Few pharmacies currently regulate its sale, and no regulations exist to prevent purchasing at non-pharmacy online outlets [105] . Interested pharmacies can implement policies to reduce excessive access and prevent harm. However, collateral purchasing at other retail stores or pharmacies may still occur [105] .
Dextromethorphan
As an analogue of codeine and a semisynthetic morphine derivative, dextromethorphan is a component of many cough and cold medicines. At therapeutic doses, dextromethorphan produces minimal analgesic and antitussive effects. At high doses, acting as a N-methyl-D-aspartate receptor antagonist, it produces the hallucinogenic and dissociative effects that are recreationally sought. Neurobehavioural effects are dose-related, ranging from a mild to moderate stimulation with restlessness and euphoria (at 100–200mg doses), to a dissociated state characterised by hallucinations, paranoia, perceptual distortions, delusional beliefs, ataxia and out-of-body experiences at doses higher than 1,000mg. These experiences are referred to as ‘robo-ing’, ‘robo-copping’ or ‘robo-tripping’ (see Table) [7] , [45] , [48] , [106] , [107] , [108] .
Benzydamine
BZY acts as an analgesic and antipyretic, and is used for the topical treatment of inflammations of the oral and vaginal mucosae. BZY has been reported to be misused in several countries, including Brazil, Italy, Romania, Poland and Turkey, at high doses (i.e. 500–3,000mg) to reach stimulant effects on the CNS (e.g. euphoria, hyperreactivity, insomnia, abnormal behaviour, and psychotic symptoms, including paranoia and visual hallucinations) [109] , [110] , [111] , [112] , [113] , [114] , [115] . BZY diversion issues might involve young people and the concomitant use of alcohol/cannabis [38] , [39] , [43] .
Even though the molecular mechanism underlying the psychoactive and reinforcing effects of BZY is still unknown, a central cannabinoidergic mechanism of action has been hypothesised (see Table) [48] , [116] . Informal self-reports, hosted by internet drug fora and social networks, have contributed to the diffusion of BZY abuse, providing information about routes of administration, dosages and substance preparation from commercial products, as well as advice about other psychotropic substances to be used in combination with BZY to both enhance its pleasurable effects and dampen undesired ones [38] , [39] , [43] .
Antihistamine drugs
Promethazine
As a histamine (H) 1 receptor antagonist, promethazine is commonly used for symptomatic relief from nausea and vomiting, allergic conditions, motion sickness and the common cold. Often available with codeine in common cough suppressants, its abuse potential appears related to its calming and sedating effect, and enhancement of other coâ€ingested substances, such as benzodiazepines and opioids [34] , [48] , [117] .
The abuse of promethazine mixed with a soft drink and candy, with some variants, including purple-coloured alcohol (‘purple drank’), has become popular among young people for its euphoric effects and easy accessibility [12] , [28] , [37] . Despite being preferred to other substances, such as benzodiazepines, for the treatment of anxiety and sleep disorders in substance-dependent patients, promethazine has been reported to be misused among people with a SUD or an opioid dependence as a substitute for another drug (e.g. if the desired drug is unavailable or too costly) or to augment the effects of inadequate opioid dosing (i.e. to delay the onset of opioid withdrawal) (see Table) [118] , [119] , [120] , [121] .
Chlorphenamine
As a first generation H 1 -receptor antagonist, chlorphenamine is used as a cheap sleep aid or anxiolytic [13] . Chlorphenamine has potent antimuscarinic properties, and its abuse has been related to pleasurable feelings such as euphoria, which reinforces the repetitive use of the drug and the possibility of developing drug dependence, but might also cause psychotic symptoms in predisposed individuals (e.g. people with mental illnesses or individuals concomitantly abusing other drugs) [122] .
Together with dextromethorphan in cough and cold suppressants, or simultaneously consumed with serotoninergic drugs, it might cause significant serotonin toxicity [107] , [123] , [124] , [125] . A fatality has been registered involving chlorphenamine used concomitantly with an opioid [126] . The abuse of chlorphenamine has been described by data collected from the Texas Poison Center Network Toxic Exposure Surveillance System, and its intended use or abuse appears to be increasing, particularly among young people [127] .
Diphenhydramine
Diphenhydramine is an OTC drug acting on peripheral and central H 1 receptors, causing reduction of allergic symptoms and sedation, respectively [128] . The abuse of diphenhydramine appears related to multiple potential mechanisms of action, including a potent competitive antagonism on muscarinic receptors, causing sinus tachycardia, xerostomia, mydriasis, blurred vision, ileus, urinary retention, CNS depression, agitation, hyperactivity or psychosis [107] , [128] .
At high dosage and concomitantly assumed together with other drugs (e.g. alcohol, cannabis and stimulants), diphenhydramine can have a stimulatory effect in children and young adults, such as elevated mood, increased energy levels and mild euphoria, instead of the sedating properties seen in adults [128] . Increased dopaminergic neurotransmission in the mesolimbic pathway is thought to cause rewarding properties and drug-seeking behaviour [107] , [128] . There are no examples of at-risk groups that are prone to this misuse.
Hyoscine butylbromide
Also known as scopolamine butylbromide, hyoscine butylbromide is a plant-derived anticholinergic agent, commonly used as an antispasmodic drug [35] . A dose of 10mg or more is used to control intestinal and other smooth muscle spasms, for the symptomatic relief of irritable bowel syndrome and as a premedication in anaesthesia [35] . Its use and abuse as a psychoactive substance has previously been reported among young people, who obtain it from proprietary products (e.g. Buscopan [Sanofi]) [35] .
At supratherapeutic dosages (from 1.2mg as a single dose, while the recommended dose for adults is one to two tablets of 0.3mg as a single dose), it exerts potent CNS effects, including restlessness, excitement, euphoria, disorientation, irritability and characteristic delirium-like states with auditory, visual and tactile hallucinations, altered mood, insomnia and cognitive dysfunctions [33] , [35] , [129] . An advisory warning was issued in 2016 by the European Monitoring Centre for Drugs and Drug Addiction regarding 17 intoxications involving cocaine containing scopolamine, although it has not been formally notified as an NPS (see Table) [130] , [131] .
The non-medical use of prescription drugs and OTC medications for recreational purposes is a global health concern because of the unpredictable effects of some drugs used in abnormal dosages and unlicensed administration, but also owing to the possibility of their diversion in the context of polydrug misuse [13] . It is particularly concerning that drugs that have not been considered as being potentially abused, such as loperamide, might be diverted at high dosages, and possibly cause serious cardiotoxic effects and fatalities [102] , [103] .
Abuse of drugs during the COVID-19 pandemic
The COVID-19 outbreak has challenged public health policies owing to additional concerns relating to drug users and people with SUDs [132] , [133] . Individuals in this vulnerable category might be exposed to additional risks, such as physical problems (e.g. hepatic/renal dysfunctions, neuropathies, obesity, cardiovascular diseases), psychological comorbidities (e.g. mood and anxiety disorders, psychoses); homelessness; incarceration; economic difficulties; and other socioeconomic issues deriving from drug addiction [133] , [134] , [135] , [136] , [137] , [138] . Moreover, the COVID-19 pandemic is impacting drug markets. There have been reports of supply shortages of numerous drugs (e.g. opioids) at the street level; price increases for consumers on the black market; and reductions in purity [133] , [134] , [139] . These issues, in combination with a general economic loss, can encourage shifts to more risky drug-using behaviours, such as the:
- Use of domestically produced substances;
- Use of prescription/OTC drugs;
- Mixing with cheaper drugs (such as ‘street benzos’) and synthetic cannabinoids [139] .
Access to drug services is disrupted by quarantine, social distancing and other restrictive measures adopted to stop the spread of COVID-19 [133] , [134] , [135] , [137] , [140] . In addition, community pharmacies are being challenged by staff shortages, service disorganisation and self-isolation, meaning that the recent crisis creates an urgent requirement for expanded drug service provision, to protect vulnerable populations and minimise additional burdens on the health system [133] , [137] , [141] .
Pharmacists’ role in drug abuse prevention, education and assistance
As more users turn from street drugs to prescription/OTC products, pharmacists must increase their vigilance when supplying medicines, and be aware of medicines’ potential to end up on the black market [57] . Pharmacists have long taken responsibility for assuming an important role in substance abuse prevention and education, and they are enhancing their services during and after the pandemic to support their patients [142] . As healthcare providers, they should participate in, or contribute to, the development of specific prevention and assistance programmes within healthcare organisations or public services [143] , [144] . They should also avoid potentially risky prescribing practices (e.g. prescribing larger quantities of pain medication than is clinically needed), and collaborate with outpatient and ambulatory care providers to prevent substance abuse following discharge.
Pharmacists should engage in open communication to provide reassurance to patients and develop a trusting relationship, especially in vulnerable populations who might be less confident in communicating diversion and misuse issues to healthcare professionals. Pharmacists might be able to help identify patients who may have problems related to substance abuse, and refer them to the appropriate service (e.g. mental or addiction services) [145] , [146] , [147] , [148] . Additionally, pharmacists should be involved in ensuring safe and effective medication-use systems, including the development of the pharmacotherapeutic elements of drug detoxification protocols and organisational responsibilities for medication supply, distribution and control [145] .
Implication for practice
Pharmacists can prevent and control drug diversion behaviours, thereby reducing the negative impacts of their misuse by:
- Giving clear information about the effects medications may have, providing advice about any possible drug interactions;
- Making drug records that might prevent consultations with multiple doctors and subsequent duplicate prescriptions (‘doctor shopping’) for a drug with misuse potential.
It is vital that pharmacists ensure the continuity of care for people who use drugs and people with drug use disorders by facilitating access to community maintenance programmes (e.g. provision of methadone or buprenorphine to opioid users) [149] , [150] . Harm-avoiding interventions could be adopted, including guidance for facilitating controlled substance prescribing [132] , [137] , [151] . Telehealth, for monitoring drug-dependent patients while providing access to virtual support groups through online meetings during the pandemic, could also be put in place [132] , [141] , [152] .
In the context of a trusted pharmacist–patient relationship, pharmacists should inform at-risk individuals of drug dosages and drug interactions, and counsel them on harmful combinations among medications, as well as interactions between medications and alcohol or other illicit substances [153] . The use of drug combinations, including several CNS-depressants (e.g. benzodiazepines, opioids, gabapentinoids), together with OTC products, alcohol or other illicit substances should be discouraged [18] , [154] , [155] .
Finally, clinicians and pharmacists should be aware of potential pressures from patients to prescribe more drugs than needed; of excessive sales of prescription/OTC products, which might be diverted and abused; and of the risk of eventual aggression towards pharmacy staff [156] . Developing multidisciplinary support platforms, including both health and social support, could help reduce mental distress owing to misinformation among users, as could teaching problem-solving strategies to cope with drug abuse (e.g. the management of stress to prevent relapses during the pandemic) [157] . Telemental health might provide users with prevention interventions, through telepsychiatry, digital platforms, dedicated hotlines and mental health apps [158] , [159] , [160] .
The abuse of prescription and OTC drugs has become of increasing public concern across the globe. The current drug scenarios are greatly challenging healthcare providers and pharmacists, particularly during the COVID-19 pandemic. These healthcare professionals are recommended to be vigilant and develop strategies to ensure continuity of care for people who use drugs and people with drug use disorders, and prevent possible medicines misuse and diversion.
Author details
Stefania Chiappini is an MD, psychiatrist and a PhD student; Amira Guirguis is a PhD, MPharm; John Martin Corkery is a BA Hons, MSc; and Fabrizio Schifano is an MD, psychiatrist, and chair in clinical pharmacology and therapeutics. All work within the Psychopharmacology, Drug Misuse and Novel Psychoactive Substances Research Unit at the School of Life and Medical Sciences, University of Hertfordshire, UK. Amira Guirguis is also a senior lecturer and MPharm programme director at Swansea University Medical School, Institute of Life Sciences.
Corresponding author: Stefania Chiappini, [email protected]
Financial disclosure and conflict of interest statement
Fabrizio Schifano was a member of the UK Advisory Council on the Misuse of Drugs (ACMD) from 2011 to 2019; he is currently an EMA Advisory Board (psychiatry) member. John Martin Corkery is a member of the ACMD’s Novel Psychoactive Substances and Technical Committees. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
[1] Graddy R, Buresh ME & Rastegar DA. New and emerging illicit psychoactive substances. Med Clin North Am 2018;102(4):697–714. doi: 10.1016/j.mcna.2018.02.010
[2] Hughes GF, McElnay JC, Hughes CM & McKenna P. Abuse/misuse of non-prescription drugs. Pharm World Sci 1999;21(6):251–255. doi: 10.1023/a:1008788726842
[3] Schifano F. Recent changes in drug abuse scenarios: the new/novel psychoactive substances (NPS) phenomenon. Brain Sci 2018;13;8(12)pii:E221. doi: 10.3390/brainsci8120221
[4] Schifano F, Chiappini S, Corkery JM & Guirguis A. Abuse of prescription drugs in the context of novel psychoactive substances (NPS): a systematic review. Brain Sci 2018;22;8(4)pii:E73. doi: 10.3390/brainsci8040073
[5] Chiappini S & Schifano F. What about “pharming”? Issues regarding the misuse of prescription and over-the-counter drugs. Brain Sci 2020;10,736. doi: 10.3390/brainsci10100736
[6] Lessenger JE & Feinberg SD. Abuse of prescription and over-the-counter medications. J Am Board Fam Med 2008;21:45–54. doi: 10.3122/jabfm.2008.01.070071
[7] Levine DA. “Pharming”: the abuse of prescription and over-the-counter drugs in teens. Curr Opin Pediatr 2007;19(3):270–274. doi: 10.1097/MOP.0b013e32814b09cf
[8] Wood D. Drug diversion. Aust Prescr 2015;38:164–166. doi: 10.18773/austprescr.2015.058
[9] Schifano F, Napoletano F, Chiappini S et al . New emerging psychoactive substances and associated psychopathological consequences. Psychol Med 2019;22:1–13. doi: 10.1017/S0033291719001727
[10] McCabe SE, Veliz PT, Dickinson K et al . Trajectories of prescription drug misuse during the transition from late adolescence into adulthood in the USA: a national longitudinal multicohort study. Lancet Psychiatry 2019;6(10):840–850. doi: 10.1016/S2215-0366(19)30299-8
[11] Scherbaum N, Schifano F & Bonnet U. New psychoactive substances (NPS): a challenge for the addiction treatment services. Pharmacopsychiatry 2017;50(3):116–122. doi: 10.1055/s-0043-102059
[12] Burns JM & Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil 2013;4:75–82. doi: 10.2147/SAR.S36761
[13] Cooper RJ. Over-the-counter medicine abuse: a review of the literature. J Subst Use 2013;18(2):82–107. doi: 10.3109/14659891.2011.615002
[14] Jouanjus E, Falcou A, Deheul S et al . Detecting the diverted use of psychoactive drugs by adolescents and young adults: a pilot study. Pharmacoepidemiol Drug Saf 2018;27:1286–1292. doi: 10.1002/pds.4624
[15] McCarthy M. Prescription drug abuse up sharply in the USA. Lancet 2007;369:1505–1506. doi: 10.1016/S0140-6736(07)60690-4
[16] Advisory Council on the Misuse of Drugs. Diversion and illicit supply of medicines. 2016. Available at: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/580296/Meds_report-_final_report_15_December_LU__2_.pdf (accessed November 2020)
[17] United Nations Office on Drugs and Crime. The non-medical use of prescription drugs. Policy direction issues. 2011. Available at: http://www.unodc.org/docs/youthnet/Final_Prescription_Drugs_Paper.pdf (accessed November 2020)
[18] United Nations Office on Drugs and Crime. World drug report 2019. 2019. Available at: https://wdr.unodc.org/wdr2019/ (accessed November 2020)
[19] National Institute on Drug Abuse. Drug misuse and addiction. 2020. Available at: https://www.drugabuse.gov/publications/drugs-brains-behavior-science-addiction/drug-misuse-addiction (accessed November 2020)
[20] Arillotta D, Schifano F, Napoletano F et al . Novel opioids: systematic web crawling within the e-psychonauts’ scenario. Front Neurosci 2020;18;14:149. doi: 10.3389/fnins.2020.00149
[21] Orsolini L, Corkery JM, Chiappini S et al. New/designer benzodiazepines: an analysis of the literature and psychonauts’ trip reports. Curr Neuropharmacol 2020;18(9)809–837 . doi: 10.2174/1570159X18666200110121333
[22] Corazza O & Roman-Urrestarazu A. Handbook of Novel Psychoactive Substances: What Clinicians Should Know about NPS. New York: Routledge; 2018.
[23] Wilens TE & Kaminski TA. Prescription stimulants: from cognitive enhancement to misuse. Pediatr Clin North Am 2019;66(6):1109–1120. doi: 10.1016/j.pcl.2019.08.006
[24] Corazza O, Bersani FS, Brunoro R et al. The diffusion of performance and image-enhancing drugs (PIEDs) on the internet: the abuse of the cognitive enhancer piracetam. Subst Use Misuse 2014;49(14):1849–1856. doi: 10.3109/10826084.2014.912232
[25] Medical Dictionary for Regulatory Activities (MedDRA) Version 21. 2018. Available at: https://www.meddra.org/sites/default/files/guidance/file/smq_intguide_21_0_english.pdf (accessed November 2020)
[26] American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition (DSM-IV) . Washington DC: American Psychiatric Association; 1994.
[27] American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition (DSM-5) . Washington DC: American Psychiatric Association; 2013.
[28] Agnich LE, Stogner JM, Miller BL & Marcum CD. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict Behav 2013;38(9):2445–2449. doi: 10.1016/j.addbeh.2013.03.020
[29] Bonnet U & Scherbaum N. How addictive are gabapentin and pregabalin? A systematic review. Eur Neuropsychopharmacol 2017;27(12):1185–1215. doi: 10.1016/j.euroneuro.2017.08.430
[30] Chiappini S & Schifano F. Is there a potential of misuse for quetiapine? Literature review and analysis of the European Medicines Agency/European Medicines Agency adverse drug reactions database. J Clin Psychopharmacol 2018;38(1):72–79. doi: 10.1097/JCP.0000000000000814
[31] Evans EA & Sullivan MA. Abuse and misuse of antidepressants. Subst Abuse Rehabil 2014;5:107–120. doi: 10.2147/SAR.S37917
[32] Francesconi G, Orsolini L, Papanti D et al . Venlafaxine as the ‘baby ecstasy’? Literature overview and analysis of web-based misusers’ experiences. Hum Psychopharmacol 2015;30: 255–261. doi: 10.1002/hup.2476
[33] Jalali F, Afshari R & Babaei A. Smoking crushed hyoscine/scopolamine tablets as drug abuse. Subst Use Misuse 2014;49(7):793–797. doi: 10.3109/10826084.2014.880178
[34] Jensen LL, Rømsing J & Dalhoff K. A Danish survey of antihistamine use and poisoning patterns. Basic Clin Pharmacol 2017;120:64–70. doi: 10.1111/bcpt.12632
[35] Kummer S, Rickert A, Daldrup T & Mayatepek E. Abuse of the over-the-counter antispasmodic butylscopolamine for the home synthesis of psychoactive scopolamine. Eur J Pediatr 2016;175(7):1019–1021. doi: 10.1007/s00431-015-2683-5
[36] Lähteenmäki R, Neuvonen PJ, Puustinen J et al . Withdrawal from long-term use of zopiclone, zolpidem and temazepam may improve perceived sleep and quality of life in older adults with primary insomnia. Basic Clin Pharmacol Toxicol 2019;124(3):330–340. doi: 10.1111/bcpt.13144
[37] Miuli A, Stigliano G, Lalli A et al . “Purple drank” (codeine and promethazine cough syrup): a systematic review of a social phenomenon with medical implications. J Psychoactive Drugs 2020;1–10. doi: 10.1080/02791072.2020.1797250
[38] Opaleye ES, Noto AR, Sanchez Zv et al . Recreational use of benzydamine as a hallucinogen among street youth in Brazil. Braz J Psychiatry 2009;31(3):208–213. doi: 10.1590/s1516-44462009000300005
[39] Opaleye ES, Sanchez ZM, Moura YG et al . An anti-inflammatory as a recreational drug in Brazil. Addiction 2011;106(1):225. doi: 10.1111/j.1360-0443.2010.03196.x
[40] Reeves RR, Ladner ME, Perry CL et al . Abuse of medications that theoretically are without abuse potential. South Med J 2015;108(3):151–157. doi: 10.14423/SMJ.0000000000000256
[41] Schifano F & Chiappini S. Is there such a thing as a ‘lope’ dope? Analysis of loperamide-related European Medicines Agency (EMA) pharmacovigilance database reports. PLoS One 2018;13(10):e0204443. doi: 10.1371/journal.pone.0204443
[42] Schifano F & Chiappini S. Pregabalin: a range of misuseâ€related unanswered questions. CNS Neurosci Ther 2019;25:659–660. doi: 10.1111/cns.13115
[43] Schifano F, Corazza O, Marchi A et al . Analysis of online reports on the potential misuse of benzidamine. Riv Psichiatr 2013;48(3):182–186. doi: 10.1708/1292.14286
[44] Stahl SM & Grady MM. Stahl’s Essential Psychopharmacology: Prescriber’s Guide. Cambridge: Cambridge University Press; 2017.
[45] Stanciu CN, Penders TM & Rouse EM. Recreational use of dextromethorphan, “robotripping”: a brief review. Am J Addict 2016;25(5):374–377. doi: 10.1111/ajad.12389
[46] Stassinos GL & Klein-Schwartz W. Bupropion “abuse” reported to US poison centers. J Addict Med 2016;10(5):357–362. doi: 10.1097/ADM.0000000000000249
[47] Vento AE, Kotzalidis GD, Cacciotti M et al. Quetiapine abuse fourteen years later: where are we now? A systematic review. Subst Use Misuse 2020;55(2):304–313. doi: 10.1080/10826084.2019.1668013
[48] Zaprutko T, Koligat D, Michalak M et al. Misuse of OTC drugs in Poland. Health Policy 2016;120(8):875–881. doi: 10.1016/j.healthpol.2016.06.008
[49] Hernandez SH & Nelson LS. Prescription drug abuse: insight into the epidemic. Clin Pharmacol Ther 2010;88(3):307–317. doi: 10.1038/clpt.2010.154
[50] Lipari RN, Williams M & Van Horn SL. Why do adults misuse prescription drugs? In: The CBHSQ report. Rockville (MD): substance abuse and mental health services administration (US). 2017. PMID: 28968046
[51] McCabe SE, Cranford JA & West BT. Trends in prescription drug abuse and dependence, co-occurrence with other substance use disorders, and treatment utilization: results from two national surveys. Addict Behav 2008;33(10):1297–1305. doi: 10.1016/j.addbeh.2008.06.005
[52] Fortuna RJ, Robbins BW, Caiola E et al. Prescribing of controlled medications to adolescents and young adults in the United States. Pediatrics 2010;126(6):1108–1116. doi: 10.1542/peds.2010-0791
[53] Kelly BC, Wells BE, Leclair A et al. Prescription drug misuse among young adults: looking across youth cultures. Drug Alcohol Rev 2013;32(3):288–294. doi: 10.1111/dar.12016
[54] D’Souza RS & Eldrige JS. Prescription drug monitoring program. In: StatPearls . Treasure Island (FL): StatPearls Publishing; 2020.
[55] Jouanjus E, Guernec G, Lapeyreâ€Mestre M & the French Addictovigilance Network. Medical prescriptions falsified by the patients: a 12â€year national monitoring to assess prescription drug diversion. Fundam Clin Pharmacol 2018;32(3):306–322. doi: 10.1111/fcp.12356
[56] Soussan C, Andersson M & Kjellgren A. The diverse reasons for using novel psychoactive substances: a qualitative study of the users’ own perspectives. Int J Drug Policy 2018;52:71–78. doi: 10.1016/j.drugpo.2017.11.003
[57] Sukkar E & Osborne K. Pharmacists can help prevent misuse of prescription drugs by young people. Pharm J 2017;298:7902. doi: 10.1211/PJ.2017.20202998
[58] Evoy KE, Teng C, Encarnacion VG et al . Comparison of quetiapine abuse and misuse reports to the FDA adverse event reporting system with other second-generation antipsychotics. Subst Abuse 2019;13:1178221819844205. doi: 10.1177/1178221819844205
[59] Klein L, Bangh S & Cole JB. Intentional recreational abuse of quetiapine compared to other second-generation antipsychotics. West J Emerg Med 2017;18(2):243–250. doi: 10.5811/westjem.2016.10.32322
[60] Mattson ME, Albright VA, Yoon J & Council CL. Emergency department visits involving misuse and abuse of the antipsychotic quetiapine: results from the Drug Abuse Warning Network (DAWN). Subst Abuse 2015;9:39–46. doi: 10.4137/SART.S22233
[61] Heilbronn C, Lloyd B, McElwee P et al . Trends in quetiapine use and non-fatal quetiapine-related ambulance attendances. Drug Alcohol Rev 2013;32(4):405–411. doi: 10.1111/dar.12028
[62] Kim S, Lee G, Kim E et al . Quetiapine misuse and abuse: is it an atypical paradigm of drug seeking behavior? J Res Pharm Pract 2017;6(1):12–15. doi: 10.4103/2279-042X.200987
[63] Reeves RR & Brister JC. Additional evidence of the abuse potential of quetiapine. South Med J 2007;100(8):834–836. doi: 10.1097/SMJ.0b013e3180f62d53
[64] Electronic medicines compendium. Gabapentin. 2020. Available at: https://www.medicines.org.uk/emc/product/2362/smpc (accessed November 2020)
[65] Electronic medicines compendium. Pregabalin. 2020. Available at: https://www.medicines.org.uk/emc/product/1761/smpc (accessed November 2020)
[66] Chiappini S & Schifano F. A decade of gabapentinoid misuse: an analysis of the European Medicines Agency’s ‘suspected adverse drug reactions’ database. CNS Drugs 2016;30(7):647–654. doi: 10.1007/s40263-016-0359-y
[67] Schifano F. Misuse and abuse of pregabalin and gabapentin: cause for concern? CNS Drugs 2014;28(6):491–496. doi: 10.1007/s40263-014-0164-4
[68] Al-Husseini A, Wazaify M & Van Hout MC. Pregabalin misuse and abuse in Jordan: a qualitative study of user experiences. Int J Ment Health Addict 2018;16(3):642–654. doi: 10.1007/s11469-017-9813-4
[69] Baird CR, Fox P & Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: a survey among substance misusers. Eur Addict Res 2014;20(3):115–118. doi: 10.1159/000355268
[70] Buttram ME & Kurtz SP. Preliminary evidence of pregabalin misuse among prescription and/or illicit opioid (mis)users. J Psychoactive Drugs 2020;52(2):172–175. doi: 10.1080/02791072.2020.1734695
[71] Crossin R, Scott D, Arunogiri S et al . Pregabalin misuse-related ambulance attendances in Victoria, 2012–2017: characteristics of patients and attendances. Med J Aust 2019;210(2):75–79. doi: 10.5694/mja2.12036
[72] Advisory Council on the Misuse of Drugs. Addendum to advice on the anticonvulsant drugs pregabalin and gabapentin. 2018. Available at: https://www.gov.uk/government/publications/advice-on-the-anticonvulsant-drugs-pregabalin-and-gabapentin/addendum-to-advice-on-the-anticonvulsant-drugs-pregabalin-and-gabapentin-october-2018 (accessed November 2020)
[73] Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol 2013;9(2):155–162. doi: 10.1007/s13181-013-0292-0
[74] Hockenhull J, Black JC, Haynes CM et al . Nonmedical use of benzodiazepines and Z-drugs in the UK. Br J Clin Pharmacol 2020. doi: 10.1111/bcp.14397
[75] Griffiths RR & Johnson MW. Relative abuse liability of hypnotic drugs: a conceptual framework and algorithm for differentiating among compounds. J Clin Psychiatry 2005;66 Suppl 9:31–41. PMID: 16336040
[76] Rousselet M, Feuillet F, Gerardin M et al . The French addictovigilance network clinical assessment: Z-drugs, true false twins. Expert Opin Drug Saf 2017;16(9):1063–1069. doi: 10.1080/14740338.2017.1346084
[77] Jaffe JH, Bloor R, Crome I et al . A postmarketing study of relative abuse liability of hypnotic sedative drugs. Addiction 2004;99:165–173. doi: 10.1111/j.1360-0443.2003.00631.x
[78] Advisory Council on the Misuse of Drugs. Advice on the control of Z-drugs (zaleplon, zolpidem and zopiclone). 2013. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/237037/ACMD_advice_Z_drugs.pdf (accessed November 2020)
[79] Marsden J, White M, Annand F et al . Medicines associated with dependence or withdrawal: a mixed-methods public health review and national database study in England. Lancet Psychiatry 2019;6(11):935–950. doi: 10.1016/S2215-0366(19)30331-1
[80] Electronic medicines compendium. Zyban 150mg prolonged release tablets. 2019. Available at: https://www.medicines.org.uk/emc/medicine/2948/ (accessed November 2020)
[81] Hu LY, Lu T & Chen YT. Have we underestimated the possibility of bupropion sustained-release addiction? Aust N Z J Psychiatry 2016;50,925–926. doi: 10.1177/0004867416632928
[82] McCormick J. Recreational bupropion abuse in a teenager. Br J Clin Pharmacol 2002;53(2):214. doi: 10.1046/j.0306-5251.2001.01538.x
[83] Reeves RR & Ladner ME. Additional evidence of the abuse potential of bupropion. J Clin Pyschopharmacol 2013;33,584–585. doi: 10.1097/JCP.0b013e318295fe2f
[84] Stall N, Godwin J& Juurlink D. Bupropion abuse and overdose. CMAJ 2014;186(13):1015. doi: 10.1503/cmaj.131534
[85] Namdari B. Venlafaxine abuse in a patient with schizophrenia and prior history of substance dependence: a case report. J Addict Dis 2013;32(4):393–395. doi: 10.1080/10550887.2013.849974
[86] Bosse GM, Spiller HA & Collins AM. A fatal case of venlafaxine overdose. J Med Toxicol 2008;4(1):18–20. doi: 10.1007/BF03160945
[87] Leonard JB & Klein-Schwartz W. Characterization of intentional-abuse venlafaxine exposures reported to poison control centers in the United States. Am J Drug Alcohol Abuse 2019;45(4):421–426. doi: 10.1080/00952990.2019.1599382
[88] Benotsch EG, Koester S, Martin AM et al . Intentional misuse of over-the-counter medications, mental health, and polysubstance use in young adults. J Community Health 2014;39(4):688–695. doi: 10.1007/s10900-013-9811-9
[89] Le VT, Norris Turner A, McDaniel A et al . Nonmedical use of over-the-counter medications is significantly associated with nonmedical use of prescription drugs among university students. J Am Coll Health 2018;66(1):1–8. doi: 10.1080/07448481.2017.1356312
[90] Cooper RJ. ‘I can’t be an addict. I am.’ Over-the-counter medicine abuse: a qualitative study. BMJ Open 2013;3(6):e002913. doi: 10.1136/bmjopen-2013-002913
[91] MacDonald R, Heiner J, Villarreal J & Strote J. Loperamide dependence and abuse. BMJ Case Rep 2015;2015:bcr2015209705. doi: 10.1136/bcr-2015-209705
[92] Powell JW & Presnell SE. Loperamide as a potential drug of abuse and misuse: fatal overdoses at the Medical University of South Carolina. J Forensic Sci 2019;64(6):1726–1730. doi: 10.1111/1556-4029.14115
[93] Ali M, Mujahid A, Bulathsinghala CP & Surani S. Cardiac arrhythmia secondary to loperamide abuse and toxicity. Cureus 2020;12(2):e6936. doi: 10.7759/cureus.6936
[94] Antoniou T & Juurlink DN. Loperamide abuse. CMAJ 2017;189(23):E803. doi: 10.1503/cmaj.161421
[95] Atoot A, Sholem S, Khaddash I & Zuberi J. Transient brugada pattern induced by loperamide abuse. Cureus 2020;12(5):e8037. doi: 10.7759/cureus.8037
[96] Katz KD, Cannon RD, Cook MD et al . Loperamide-induced torsades de pointes: a case series. J Emerg Med 2017;53(3):339–344. doi: 10.1016/j.jemermed.2017.04.027
[97] Marraffa JM, Holland MG, Sullivan RW et al . Cardiac conduction disturbance after loperamide abuse. Clin Toxicol (Phila) 2014;52(9):952–957. doi: 10.3109/15563650.2014.969371
[98] Nattel S. An emerging malignant arrhythmia epidemic due to loperamide abuse: underlying mechanisms and clinical relevance. JACC Clin Electrophysiol 2016;2(7):790–792. doi: 10.1016/j.jacep.2016.10.009
[99] Rasla S, Parikh P, Hoffmeister P et al . Unexpected serious cardiac arrhythmias in the setting of loperamide abuse. R I Med J (2013). 2017;100(4):33–36. PMID: 28375418
[100] Stefek B, Wolfe LT & Cohen M. Brugada syndrome associated with adolescent loperamide abuse. Pediatrics 2018;142(4):e20181423. doi: 10.1542/peds.2018-1423
[101] Wightman RS, Hoffman RS, Howland MA et al . Not your regular high: cardiac dysrhythmias caused by loperamide. Clin Toxicol (Phila) 2016;54(5):454–458. doi: 10.3109/15563650.2016.1159310
[102] Vakkalanka JP, Charlton NP & Holstege CP. Epidemiologic trends in loperamide abuse and misuse. Ann Emerg Med 2017;69(1):73–78. doi: 10.1016/j.annemergmed.2016.08.444
[103] Eggleston W, Marraffa JM, Stork CM et al . Notes from the field: cardiac dysrhythmias after loperamide abuse — New York, 2008–2016. MMWR Morb Mortal Wkly Rep 2016;65(45):1276–1277. doi: 10.15585/mmwr.mm6545a7
[104] Food and Drug Administration. FDA limits packaging for anti-diarrhea medicine loperamide (Imodium) to encourage safe use. 2019. Available at: https://www.fda.gov/drugs/drug-safety-and-availability/fda-limits-packaging-anti-diarrhea-medicine-loperamide-imodium-encourage-safe-use (accessed November 2020)
[105] Feldman R & Everton E. National assessment of pharmacist awareness of loperamide abuse and ability to restrict sale if abuse is suspected. J Am Pharm Assoc . 2020;S1544–3191(20)30264-8. doi: 10.1016/j.japh.2020.05.021
[106] Martinak B, Bolis RA, Black JR et al . Dextromethorphan in cough syrup: the poor man’s psychosis. Psychopharmacol Bull 2017;47(4):59–63. PMID: 28936010
[107] Sansgiry SS, Bhansali AH, Bapat SS & Xu Q. Abuse of over-the-counter medicines: a pharmacist’s perspective. Integr Pharm Res Pract . 2017;6:1–6. doi: 10.2147/IPRP.S103494
[108] Schwartz RH. Adolescent abuse of dextromethorphan. Clin Pediatr (Phila) 2005;44(7):565–568. doi: 10.1177/000992280504400702
[109] Acar YA, Kalkan M, Cetin R et al . Acute psychotic symptoms due to benzydamine hydrochloride abuse with alcohol. Case Rep Psychiatry 2014;290365. doi: 10.1155/2014/290365
[110] Anand JS, Lukasik-GlÄ™bocka M & Korolkiewicz RP. Recreational abuse with benzydamine hydrochloride (tantum rosa). Clin Toxicol 2007;45(2):198–199. doi: 10.1080/15563650600981210
[111] Ballesteros S, Ramón MF & MartÃnez-Arrieta R. Ingestions of benzydamine-containing vaginal preparations. Clin Toxicol (Phila) 2009;47(2):145–149. doi: 10.1080/15563650801938670
[112] Can B, Oz I, Ozer H & Simsek T. Hallucinations after ingesting a high dose of benzydamine hydrochloride. Clin Psychopharmacol Neurosci 2016;14(4):407–408. doi: 10.9758/cpn.2016.14.4.407
[113] DoÄŸan M, Yılmaz C, Çaksen H & Güven AS. A case of benzydamine HCL intoxication. East J Med 2006;11(1):26–28. Available at: https://eastjmed.org/jvi.aspx?pdir=ejm&plng=eng&un=EJM-65807&look4= (accessed November 2020)
[114] Gürü M, Åžafak Y, Cengiz GF & Kuru E, Örsel S. Chronic psychosis related to benzydamine hydrochloride abuse. Neurocase 2019;25(3–4):156–158. doi: 10.1080/13554794.2019.1617318
[115] Rotolo MC, Pellegrini M, Solimini R et al . ‘Smart drugs’, the new drugs on the web: two cases of acute intoxication. Biochim Clin 2014;38(3):268–271. Available at: https://www.researchgate.net/publication/281928450_Smart_drugs_the_new_drugs_on_the_web_Two_cases_of_acute_intoxication (accessed November 2020)
[116] Avvisati R, Meringolo M, Stendardo E et al . Intravenous self-administration of benzydamine, a non-steroidal anti-inflammatory drug with a central cannabinoidergic mechanism of action. Addict Biol 2018;23(2):610–619. doi: 10.1111/adb.12516
[117] Tsay ME, Procopio G, Anderson BD & Klein-Schwartz W. Abuse and intentional misuse of promethazine reported to US poison centers: 2002 to 2012. J Addict Med 2015;9(3):233–237. doi: 10.1097/ADM.0000000000000124
[118] Chiappini S, Corkery JM, Schifano F & Guirguis A. Beyond the purple drank. Study of promethazine abuse according to the European Medicines Agency (EMA) adverse drug reactions (ADR) reports. J Psychopharmacol 2020; In press.
[119] Clatts M, Giang Le M, Goldsamt L & Colón-López V. Nonmedical use of promethazine hydrochloride among heroin injectors in Vietnam: unrecognized risks and unintended consequences. Subst Use Misuse 2010;45(4):515–527. doi: 10.3109/10826080903452520
[120] Lynch KL, Shapiro BJ, Coffa D et al . Promethazine use among chronic pain patients. Drug Alcohol Depend 2015;150:92–97. doi: 10.1016/j.drugalcdep.2015.02.023
[121] Shapiro BJ, Lynch KL, Toochinda T et al . Promethazine misuse among methadone maintenance patients and community-based injection drug users. J Addict Med 2013;7(2):96–101. doi: 10.1097/ADM.0b013e31827f9b43
[122] Banerji S & Anderson IB. Abuse of coricidin HBP cough & cold tablets: episodes recorded by a poison center. Am J Health Syst Pharm 2001;58(19):1811–1814. doi: 10.1093/ajhp/58.19.1811
[123] Dickerson DL, Schaepper MA, Peterson MD & Ashworth MD. Coricidin HBP abuse: patient characteristics and psychiatric manifestations as recorded in an inpatient psychiatric unit. J Addict Dis 2008;27(1):25–32. doi: 10.1300/J069v27n01_03
[124] Kirages TJ, Sulé HP & Mycyk MB. Severe manifestations of coricidin intoxication. Am J Emerg Med 2003;21(6):473–475. doi: 10.1016/s0735-6757(03)00168-2
[125] Monte AA, Chuang R & Bodme M. Dextromethorphan, chlorphenamine and serotonin toxicity: case report and systematic literature review. Br J Clin Pharmacol 2010;70:6:794–798. doi: 10.1111/j.1365-2125.2010.03747.x
[126] Kinoshita H, Tanaka N, Jamal M et al . A fatal case due to cough syrup abuse. Soud Lek 2012;57(4):69–70. PMID: 23121038
[127] Baker SD & Borys DJ. A possible trend suggesting increased abuse from coricidin exposures reported to the Texas Poison Network: comparing 1998 to 1999. Vet Hum Toxicol 2002;44(3):169–171. PMID: 12046973
[128] Saran JS, Barbano RL, Schult R et al . Chronic diphenhydramine abuse and withdrawal. A diagnostic challenge. Neurol Clin Pract 2017;7(5):439–441. doi: 10.1212/CPJ.0000000000000304
[129] Cheng SW, Hu WH, Hung DZ & Yang DY. Anticholinergic poisoning from a large dose of Scopolia extract. Vet Hum Toxicol 2002;44(4):222–223. PMID: 12136971
[130] Ham S, Tae KK, Kim K et al . Drug abuse and psychosis: new insights into drug-induced psychosis. Exp Neurobiol 2017;26(1):11–24. doi: 10.5607/en.2017.26.1.11
[131] Lakstygal AM, Kolesnikova TO, Khatsko SL et al . DARK classics in chemical neuroscience: atropine, scopolamine, and other anticholinergic deliriant hallucinogens. ACS Chem Neurosci 2019;10(5):2144–2159. doi: 10.1021/acschemneuro.8b00615
[132] Enforcement Administration. COVID-19 information page. 2020. Available at: www.deadiversion.usdoj.gov/coronavirus.html (accessed November 2020)
[133] European Monitoring Centre for Drug and Drug Addiction. The implications of COVID-19 for people who use drugs (PWUD) and drug service providers. 2020. Available at: http://www.emcdda.europa.eu/publications/topic-overviews/covid-19-and-people-who-use-drugs_en (accessed November 2020)
[134] Chiappini S, Guirguis A, John A et al . COVID-19: the hidden impact on mental health and drug addiction. Front Psychiatry 2020;11:767. doi: 10.3389/fpsyt.2020.00767
[135] Department of Health and Social Care and Public Health England. COVID-19: guidance for commissioners and providers of services for people who use drugs or alcohol. 2020. Available at: https://www.gov.uk/government/publications/covid-19-guidance-for-commissioners-and-providers-of-services-for-people-who-use-drugs-or-alcohol/covid-19-guidance-for-commissioners-and-providers-of-services-for-people-who-use-drugs-or-alcohol (accessed November 2020)
[136] Pfefferbaum B & North CS. Mental health and the covid-19 pandemic. NEJM 2020;383(6):510–512. doi: 10.1056/NEJMp2008017
[137] Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med 2020. doi: 10.7326/M20-1212
[138] Zhu S, Wu Y, Zhu CY et al . The immediate mental health impacts of the COVID-19 pandemic among people with or without quarantine managements. Brain Behav Immun 2020;pii:S0889–1591(20)30601-2. doi: 10.1016/j.bbi.2020.04.045
[139] United Nations. COVID-19 causes some illegal drug prices to surge, as supplies are disrupted worldwide. 2020. Available at: https://news.un.org/en/story/2020/05/1063512 (accessed November 2020)
[140] Volkow ND. Coping with the collision of public health crises: COVID-19 and substance use disorders. 2020. Available at: https://directorsblog.nih.gov/2020/04/21/coping-with-the-collision-of-public-health-crises-covid-19-and-substance-use-disorders/ (accessed November 2020)
[141] Green TC, Bratberg J & Finnell DS. Opioid use disorder and the COVID 19 pandemic: a call to sustain regulatory easements and further expand access to treatment. Subst Abus 2020;41(2):147–149. doi: 10.1080/08897077.2020.1752351
[142] Hogue MD, Hogue HB, Lander RD et al . The nontraditional role of pharmacists after Hurricane Katrina: process description and lessons learned. Public Health Rep 2009;124(2):217–223. doi: 10.1177/003335490912400209
[143] Substance Abuse and Mental Health Services Administration. COVID-19 and opioid treatment programs. 2020. https://www.samhsa.gov/sites/default/files/sample-otp-covid-19-faqs.pdf (accessed November 2020)
[144] Wazaify M, Hughes CM & McElnay JC. The implementation of a harm minimisation model for the identification and treatment of over-the-counter drug misuse and abuse in community pharmacies in Northern Ireland. Patient Educ Couns 2006;64(1–3):136–141. doi: 10.1016/j.pec.2005.12.008
[145] American Society of Health-System Pharmacists. ASHP statement on the pharmacist’s role in substance abuse prevention, education, and assistance. Am J Health-Syst Pharm 2016;73:e267–270. doi: 10.2146/sp140002
[146] Cooper RJ. Surveillance and uncertainty: community pharmacy responses to over the counter medicine abuse. Health Soc Care Community 2013;21(3):254–262. doi: 10.1111/hsc.12012
[147] Sheridan J, Manning V, Ridge G et al . Community pharmacies and the provision of opioid substitution services for drug misusers: changes in activity and attitudes of community pharmacists across England 1995–2005. Addiction 2007;102(11):1824–1830. doi: 10.1111/j.1360-0443.2007.02016.x
[148] Wagner AG & Guerra de Andrade A. Pharmacist professionals in the prevention of drug abuse: updating roles, and opportunities. Brazil J Pharmac Sciences 2010;46(1). doi: 10.1590/S1984-82502010000100003
[149] Becker WC & Fiellin DA. When epidemics collide: coronavirus disease 2019 (COVID-19) and the opioid crisis. Ann Intern Med 2020. doi: 10.7326/M20-1210
[150] Simeone R. Doctor shopping behavior and the diversion of prescription opioids. Subst Abuse 2017;11. doi: 10.1177/1178221817696077
[151] Levander XA & Wakeman SE. Covid-19 will worsen the opioid overdose crisis is we don’t prepare now. STAT . 2020. Available at: https://www.statnews.com/2020/03/17/covid-19-will-worsen- the-opioid-overdose-crisis-if-we-dont-prepare-now / (accessed November 2020)
[152] World Health Organization. Alcohol and COVID-19: what you need to know. 2020. Available at: http://www.euro.who.int/__data/assets/pdf_file/0010/437608/Alcohol-and-COVID-19-what-you-need-to-know.pdf (accessed November 2020)
[153] Spoth R, Trudeau L, Shin C & Redmond C. Long-term effects of universal preventive interventions on prescription drug misuse. Addiction 2008;103(7):1160–1168. doi: 10.1111/j.1360-0443.2008.02160.x
[154] Chary M, Yi D & Manini AF. Candyflipping and other combinations: identifying drug–drug combinations from an online forum. Front Psychiatry 2018;9:135. doi: 10.3389/fpsyt.2018.00135
[155] Clinical Guidelines on Drug Misuse and Dependence Update 2017 Independent Expert Working Group. Drug misuse and dependence: UK guidelines on clinical management. Department of Health. 2017. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/673978/clinical_guidelines_2017.pdf (accessed November 2020)
[156] Crowley DM, Jones DE, Coffman DL & Greenberg MT. Can we build an efficient response to the prescription drug abuse epidemic? Assessing the cost effectiveness of universal prevention in the PROSPER trial. Prev Med 2014;62:71–77. doi: 10.1016/j.ypmed.2014.01.029
[157] Rajkumar RP. COVID-19 and mental health: a review of the existing literature. Asian J Psychiatr 2020;52:102066. doi: 10.1016/j.ajp.2020.102066
[158] Ransing R, Adiukwu F, Pereira-Sanchez V et al . Mental health interventions during the COVID-19 pandemic: a conceptual framework by early career psychiatrists. Asian J Psychiatry 2020;51:102085. doi: 10.1016/j.ajp.2020.102085
[159] Reger MA, Stanley IH & Joiner TE. Suicide mortality and coronavirus disease 2019 — a perfect storm? JAMA Psychiatry 2020. doi: 10.1001/jamapsychiatry.2020.1060
[160] Smith K, Ostinelli E & Cipriani A. Covid-19 and mental health: a transformational opportunity to apply an evidence-based approach to clinical practice and research. Evid Based Ment Health 2020;23:45–46. doi: 10.1136/ebmental-2020-300155
You may also be interested in

Drug-related deaths in Scotland rose by 10% in 2023
More than 300 people took overdose of propranolol in one year, report reveals

Projects awarded £5m funding for AI and drone technology to tackle UK drug deaths
Impact of federal negotiation of prescription drug prices
Subscribe to the economic studies bulletin, anna anderson-cook and anna anderson-cook senior fellow - arnold ventures richard g. frank richard g. frank director - center on health policy , senior fellow - economic studies.
August 19, 2024
Key takeaways:
- Negotiations for the Medicare Part D prices of 10 prescription drugs—enabled by The Inflation Reduction Act—has just been completed.
- The Centers for Medicare and Medicaid Services (CMS) reported a $6 billion reduction in spending associated with the new negotiated prices, evaluated at 2023 volumes of prescriptions.
- Using publicly-available data, the authors estimate savings consistent with CMS reporting, and find that 51.4% of the estimated savings ($3.28 billion) is accounted for by 3 drugs.
- 20 min read
The much anticipated first set of negotiations on the prices of brand-name prescription drugs for the Medicare Part D program has been completed. The 2026 Maximum Fair Prices (MFP) for 10 selected drugs have been established. The Inflation Reduction Act (IRA) included provisions that enabled the secretary of the Department of Health and Human Services to negotiate the prices for certain prescriptions drugs by amending the “non-interference clause” in Medicare Modernization Act that forbid Medicare from negotiating prescription drug prices. The drugs that were selected for negotiation had to be single source products that would be on the market for 9 years if they were a small molecule product or 13 years if they were a biological product by January 2026 when the MFP takes effect. The drugs selected for negotiation were among the 50 products with the highest level of Part D spending. 1
The Act sets out a variety of parameters that govern the first round of negotiations. One key feature is that the Act establishes an upper limit that the negotiation can take in arriving at the MFP that would become the transaction price in 2026. That upper limit is defined as a percentage of the non-federal average manufacturer (NFAMP) prices. 2 For selected drugs that have been approved less than 16 years earlier, that upper limit is equal to 75% of the NFAMP. For those more than 16 years post FDA approval the upper limit is 40% of the NFAMP. 3
On August 29, 2023, the Department of Health and Human Services announced the 10 drugs selected for negotiations. Table 1 lists the selected drugs, the year they were approved, the conditions they treat, the number of Medicare enrollees in Part D using each drug, and the gross Part D spending during calendar year 2023. Note that in 2026 no drug will have been approved for marketing less than 11 years and one will be 28 years post approval. The Table shows that those drugs account for nearly $50.5 billion in gross Part D drug spending and were purchased on behalf of 9.7 million Medicare beneficiaries.

In this paper we provide an estimate of the savings realized through the negotiation process. The Centers for Medicare and Medicaid Services (CMS) has reported that the reduction in spending associated with the new negotiated prices amounts to $6 billion evaluated at 2023 volumes of prescriptions. 4 Our analysis examines the differences in the prices that were realized through negotiation by Part D plans and those obtained through the IRA’s negotiation program for the 10 drugs selected for those negotiations. 5 We make use of publicly available data as the detailed price measures for the pre-IRA negotiation period, which would yield would more precise estimates, are confidential. We therefore combine data from multiple sources to approximate pre-IRA net prices to estimate pre-period spending. We then make use of the announced MFPs and apply them to recently reported volume metrics to obtain expected spending for the 10 drugs at the negotiated prices. In the next section, we briefly explain our estimation methods, followed by a presentation of our estimates. The last section discusses the estimates and important issues in interpreting the numbers. We also provide an appendix with a detailed example for one drug that illustrates the details of our estimation method.
Approach to estimating savings
In its simplest conception, our estimates of savings begin by estimating total net spending attributable to negotiations in Part D for the 10 drugs selected for negotiation as well as total spending net of manufacturer rebates in the absence of the IRA. The difference between those amounts is equal to estimated reduction in net spending for each drug that can be attributed to the introduction of federal price negotiations. Our approach is similar to that used by CMS; however, we rely only on publicly available data.
To accomplish this, we must first estimate the net spending by Medicare for the drugs selected for negotiation. We do so by reviewing and combining information from several data sources. They include data on sales from the CMS Drug Spending Dashboard and the CMS Fact Sheet on Negotiation, 6 rebates by therapeutic class reported by MedPac, and information on the relationship between Wholesale Acquisition Costs (WAC) and Medicare Part D gross sales process developed by the Congressional Budget Office. 7 As a check against these data, we also examined SSR Health data on net prices for the selected drugs. We make use of MedPac reporting on pre-IRA rebates by therapeutic class. Because SSR Health estimates of rebates incorporate a variety of revenue adjustments unrelated to manufacturer rebates—like discount coupons and other consumer discounts—we make use of the MedPac maximum rebates by therapeutic class for each drug to refine the net sales estimates prior to the IRA. 8 We believe that using the MedPac maximum rebate estimate is a conservative approach (resulting in lower net sales estimates in the pre-period) because several of the drugs selected for negotiation are mostly specialty drugs and those have been shown to have lower than average rebates for brand name drugs. Finally, the estimates of net spending were based on sales volumes for calendar year 2023. These sources of data and information are assembled to obtain drug specific Medicare Part D net spending estimates. A detailed example of how the information is used is reported in Appendix A.

Table 2 reports key data elements that were used in estimating sales resulting from negotiated prices. The table reports the following data elements for each of the 10 selected drugs:
- Gross Part D sales for the pre-IRA baseline (calendar year 2023)
- Pre-IRA manufacturer rebates to Part D plans as a percentage of gross sales
- Classification of each drug as a long or short monopoly drug
- The estimated net of manufacturer rebate sales for each drug in the pre-IRA baseline period
- The discount off of Part D gross drug costs at the Maximum Fair Price
- Estimated sales based on reported MFP under the IRA
- Estimated savings from the pre-IRA baseline.
The estimated impact on the government negotiations compared to sole reliance on prescription drug plans for the 10 drugs amounted to $6.37 billion. On average that meant that the average MFP was 22% below the pre-IRA price net of manufacturer rebates. As the Table highlights, 51.4% of the estimated savings or $3.28 billion is accounted for by 3 drugs (Enbrel, Stelara and Eliquis). Several drugs had large rebates in the pre-IRA period (e.g., Jardiance, Januvia, Farxiga, and Novolog), as noted in the third column of Table 2. It is therefore notable that the negotiations resulted in prices that were likely to be below the statutory ceiling price for those products. 9 For example, Januvia saw an 18-percentage point increase in the net of manufacturer rebate price due to negotiation. The Table also reveals that the largest price concessions were obtained for drugs that had the lowest pre-IRA rebates because of limited pre-IRA competition. This is the case for Stelara, Enbrel, and Imbruvica.
Comments on interpretation
The estimates presented represent savings stemming from the differences in sales based on prices negotiated by prescription drug plans pre-IRA and prices negotiated by the government due to the IRA’s creation of the drug negotiation program. We have also used the upper end of the rebate ranges for each class for most of the selected drugs; thus, they are likely to result in a lower baseline net spending estimate. We believe the estimates will most often represent lower bounds on the impact of allowing the government to negotiate some prices. The estimates are not meant to provide a full budgetary impact of the IRA but to focus on how allowing the government to negotiate affects prices relative to relying solely on Part D prescription drug plans. In that sense the estimates show that the government negotiations are especially significant for drugs where market forces were most limited and therefore had the least impact on producing price concessions. This was the intent of the policy design.
Finally, because the estimates rely on publicly available data and not the actual rebates and non-federal manufacturer prices, they are meant to provide an approximation of how the $6 billion in savings is distributed across the 10 selected drugs. Our methods of analysis are similar to those used by CMS, and so it is notable that the reliance on publicly available information leads us to roughly the same estimates of aggregate savings and the percentage reduction net spending as those reported by CMS. Thus, the estimates measure the ability of CMS to effectively negotiate prices and point to what can be expected from future rounds of negotiation as the program grows.
Related Content
Gerard F. Anderson, Richard G. Frank
April 17, 2023
Rena M. Conti, Richard G. Frank, Jonathan Gruber
December 1, 2021
Rena M. Conti, Jonathan Gruber
November 15, 2021
Methodology for estimating savings under IRA negotiations for Enbrel
This Appendix describes our methodology for estimating savings under IRA negotiations. We compare the estimated prices that were realized through manufacturer negotiations with Part D plans to those obtained through the IRA’s negotiation program. The reduction in net spending applying the MFP is our estimate of savings.
Our methodology follows that used by CMS which compares Part D spending net of manufacturer rebates in 2023 to spending applying the MFP to the 10 selected drugs. CMS finds that, in the aggregate, the difference in net spending at the lower MFP prices is about $6 billion and net spending is reduced by 22%. Our methods rely on publicly available data to estimate savings. We can closely approximate the total estimated savings by CMS across the 10 selected drugs (we estimate $6.3 billion in savings and a reduction in net spending of 22%). Our methods demonstrate how publicly available data can be used to approximate savings under IRA negotiations in the aggregate as well as give an approximation of how the savings are distributed across the 10 selected drugs.
This Appendix describes each step taken to estimate savings for Enbrel. The starting point for the estimate is gross Part D sales of Enbrel as reported by CMS for calendar year 2023. We estimate how much lower net spending would have been for the 10 selected drugs in 2023 if the drugs had been purchased at the MFP compared to our estimated Part D price net of manufacturer rebates.
We rely on average rebates as a share of gross drug costs under Part D reported by Medpac by therapeutic class to estimate manufacturer rebates for the selected drugs. To estimate net spending under the IRA we start with the difference between the WAC price and the MFP as announced by CMS. 10 Since WAC prices are actually somewhat higher than the prices that Part D plans pay to pharmacies for brand-name drugs, we propose a method for converting discounts off of the WAC price to discounts off of the average price paid to pharmacies under Part D. Results from a CBO report on the relationship between WAC prices and the average prices paid to pharmacies under Medicare Part D for brand-name drugs are used to make this adjustment.
Estimated net spending in 2023 after manufacturer rebates for Enbrel
Total gross spending over calendar year 2023 was $2.95 billion for Enbrel. This section explains how we estimate net spending after accounting for manufacturer rebates to Part D plans.
Manufacturer rebates. To approximate manufacturer rebates as a share of gross sales, we rely on a Medpac analysis that estimates average manufacturer rebates to Part D plans by therapeutic class. We use the high end of the range on average rebates for the therapeutic class for each of the 10 selected drugs to approximate manufacturer rebates. 11
Medpac reports a range for average manufacturer rebates as a share of gross sales by therapeutic class. Estimates of rebates as a share of gross sales for individual drugs within the therapeutic class could be outside of Medpac’s reported range. However, we believe that making the conservative assumption of taking the upper end of this range is the best available data source for our estimates.
For disease modifying agents that treat Rheumatoid arthritis such as Enbrel, Medpac reports that manufacturer rebates to Part D plans averaged between 20% and 29% in 2022. We take a conservative approach by using the upper end of this range and assume that manufacturer rebates to Part D plans for Enbrel are 29% of gross Part D sales. (Assuming higher rebates to Part D plans will reduce estimated savings).
We note that for drugs that treat diabetes, the Medpac analysis states that rebates averaged over 50% of gross sales. Since a specific upper bound is not provided by the Medpac report for this therapeutic class, we combine that information with reports on rebates on insulin products from Senate Finance Committee reports and assume that manufacturer rebates on the selected drugs that treat diabetes average 60% of gross sales. 12
While estimates of net price produced by SSR Health are frequently used by researchers, we choose not to rely on those data on rebates and discounts (even though their rebate estimates are drug specific) because our assessment of those data indicate that the rebates and discounts reported by the SSR Health are greater than the size of rebates received by Part D plans from manufacturers. The SSR Health discounts are average nationwide discounts across all payer types and therefore include purchasers that pay much lower net prices than Medicare such as Medicaid and the 340B program. Those programs receive statutory discounts that are quite large. The SSR Health data also include other types of discounts that do not apply to Medicare such as patient coupon cards.
Estimating net spending without the IRA . After accounting for manufacturer rebates, we estimate that net spending on Enbrel would be 29% lower than gross sales or $2,096 million (Appendix Table 1).
Estimating spending for Enbrel at the MFP
CMS has announced the MFPs for the 10 selected drugs. 13 The MFP for Enbrel is 67% below the WAC price. The next step involved converting that amount to a discount off the average price paid to pharmacies for Enbrel under Medicare Part D. This allows us to estimate the MFP discount off of gross part D sales of the drug in 2023.
WAC prices are inflated—that is—they are higher than the prices paid to pharmacies under Medicare Part D. CBO estimates that prices paid to pharmacies for specialty brand name drugs under Part D are 95% of WAC prices. 14 After accounting for the inflated WAC prices, a 67% discount off of the WAC price translates to a 65% discount off of the average price paid to pharmacies under Part D (see Appendix Table 3). 15
Because WAC prices exceed the prices used by Medicare as the basis for rebate, the rebates or discounts expressed as a percentage of the WAC overstate the percentage gap between the amount paid to pharmacies under Part D and net sales. The higher the discount off WAC, the smaller the impact of the necessary adjustment to the discount percentage (Appendix Table 4).
We now examine the case of Enbrel more closely. The cost of a prescription for Enbrel (30-day supply) at WAC prices is $7,105 (as reported by CMS). 16 CMS also reports that the MFP is 67% below the WAC price. So, a 30-day supply of this medication at the MFP would cost $2,355. Because Gross Part D drug costs average 95% of WAC prices for specialty brand-name drugs, the cost of a 30-day supply of Enbrel valued at the prices paid to pharmacies under Part D at the point of sale is $6,750. The difference between the cost of the prescription at the prices paid to pharmacies and the cost of the prescription for Enbrel at the MFP is $4,395. So, the MFP is 65% below the gross cost of the prescription under Part D.
Based on the CMS announcement, we estimate that the MFP for Enbrel is 65% below the value of gross Part D drug costs in 2023. Therefore, the net sales of Enbrel at the MFP would have been $1,025 billion if the drug had been available at the MFP in 2023.
Estimated savings under IRA negotiations
In the absence of the IRA, net sales for Enbrel after accounting for manufacturer rebates to Part D plans are estimated to be $2,096 million. Since the MFP is 65% below the price paid to pharmacies under Part D, then total sales of Enbrel at the MFP would be $1,025 million. Savings under the IRA would be equal to $2,096 million – $1,025 million = $1,070 million. This estimate shows that net spending at the MFP is about 50% lower than the net spending under Part D for Enbrel in 2023 after accounting for manufacturer rebates.
Considerations
This estimate uses gross Part D sales over the 2023 calendar year period to estimate savings under the IRA negotiations relative to the net prices paid by Part D plans after accounting for negotiated rebates with manufacturers. We know that the volume of these selected drugs purchased under Part D in 2026 will differ and have not attempted to adjust our estimates to account for changes in volume between 2023 and 2026. Most of the selected drugs are increasing in sales volume, therefore savings is likely to be higher in 2026 than $6 billion.
| Description | Rebates/Discounts | Calculation or Source | $ millions |
|---|---|---|---|
| Gross Part D Sales | none | CMS Fact Sheet for Selected Drugs | $2,952 |
| Manufacturer Rebates to Part D Plans | 29% of Gross Sales | =29% of $2,952 | $856 |
| Total NET Part D Sales – NO IRA | =$2,952 - $856 | $2,096 |
| Description | Rebates/Discounts | Calculation or Source | $ millions |
|---|---|---|---|
| Gross Part D Sales | none | CMS Fact Sheet for Selected Drugs | $2,952 |
| MFP Discount | 67% off WAC = 65% off Gross Sales (see Table 3) | 65.3% of $2,952 | $1,927 |
| Sales Under IRA at the MFP | $2,950 - $1,927 | $1,025 | |
| Savings Under IRA | $2,096 - $1,025 | $1,070 (The IRA lowers net spending by an estimated 51% for Enbrel) |
| Description | Amount | Calculation/Source | Explanation |
|---|---|---|---|
| WAC price for 30-day supply CY 2023 | $7,105 | CMS Fact Sheet | Cost of 30 day supply at WAC |
| MFP discount off WAC | 67% which equals $4,750 | = 67% of $7,105 | MFP discount is 67% of the WAC |
| Cost of 30 day supply at the MFP | $2,355 | = $7,105 - $4,750 | Prescription cost at WAC less MFP Discount |
| Part D prescription cost at price paid to pharmacies | $6,750 | = 95% of $7,105 | CBO finds the cost of a prescription at gross Part D prices is 95% of the cost of a prescription at WAC prices for specialty brand-name drugs |
| MFP dollar discount amount off price paid to pharmacies under Part D | $4,395 | =$6,750 – $2,355 | The difference between the price paid to pharmacies and the MFP |
| MFP Percentage Discount off prices paid to pharmacies under Part D | 65% | =$4,395/$6,750 | This 65% can be applies to the value of gross Part D drug costs to estimate sales at the MFP. |
| Rebate As Share of WAC | Net Price | Average Price Paid to Pharmacies (Source: CBO) | Rebate Amount After Adjusting for Inflated WAC Price | Rebates Expressed as a Share of Gross Drug Costs |
|---|---|---|---|---|
| 10% | 90% of WAC | 95% of WAC | (95% - 90%) of WAC = 5% of WAC | (5%/95%) = 5.3% |
| 20% | 80% of WAC | 95% of WAC | (95% - 80%) of WAC = 15% | (15%/95%) = 15.8% |
| 30% | 70% of WAC | 95% of WAC | (95% - 70%) of WAC = 25% of WAC | (25%/95%) = 26.3% |
| 40% | 60% of WAC | 95% of WAC | (95% - 60%) of WAC = 35% of WAC | (35%/95%) = 37% |
| 67% | 33% of WAC | 95% of WAC | (95% - 33%) of WAC = 62% of WAC | (62%/95%) = 65% |
The authors thank Loren Adler for helpful comments on an earlier draft of the paper.
Anna Anderson-Cook is currently a Senior Fellow at Arnold Ventures. She previously spent over 20 years as a Principal Analyst at the Congressional Budget Office focusing on research and cost estimates related to prescription drug policy. Arnold Ventures supports Brookings’ Center on Health Policy through its grants program and Dr. Cook’s contributions to this paper are based on her expertise and analysis alone.
The Brookings Institution is financed through the support of a diverse array of foundations, corporations, governments, individuals, as well as an endowment. A list of donors can be found in our annual reports published online here . The findings, interpretations, and conclusions in this report are solely those of its author(s) and are not influenced by any donation.
- For a complete description of the requirements for a negotiation eligible drug see Section 1192 of the Inflation Reduction Act or a summary produced by the Kaiser family Foundation at https://www.kff.org/medicare/issue-brief/explaining-the-prescription-drug-provisions-in-the-inflation-reduction-act/
- For some drugs the upper limit may be the Part D price net of manufacturer rebates if that is lower.
- In 2030 a third category of upper price limit is defined as 65% of FAMP for drugs approach 12 years earlier but less than 16.
- Medcare Drug Price Negotiation Program: Negotiated Prices for Initial Price Applicability Year 2026 (cms.gov)
- We follow the CMS approach to assessment by using the 2022-2023 volume of prescriptions for making price impact comparisons.
- https://www.cms.gov/files/document/fact-sheet-medicare-selected-drug-negotiation-list-ipay-2026.pdf
- Congressional Budget office, A Comparison of Brand Name Drug Prices Among Selected Federal Programs, February 2021. Available at https://www.cbo.gov/system/files/2021-02/56978-Drug-Prices.pdf .
- https://www.medpac.gov/wp-content/uploads/2024/07/July2024_MedPAC_DataBook_Sec10_SEC.pdf
- We cannot estimate the difference between the MFP and the ceiling price for higher rebate drugs where the ceiling price will be the net Part D price. We can only roughly estimate this since the Non-FAMPs are confidential
- Other sources used by researchers such as SSR Health also report discounts and rebates off WAC.
- See https://www.medpac.gov/wp-content/uploads/2024/07/July2024_MedPAC_DataBook_Sec10_SEC.pdf . Page 167. Entresto was not included in the therapeutic classes for which Medpac reported rebates. We assumed that Part D rebates for Entresto were similar to anticoagulants.
- Grassley-Wyden Insulin Report (FINAL 1).pdf (senate.gov)
- https://www.cms.gov/files/document/fact-sheet-negotiated-prices-initial-price-applicability-year-2026.pdf
- See Table 2 in the CBO report https://www.cbo.gov/publication/56978
- This estimated relationship between WAC and gross Part D drug costs is based on 2017 data. The results should still roughly apply to 2023 gross Part D drug costs used in this analysis. However, in future years, the gap between WAC prices and the prices paid to pharmacies under Medicare Part D is likely to grow. That is likely to occur because the new pharmacy DIR policy is expected to lower the amount paid to pharmacies at the point of sale by 7% on average starting in 2024 as more of the discounts provided by pharmacies are passed through at the point of sale. (See page 148 of the 2024 Medicare Trustees Report https://www.cms.gov/oact/tr/2024).
- See the CMS Fact Sheet on the negotiated prices https://www.cms.gov/files/document/fact-sheet-negotiated-prices-initial-price-applicability-year-2026.pdf
Economic Studies
Center on Health Policy
Wendell Primus, Vani Agarwal, Caitlin Rowley
July 22, 2024
Top of the Hill, Washington DC
10:00 am - 12:00 pm EDT
Gabriel R. Sanchez
July 3, 2024
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List

Addressing the challenge of high-priced prescription drugs in the era of precision medicine: A systematic review of drug life cycles, therapeutic drug markets and regulatory frameworks
Toon van der gronde.
1 Department of Pharmaceutical Sciences, Utrecht Institute for Pharmaceutical Sciences (UIPS), Utrecht University, Utrecht, the Netherlands
Carin A. Uyl-de Groot
2 Institute for Medical Technology Assessment, Department of Health Policy & Management, Erasmus University, Rotterdam, the Netherlands
Toine Pieters
Associated data.
All relevant data are within the paper and its supporting information files.
Recent public outcry has highlighted the rising cost of prescription drugs worldwide, which in several disease areas outpaces other health care expenditures and results in a suboptimal global availability of essential medicines.
A systematic review of Pubmed, the Financial Times, the New York Times, the Wall Street Journal and the Guardian was performed to identify articles related to the pricing of medicines.
Changes in drug life cycles have dramatically affected patent medicine markets, which have long been considered a self-evident and self-sustainable source of income for highly profitable drug companies. Market failure in combination with high merger and acquisition activity in the sector have allowed price increases for even off-patent drugs. With market interventions and the introduction of QALY measures in health care, governments have tried to influence drug prices, but often encounter unintended consequences. Patent reform legislation, reference pricing, outcome-based pricing and incentivizing physicians and pharmacists to prescribe low-cost drugs are among the most promising short-term policy options. Due to the lack of systematic research on the effectiveness of policy measures, an increasing number of ad hoc decisions have been made with counterproductive effects on the availability of essential drugs. Future challenges demand new policies, for which recommendations are offered.
A fertile ground for high-priced drugs has been created by changes in drug life-cycle dynamics, the unintended effects of patent legislation, government policy measures and orphan drug programs. There is an urgent need for regulatory reform to curtail prices and safeguard equitable access to innovative medicines.
1. Introduction
Global health care expenditures have been rising sharply, and drug costs are a major factor.[ 1 ] Recent public outcry about exceptionally high prescription drug prices have made this subject a popular media and political topic. Discussion of drug prices has moved from an academic and government level to a broader society level and now includes the evaluation of public impact. The price of medicines was one of the campaign issues in the 2016 Presidential election in the US [ 2 ; 3 ].
Many examples of high drug prices exist and are frequently discussed in the media. They include several types of therapeutic drugs and geographies. One often mentioned example is imatinib (brand name Gleevec ® ), a drug for chronic myeloid leukemia,which tripled in cost after the US Federal Drug Administration (FDA) allowed for a new indication. Novartis raised its price from $31,930 in 2005 to $118,000 per year in 2015 despite a huge increase in the volumes sold. The price hike occurred despite the fact that research costs for the new indication were included in the initial price.[ 4 – 6 ] Also in the US, the list price of sofosbuvir (Sovaldi ® ) is $84,000 for a 12-week treatment, or $1,000 a pill,[ 7 ] which has caused health plans to refuse routine coverage of this drug for hepatitis C virus (HCV) infection.[ 5 ; 8 ] Sovaldi ® alone accounted for 64% of US HCV-related spending in 2014, which totaled $12.3 billion.[ 9 ] Sovaldi ® could be cost effective, since it prevents the ultimate need for a liver transplant, but the financial impact is too high for US insurance companies to make it available for all patients with HCV infections.[ 2 ; 10 ; 11 ] In Spain and Latvia, the cost of a complete treatment of Solvadi ® was noted to be “unsustainable” by key stakeholders such as pharmacists and pharmaceutical policy experts.[ 12 ] The cost of an alternative combination of ledipasvir/sofosbuvir (Harvoni ® ), marketed by the same pharmaceutical company, Gilead Sciences, is comparable to a course of Sovaldi ® .[ 13 ] But the high prices for HCV drugs are not the exception. In another example, US patients suffering from cystic fibrosis were denied reimbursement for a new drug–ivacaftor (Kalydeco ® ), with an annual cost of $311,000[ 13 ; 14 ]–unless their health worsened on older, cheaper treatments.[ 13 ] The cost of pyrimethamine (Daraprim ® ), a 60-year old drug, rose from $13.50 to $750 per pill (a 5455% raise) after Turing Pharmaceuticals acquired the distribution licence. This has further sparked public debate.[ 15 – 17 ]. Additional price hikes in Mylan’s EpiPen ® from $94 ten years ago to $609 for a pack of two have caused additional public backlash, protests and US Congressional hearings.[ 18 – 20 ] The results of a recent trial,[ 21 ] which show that 74 patients needed to be treated for two years with the new cholesterol-lowering Evolocumab (Repatha ® ) to prevent one cardiovascular event, indicate that with the current list price of $14,523 per year, the prevention of one event would cost over $2 milion.[ 22 ] These and many more examples of high prices for medicines, however innovative, are untenable and frequently beyond the ability of individuals, health insurance companies or even governments in high-income countries to pay[ 23 ].
Governments and health insurers are struggling with the dramatic increase in costs of new medications.[ 7 ; 24 – 26 ] In December 2015, the US Senate issued a warning report on Sovaldi’s escalating drug price and its impact on the US health care system. The committee report said the Gilead Sciences pharmaceutical company had set the price as a benchmark to “raise the price floor” for its future hepatitis C-drugs like Harvoni, thus knowingly reducing the number of eligible patients for these superior treatments to cure HCV.[ 9 ] US congressional committees have opened enquiries into similar drug-pricing practices.[ 27 ] Simultaneously, on the other side of the Atlantic, the UK cost gatekeeper, the National Institute for Health and Care Excellence (NICE), initially rejected reimbursement for two costly cancer immunotherapies—nivolumab (Opdivo ® ) and trastuzumab/emtansine (Kadcyla ® )—despite fierce opposition by industry and patient groups.[ 28 ] In both cases, the costs were estimated to amount to £90,000 per patient per year.[ 29 ; 30 ] With a number of better targeted immunotherapies–that fit within highly promising precision medicine approaches–on their way to the market, the drug pricing and funding crisis is expected to deepen and reach a critical level for even the wealthiest countries.[ 31 ] The German government is planning to curb companies’ right to set launch-prices. Belgium, Luxembourg and the Netherlands are working together to seek a common approach to their price negotiations with drug firms. A January 2017 Lancet commentary co-authored by the Dutch Minister of Health Edith Schippers stated that: “We need meaningful efforts by both the pharmaceutical industry and governments to invest in new medicines, provide full transparency on costs, prices, and who pays what beforehand, and respect the legal space for governments to protect public health. If we don’t succeed in these efforts, we cannot guarantee people’s access to innovative and affordable medicines”[ 32 ].
On average, countries in the Organization for Economic Co-operation and Development (OECD) spend 17% of their health care budgets on pharmaceuticals;[ 24 ; 33 ] in some countries, this is even more.[ 25 ; 34 ] For low- and middle-income countries (LMIC), drug expenditure can be a critical public health problem[ 35 – 38 ] with some drugs out of reach for even well-insured patients.[ 26 ; 39 ] In some cases, to prevent striking increases in premiums or taxes, regulators are forced to limit access to healthcare,[ 13 ; 24 ; 40 – 42 ] which leaves patients without the best treatments.[ 43 ] Of concern, is that the pharmaceutical industry might be tempted to view these high-priced models as the direction for future drug pricing of new drugs that impact larger populations[ 13 ; 44 – 46 ].
The prescription drug price controversy is not new. In the 1990s, there were comparable heated debates on the high prices for interferons, paclitaxel (Taxol ® ) and HIV/AIDS medication.[ 1 ; 47 ; 48 ] Though the prices of these drugs were much lower than current new drug price levels, the fact that taxpayers had helped to pay for developing those innovative therapies at the time, generated public debate on fair pricing. In LMIC, where the need for HIV/AIDS medication was the highest, the fair-pricing issue was even more pressing, particularly with regard to the problematic availability of essential HIV medicines[ 38 ; 41 ].
Pharmaceutical expenditures are based on two factors: price and volume. This means that regulation can either aim to lower drug prices, or reduce usage.[ 34 ; 49 ; 50 ] On the one hand, there is a growing life expectancy (and aging population worldwide), while there are increasing medical options for disease control.[ 51 ; 52 ] Therefore, following drug innovation expectations and usage growth statistics, it is likely that costs will continue to rise.[ 53 ; 54 ] Many countries are striving towards universal health coverage, with guidance from the global public community,[ 55 – 57 ] to reduce individual catastrophic spending.[ 58 ] Although these countries are preventing individual catastrophic spending by pooling risks and costs, a sustainable solution to the problem of fast-rising drug costs is still necessary. The solution will require unprecedented measures to prevent health care costs from spiraling out of control[ 59 ].
Many articles have been written about the high cost of drugs. Most seek to define the cause of high drug prices in terms of government policies or industrial pricing strategies and propose related policy measures to combat the phenomenon. This review takes another angle, and presents a comprehensive analysis of the long-term dynamics of pharmaceutical markets, drug life cycles and the sometimes unintended, counterproductive effects of market interventions by governments and health insurers. The aim is to determine what has caused the recent exponential rise in drug prices, and what can be done in terms of measures around drug pricing to safeguard equitable access to innovative medicines.
The article is structured as follows. First, drug life-cycle dynamics are discussed. Next, government interventions aimed at reducing drug prices and their consequences are highlighted. Finally, we provide suggestions for alternative policy measures to reduce drug prices and improve access to innovative and essential medicines.
The prescription drug price controversy has been developing for several years now, but 2015 brought a significant change in public perceptions. Several new expensive drugs were introduced, and even old drugs were subject to price hikes. To find causes and possible solutions for recent price increases, a systematic review was performed.
Due to the wide selection of journals, PubMed was used as the search engine for peer-reviewed scientific articles. The PubMed search was performed on February 24, 2017. The search strategy was (Drugs OR medicines) AND (prices OR costs) NOT (Cost-effectiveness OR Clinical Trial OR treatment), published between January 1, 2014 and January 1, 2017, in English, and with full text available in PubMed. The articles were screened by TvdG based on the title and abstract to determine inclusion, and then read in full. Additional scientific articles were included based on the reference lists of selected journals since due to an inherent time-delay, systematically searching for topic-related articles in scientific peer-reviewed journals provided insufficient coverage of the controversy.
To include the most recent developments, additional searches in the databases of a select number of reputable English-edition international financial and daily online newspapers were performed. For this purpose, the Financial Times (FT), the New York Times (NYT), the Guardian and the Wall Street Journal (WSJ) were selected to ensure insight from both an American and European perspective, and from both financially focused and general newspapers.
The newspaper articles were limited to those published between January 1, 2015 and December 31, 2016. For the FT and the NYT the terms “Drug AND Pricing” were used in the LexisNexis search engine due to the availability of articles. The Guardian was searched for the exact combination “drug prices” using the Google-based search engine on their website. The WSJ was searched with ProQuest, with “Prescription AND Drug AND Prices” as search terms. The news articles selected by title only were all read and selectively added until there was a saturation of citations and information. The result of the searches and selection procedure is displayed in Fig 1 . To ensure the quality of reporting, the Prisma checklist was used.[ 60 ] This study was not registered with PROSPERO.

Schematic overview of the study selection process. FT: Financial Times, NYT: New York Times, WSJ: Wall Street Journal
Inclusion criteria
- Published in peer-reviewed journal or selected newspaper
- Published between January 1, 2014 and December 31, 2016 for scientific articles
- Published between January 1, 2015 and December 31, 2016 for newspaper articles
- Published in English
3. Life cycles and market dynamics
The justification for high prices for pharmaceuticals can be seen as part of the changing nature of drug life cycles and market dynamics. Further details on both these aspects are presented in this section.
Life cycles describe the market behavior of many products. Generally, the product life-cycle pattern is represented by a “bell shaped” graph, a parabola, as exhibited in Fig 2 . Though specifics can vary wildly, the general shape of the curve of investments during the drug development phase, exponential growth of sales after registration and decline through competition and patent term expiration is valid for most drugs.[ 61 ] Drug life cycles generally have four stages. First, there is a testing and approval trajectory. Second, after the drug is introduced there is market expansion, and the product is accompanied by growing expectations and drug indication extension. Next, drug maturity with a high sales volume is accompanied by rising criticism and disappointment regarding drug effectiveness and side-effects. Finally, there is contracting use and limited drug application. In most cases, this is a gradual process that involves the documentation of less favorable experiences and reports of the drug’s effectiveness and adverse reactions in everyday practice. Thus, a drug’s benefit-risk assessment and the resulting safety profile is under constant revision. Over time, newer and presumably better alternatives gain attention. This is part of an evolutionary process of selection and adaptation. Most brand-name medicines continue their careers as generics after their patents expire. On average this results in a 20–25 year therapeutic life-time in ‘the doctor’s bag’–the portfolio of drugs available to a doctor–due to therapeutic substitution and competition between branded drugs and generics[ 62 ].

General curve describing an innovator drug’s investments and earnings during R&D and market performance. The life cycle phases are indicated above the graph, and the phases of the R&D trajectory are below the graph. Own work.
What we tend to forget is that therapeutic drugs are inextricably linked to a dynamic mixture of opinions, practices and rituals and as such are important tokens of healing as a cultural process. Doctors, regulators, health care insurers, patients and politicians, however, prefer to believe that it is evidence-based medicine and not enthusiasm or belief about a particular drug that makes a drug more or less therapeutically effective. Dan Ariely, a behavioral economist at the Massachusetts Institute of Technology, and his research team have shown that a 10-cent pill doesn't kill pain as well as a $2.50 pill, even when they are identical placebos.[ 63 ] This may partly explain doctors’ and patients’ preferences for high-cost brand-named drugs over inexpensive, widely available, chemical and therapeutic generic equivalents[ 64 ].
3.1. Types of prescription drugs
This article refers to prescription drug prices, but there are distinct types of prescription drugs and this requires clarification. First, there are drugs that are under patent, with an exclusive producer and no direct competition. Then, there are generic drugs with an expired patent that allows for production by other manufacturers.
Biological drugs follow the life-cycle patterns of small molecules or conventional drugs, but higher prices are accepted and specific regulation of generic competition is in place. Oncological drugs are a separate category, because high prices are historically more common,[ 26 ] expected to rise,[ 5 ] and more acceptable given the severity of the indications. Laws are in place to incentivize the development and marketing of orphan drugs, which means they follow market dynamics that differ from conventional drugs. Finally, when the patent runs out, and other producers can manufacture the same drug, generics are introduced. In the case of biologicals, biosimilars compete with the innovator while following a specific set of regulations[ 65 ].
3.2. Patents and registration
The pharmaceutical industry is often characterized as a competitive sector in a free market, where the total supply and demand determine market price. However, according to business analysts, in a truly free and competitive market without patent regulation, it would be difficult to profit from new drug development.[ 66 ] This is why governments protect companies from competition during the life of a patent. In general, the term of a new patent is 20 years from the date on which the application for the patent was filed.[ 1 ; 40 ; 67 ; 68 ] This can be extended to 25 years.[ 69 ] In addition, in the US, the FDA can grant exclusive marketing rights upon a drug’s approval, which is generally concurrent with the length of a patent. The FDA usually grants new drug exclusivity for between seven years for orphan drugs[ 70 ] and five years for new chemicals, with an additional period of six months of exclusivity following pediatric approval[ 71 ; 72 ].
Patents are also granted for new chemical entities. This allows companies to charge high prices once the drug is ready for marketing.[ 1 ; 73 ] Patents then become public, which gives other producers the chance to further improve and develop the drug[ 41 ].
Patent timelines are limited, which provides an incentive for companies to shorten the drug development phase or look for disease areas with less stringent trial requirements. For example, there is more research in drugs for late-stage cancer than early-stage cancer, because of the less demanding and shorter trial trajectories[ 1 ; 74 ; 75 ].
The number of patents a company files, or alternatively the research and development (R&D) costs per patent filed, are often used as an output measure for the efficiency of drug development and the future of a firm.[ 76 ] Since most patented molecules do not make it to the market as an actual medicine, both datasets are incomplete representations of productivity[ 77 ].
In debating the patent system, some analysts state that basic human rights like health and access to essential medicines should be equitable[ 23 ] and should not be limited by property rights.[ 1 ; 6 ; 78 ; 79 ] Others use a utilitarian stance to argue that pharmaceutical companies are for-profit entities,[ 40 ; 73 ] and without patents these companies would not be incentivized to develop drugs.[ 1 ] This difference in viewpoint is illustrated by the litigation surrounding patents and compulsory licensing (see paragraph 4.2.7) in LMIC[ 73 ; 80 ; 81 ].
3.3. Developmental phase and registration
Pharmaceutical companies must register new drugs, which requires clinical studies and safety tests. This is a high-risk, high-cost and low-output endeavor. The odds of having a drug approved varies from approximately 24% (for systemic anti-infective drugs) to less than 10% (for drugs used to treat cardiovascular, gastrointestinal or metabolic disorders).[ 82 ] On average, it takes a company ten years to register a drug.[ 41 ; 68 ; 77 ] Thus, companies have to decide on projects that have a good chance of becoming registered drugs several years in the future.[ 83 ] The drug development process requires investments, estimated at between $60 million to $2.6 billion,[ 6 ; 67 ; 68 ; 77 ] though most estimations are close to $800 million from bench research to prescription medicine.[ 33 ; 66 ; 72 ; 84 ; 85 ] The wide range of cost estimates is due to the lack of clear data and various methods of calculation, and depends on the type of drug and the trial data required,[ 72 ] as well as the size of the company developing the drug.[ 86 ] Development costs are highest for large companies due to their relatively high overhead and marketing costs[ 1 ; 76 ].
Historic examples illustrate what happens when the demonstration of medicine safety during development is not adequately regulated. An exemplary case is the thalidomide drug disaster that took place between 1958 and 1962.[ 24 ; 71 ; 74 ] This drug for morning sickness resulted in malformations in the extremities (phocomelia syndrome) of thousands of babies born to women who had taken thalidomide during pregnancy.[ 71 ] Regulatory reaction to drug safety alerts often involves the introduction of more stringent regulations requiring more safety and efficacy studies, which leads to more dropouts in the development process and an increase in invested time and costs.[ 41 ; 74 ] Regulatory agencies are criticized by many parties for being either too stringent (delaying innovation and increasing costs) or not stringent enough (allowing dangerous drugs to be marketed).[ 72 ] Arthur Daemmrich, a US historian, discussed this tension between safety management and drug innovation and was the first to use the term ‘double bind trade-off phenomenon’[ 87 ].
The imperative of regulation makes it more difficult for smaller companies to register drugs, thus limiting the number of firms with the critical mass and financial means to invest in drug research. This situation limits viable competition from smaller companies[ 41 ] for Big Pharma—the collective sector of large pharmaceutical companies. That is why most new drugs that received a positive reaction from the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) between 2010 and 2012 were filed by large (59%) or intermediate-sized (28%) companies.[ 88 ] Small enterprises are important during early phases of development. However, in later phases, if the success of a new chemical entity developed by a small company is likely, a large pharma company will often buy the small company or purchase the licence for the new medicine[ 88 ].
R&D for medicines has been declining.[ 41 ; 67 ; 68 ; 89 ] Higher investments, however, will not necessarily fill R&D pipelines with new promising drug compounds. R&D has recently yielded fewer drugs than in years past, since low-hanging fruits have already been harvested.[ 66 ; 89 ; 90 ] Furthermore, there are many drugs with promising results in phase II settings that have not made it to phase III settings.
Regulatory agencies allow drugs to be released to the market based on safety and effectivity, but not with reference to price or cost-effectiveness.[ 70 ; 91 ] This means the price and reimbursement of a drug are determined only after registration approval and insurance company and/or government negotiation[ 91 ].
3.4. Post registration and reimbursement
Once a drug is registered for a specific disease indication, manufacturers can apply for reimbursement. Many public health care systems allow the government to control drug prices. Some base the acceptability of a price on the Incremental Cost Effectiveness Ratio (ICER) and budget impact.[ 10 ; 83 ; 92 ] This means companies have to assess the volume of sales[ 93 ] and the price at which they are reimbursed, and then offer a price based on that estimate.[ 90 ; 94 ] Then, negotiations take place between the company and the reimbursing agent or government to determine an acceptable price for each stakeholder.
A drug’s reimbursed price can be lower than the pharmacy retail price or list price. This makes patients aware of drug prices, since they will have to pay for the difference out of pocket. Such pricing and reimbursement schemes can be a tool to make patients switch to cheaper or generic drugs, and make manufacturers of high-priced drugs lower their prices to prevent patients from making this switch.[ 36 ; 59 ; 95 ] Manufacturers argue that patient co-payments can cause adherence problems,[ 96 ; 97 ] especially for expensive[ 6 ; 98 ] and psychiatric drugs.[ 99 ] This means physicians and patients prefer drugs without co-payments.[ 78 ; 100 ] To circumvent this situation, producers have implemented patient-assistance programs, which are discussed in paragraph 5.6.
Companies want to make the highest possible profits in each country by differentiating prices,[ 40 ] but they also want their prices to be similar across countries and close to competitors to reduce the incentive for parallel importation.[ 24 ; 101 ; 102 ] Governments worldwide want innovative new drugs to be available as quickly as possible, so their population can profit from them. High drug prices may incentivize companies to develop and launch their new drugs faster.[ 1 ; 40 ; 73 ] On the other hand governments also want to have affordable drugs for everyone at the lowest possible price, to reduce healthcare spending[ 1 ; 5 ; 78 ].
In the US, the government does not control reimbursement,[ 16 ; 33 ; 91 ] based on the assumption that the free market will drive the pharmaceutical industry to compete, which will result in lower prices.[ 92 ; 103 – 105 ] Thus, pharmaceutical companies set their own prices, which allows for market calculations aimed at maximizing profits.[ 106 – 108 ] As a consequence, US prescription drug prices are among the highest in the world.[ 6 ; 33 ; 109 ] For the uninsured, a cancer diagnosis is still a major cause of personal bankruptcy[ 110 ; 111 ].
In general, the manner in which drug list prices are determined is not transparent,[ 14 ; 57 ; 106 ; 112 ] so critics are pushing for more transparency.[ 77 ; 98 ; 113 ] Some differences in pricing between countries can be explained by differences in health care systems[ 4 ; 93 ; 114 ] and socio-economic dynamics,[ 78 ; 84 ] all of which lead to differences in ICERs. In some cases, a drug’s price is based on the old standard of care plus a premium,[ 5 ; 6 ] the uncertainty associated with the drug[ 93 ] or simply the government’s willingness to pay.[ 92 ; 115 ] Ultimately, a drug’s price is unrelated to the cost of development[ 4 ; 5 ; 51 ] or a country’s gross domestic product,[ 12 ] and only related to cost-effectiveness if the payer introduces this into negotiations[ 30 ; 53 ; 107 ].
3.5. Introduction and growth phase
When a drug is marketed, there is a level of market penetration.[ 61 ] It is important to the producer that the drug is brought into use as quickly as possible, since a patent limits the period of exclusivity and thus profit. Speeding up market penetration was historically accomplished by advertising to doctors, pharmacists and patients (see Fig 3 ).[ 116 ] In the US, doctors are paid directly for promotional activities,[ 112 ] though the Affordable Care Act (ACA) requires disclosure.

A simplified, schematic overview of stakeholders and relationships in the pharmaceutical market. Own work.
Direct-to-consumer advertising (DTCA) is controversial, since it increases demand, partially through inappropriate prescription. Doctors will prescribe drugs that are not indicated if they know they can satisfy their patients by doing so, or unnecessarily prescribe branded drugs. This increases costs without improving health.[ 117 ] Therefore, this practice has become illegal in most countries, but not in the US or New Zealand.[ 1 ; 117 – 199 ] This situation makes it harder for drug companies to create demand for their products in most countries, and reduces turnover in the early phase of developing a new drug.
In Europe, marketing to doctors and pharmacists is permitted, if it is medically substantiated.[ 120 ] This requires more expensive studies, and careful wording of the marketing message. Still, marketing is a large part of the pharmaceutical industry’s expenses. In fact, more money is spent on marketing than on R&D[ 24 ; 41 ; 121 ].
To market drugs to doctors and circumvent this regulation, trials are sometimes used as a marketing tool.[ 48 ] ‘Seeding trials’ are designed to ‘seed’ the use of a drug among patients and physicians, while they often offer no scientific purpose.[ 122 ; 123 ] Furthermore, medical centres that want to promote an image of being at the cutting edge of science are willing to test the newest available experimental treatments and compete for hosting profitable industry-sponsored clinical trials.[ 48 ] This was the case for interferons when companies were looking for an indication for this new experimental drug.[ 48 ] Numerous promising new drug compounds have followed suit[ 122 ; 123 ].
A pharmaceutical company’s marketing push is somewhat mitigated by the increased tendency to work with local guidelines.[ 25 ; 50 ; 73 ; 124 ] This often improves the quality of care for the patients, but makes it harder for new drugs to enter the doctor’s bag. In addition to the cycle of updating those guidelines–which generally happens once every five years, or less frequently[ 46 ]–new drugs have to show superiority to already available drugs. Furthermore, the price of a new drug is often higher than that of older drugs that might be available as generics, which increases the demand for proven superiority[ 41 ].
3.6. Mature phase
During a drug’s patent life, doctors and pharmacists play a crucial role in the choice for one drug over another.[ 1 ] These professionals need to inform the patient about their pharmaceutical options, and a drug’s effectiveness and costs. This is why advertising aimed at brand recognition continues during the mature phase of a drug’s life cycle[ 61 ].
Several studies have shown that if there are financial incentives for doctors to choose one drug over another, the one that is most beneficial to the doctor’s finances is most likely to be prescribed.[ 1 ; 125 ] In the US, where Medicare and Medicaid reimbursement is based on a 6% mark-up of the price of cancer drugs,[ 53 ; 110 ; 119 ] doctors have an incentive to select the more expensive option.[ 1 ; 5 ] This is another explanation for the high prices for drugs in the US. In order to help patient and doctors, the European Society for Medical Oncology and the American Society of Clinical Oncology have developed frameworks to assess the value of new cancer drugs[ 58 ; 126 ].
To combat incentives to prescribe expensive drugs, some health insurance companies in the US offer monthly payments of $350 to physicians who prescribe according to their guidelines, thus saving costs and improving patient health.[ 5 ] This scheme compensates a physician for earnings missed by prescribing less expensive drugs[ 5 ].
In this phase, companies often attempt to have their drug registered for additional indications, thus increasing the number of patients, to increase their sales volume. A larger patient base would logically make the cost per treatment lower, but this is often not the case[ 53 ; 111 ].
3.7. Declining phase
Several countries around the world have implemented preference policies, aimed at generic substitution.[ 1 ; 127 ; 128 ] This policy requires physicians to prescribe and pharmacists to dispense the cheapest available version of a drug, often generic, unless a more expensive one is medically necessary. This can be the case for drugs with a very small therapeutic window, like Tegretol ® (containing generically available carbamazepine) for the treatment of epileptic seizures.[ 127 ] For these drugs, the preference policy implies that new patients start on a generic drug, but those who have already reacted well to a branded version do not have to switch. The potential substitution rate differs per indication group[ 103 ; 127 ; 129 ; 130 ].
Generic substitution reduces the length of the life cycle of patented innovator drugs, since they are replaced by generics as soon as the patent expires. The time an innovator drug has to generate income is thus reduced, which makes higher prices during the exclusive phase necessary to generate the same turnover.
Doctors and patients are often hesitant to change the brand of the drug after patent expiry, since they are familiar with the innovator and trust it.[ 69 ; 96 ; 99 ; 128 ; 131 – 133 ] This explains why generic substitution only happens on a large scale if policy to enforce it is in place. In some countries, generic substitution by pharmacist’s initiative is mandatory;[ 78 ; 130 ] in others it is prohibited[ 99 ; 130 ].
Since roughly 2000, Big Pharma has been struggling with the patent cliff, a series of blockbuster drugs whose patents have expired. This has caused a significant loss of turnover due to generic substitution.[ 66 ; 67 ] The effect cannot be compensated for by new drug introductions, since relatively few new blockbuster drugs have been introduced.[ 103 ] This means that in order to maintain profitability, more revenue must be generated from fewer breakthrough drugs, which has led to increased prices for innovator drugs and increased merger and acquisition activity within the pharma industry. Over the last two decades, 60 pharma companies have merged into just ten pharma companies. This consolidation has helped Big Pharma gain more power to influence regulation and pricing policies while simultaneously diminishing competition (see Fig 3 )[ 134 ].
The patent expiry and substitution effect is expected to be smaller for the new generation of biologicals, because these biological drugs are more difficult to copy. Also, a drop in price in cases of biosimilar substitution is often just 20–30%,[ 65 ; 85 ; 135 ] whereas the price for conventional small molecules can fall by more than 70%[ 85 ; 99 ; 131 ].
Large pharmaceutical companies employ several tactics to extend the life cycles of their products, and reduce their loss of income due to patent expiry. Strategies to address this often include improved formulations (e.g. Seroquel ® XR, quetiapine), new indications (e.g. Zyban ® , bupropion), chiral switching (e.g. omeprazole (Prilosec ® )/esomeprazole (Nexium ® ) or citalopram (Celexa ® )/escitalopram (Lexapro ® )), combining drugs (e.g. Harvoni ® , containing ledipasvir and sofosbuvir), changing to over-the-counter (e.g. Prilosec ® , omeprazole) and finally introducing an authorized generic[ 6 ] (e.g. atorvastatin, Lipitor ® /Zarator ® )[ 67 ].
Another option is the development of pediatric dosage recommendations and formulations, which allows for extension of market exclusivity by the FDA for six months.[ 71 ; 72 ] Finally, ‘pay for delay’, the act of paying off generic competition so an innovator can maintain market exclusivity, is fiercely contested by both the European Commission and US Federal Trade Commission, but the practice has been increasing over the last few years[ 67 ].
Some drug brands are so strong that, even after the loss of market exclusivity, doctors and patients continue to privilege them over generic drugs. Examples include brands like Viagra ® , Prozac ® and Aspirin ® .[ 136 ] For over-the-counter medicines, in particular, branding is a relevant mechanism to maintain market share, since consumer name recognition is a more important factor in product choice when there is no medical professional role.
Fierce competition between generics can cause companies to offer prices that become too low to be sustained by the offering company. Sustainability can be threatened by the rising prices of raw materials and production costs,[ 137 ; 138 ] which lead to a failure of supply and shortages.[ 95 ; 131 ; 138 ] This situation can also force competitors for a specific drug to completely retreat from the market, which leads to fewer producers[ 139 ] or even a new monopolistic position for a generic,[ 137 ; 140 ] as recently seen with Lanoxin ® (digoxin) and Daraprim ® (pyrimethamine). The result is that even for generic medicines we increasingly see steep price increases.[ 141 ].
3.8. Changing life cycle dynamics, effects on drug pricing and profitability
Drug life cycle analysis indicates a trend of shortening life cycles and pharmaceutical companies experiencing more difficulty achieving high, sustainable sale volumes during the past two decades than before. Since a company’s income is based on volumes multiplied by price (equals value), the first strategy to maintain high revenues is to increase price.[ 142 ] Despite regulated pricing, this practice results in drug spending growth matching overall medical spending growth[ 39 ].
On average, the top ten pharmaceutical companies have a profit margin of 20%;[ 2 ] those noted in the S&P 1500 have a net profit margin of 16%, compared to 7% for all other companies in the index.[ 119 ] This means that even though companies experience more difficulty in achieving long-term high-volume prescription drug sales, the higher drug prices compensate for the lower product turnover and safeguard Big Pharma’s high-profit profile. This is not surprising, because pharmaceutical companies are for-profit entities that wish to maximize their profits and increase share-holder value without breaking the law.[ 24 ; 40 ; 143 ] However, this approach means they may not automatically do what is best for society. Critics argue that more regulation is needed to counterbalance Big Pharma’s only-for-profit motive and force them to do what is best for all stakeholders.[ 15 ; 53 ; 104 ; 144 ; 145 ] Through a number of interventions (some more effective than others) governments and their regulators have tried to direct either the price of drugs or the availability of innovations. Government interventions to stimulate or curtail the pharmaceutical markets and the introduction of new procedural measures concerning drug patent licences and drug registration licences are discussed in the next chapter.
4. Drug innovation, regulation and pricing interventions
As stated previously, though the pharmaceutical market is often portrayed as a competitive market, it is not truly a free market. In addition to the patent system, skewed economic dynamics create further complexities. In free markets, a consumer decides on, buys, pays for and uses a product, whereas in healthcare, a doctor decides and the pharmacy or hospital pharmacy provides, the insurance company or government pays and the patient uses the product.[ 142 ; 146 ] An overview of the stakeholders and their relationships is given in Fig 3 . Financial incentives are not aligned with consumption, so companies’ pricing power is not related to how consumers value the products.
4.1 Unintended consequences of innovation and measures to stimulate drug safety
Drug regulation usually aims at making new drugs available while keeping costs down, however, there can be unintended consequences. For example, if drug production or distribution becomes too competitive to remain lucrative, the market can become so consolidated that drugs are no longer available.[ 140 ] Even worse, temporary or sustained monopolistic positions can arise due to market failure which can cause an increase in prices.[ 104 ; 106 ; 147 ] Hence, after specifically designing a policy to stimulate innovation and safety or reducing prices, potential consequences should be carefully monitored.
4.1.1 Orphan and priority drug regulations and potential consequences
There are cost-reduction strategies that could work by changing the trajectory of a drug’s development.[ 53 ; 68 ] The first option is to speed up innovation and regulatory approval, so that companies have less waiting time before marketing a drug and thus enjoy a longer profit-generating post-marketing patent life.[ 5 ] One way to do this is to accept surrogate parameters as trial endpoints to prove efficacy, which saves time.[ 135 ] Another innovative option is to harmonize regulation between countries, so companies only have to prove efficacy once.[ 135 ] However, because drugs are not necessarily priced based on investment costs, the effects of this approach on drug pricing might not be significant, or as we will see, harmonized regulations may actually be counterproductive.
One clear example where regulation is in place to speed up innovation is the field of orphan drugs. Generally, low patient volumes make it unattractive for pharmaceutical companies to invest in the development of orphan drugs.[ 148 – 150 ] Fabry disease, for example, has a prevalence of approximately 1 per 100,000 persons, thus making it unattractive for companies to develop drugs for these patients without further incentives.[ 149 ] That is why the orphan drug regulation was designed.
The benefits of developing drugs with an orphan status are exclusive licensing for seven years, faster assessments and lower taxes in the US. European regulatory bodies offer exclusive licensing for ten years, lower regulatory fees and scientific advice.[ 148 ; 150 ] Orphan drug legislation has worked, yielding at least 73 drugs for orphan indications in the European Union since the law passed in 2000[ 151 ] and 335 in the US since the FDA set regulations in 1983 and 2002[ 152 ].
Another incentive for companies in the US that develop orphan drugs is a priority review voucher, which is also available for tropical disease drugs. This voucher is awarded after a company develops a drug for an orphan disease and releases the patent. When applied, it allows companies to request an expedited review process for a new drug, which can speed up the regulatory process by several months. This approach allows a pharmaceutical company to stretch the patent period, and thus the mature, beneficial part of the life cycle of a subsequent blockbuster drug.[ 153 ] A well-used voucher could increase a company’s income by up to $300 million according to some estimates.[ 1 ; 154 ] The company can also sell the voucher to another company that is about to launch a similar drug for roughly the same price[ 154 ].
The moral argument for orphan drug regulation is that society, and medical science in particular, has an obligation to pursue new therapies for everyone, including people who suffer from orphan diseases.[ 149 ; 152 ] The downside of this policy is the non-utilitarian outcome that money is being used for diseases that very few people have, and that the same money could have been used for more relevant research reaching a larger population[ 1 ; 145 ; 151 ; 152 ].
Given that no price ceiling or maximum budget impact is imposed on an orphan drug’s regulatory design, orphan drugs are often very expensive. Prices are unrelated to effectiveness or prevalence, which means that regulatory bodies frequently label these drugs as not cost-effective.[ 148 ; 151 ] Also, the evidence for the effectiveness of orphan drugs is often lower in quality than required for regular drugs, and more side effects are tolerated than for other drugs[ 148 ].
In Europe, market exclusivity can be withdrawn after five years if a product has generated adequate profit. However, this has not happened for a single orphan drug to date.[ 149 ; 155 ] Even for orphan drugs that have lost exclusivity, no generic producer has ever created a competing product, and it is doubtful whether or not this would ever be attractive given the low patient numbers[ 151 ; 156 ].
Orphan drug regulation is an example of a policy working better than expected, thereby increasing healthcare budgets. Companies have tried to abuse this regulation for profitability purposes. For example, there was a request that malaria be designated for an orphan drug indication in Europe[ 71 ].
Orphan drug regulation is also used by pharmaceutical companies to register drugs so that multiple indications–not always orphan indications–can be added. For example, Gleevec ® (imatinib) has been marketed for several orphan indications,[ 149 ] yielding a turnover of $4.7 billion in 2012.[ 6 ] Rituximab, the world’s second most profitable drug, also holds multiple orphan drug indications, in addition to the use for common rheumatoid arthritis.[ 155 ] Although this approach increases drug volumes, companies usually do not reduce prices.[ 152 ; 157 ] Finally, pharmaceutical companies split common indications into groups that are small enough for their drugs to qualify for an orphan drug label[ 70 ; 149 ; 155 ].
Regulations for orphan drugs are quite effective, but should include compulsory price-ceiling measures to prevent tax-payers from paying twice, first for some of the R&D costs and second for reimbursement of overpriced drugs. Furthermore, the orphan drug label should only be applicable if a drug has not been granted access for another indication already. Currently, Revatio ® (sildenafil, also known as Viagra ® , for erectile dysfunction) is being sold as an orphan drug for pulmonary arterial hypertension[ 149 ].
4.1.2 The FDA’s unapproved drugs initiative and consequences
In June 2006, the FDA announced a new drug safety initiative with the goal of removing unapproved ‘old’ generic drugs with problematic safety profiles from the market . The FDA states that it uses a risk-based enforcement program in order to focus on products that pose the highest threat to public health and “without imposing undue burdens on consumers, or unnecessarily disrupting the market”.[ 158 ] However, the program has had unintended consequences. If a product is not officially approved by the FDA, the agency can require a New Drug Application from the manufacturer, which is reviewed to determine if the drug meets FDA standards. Inexpensive generic drugs that have been on the market for decades are studied anew, drug applications are filed and exclusive patent rights to sell the drug are given to the first manufacturer who meets the new FDA effectiveness standard. This manufacturer can then decide what to charge—with no competition. Exemplary is the patent flipping of the formerly inexpensive drug colchicine (less than $1 for 30 pills), used to treat flare-ups of gout. Now, after FDA review, just one manufacturer has the patent rights to market colchicine as Colcrys ® , and the retail price is almost $200 for 30 pills. How drug pricing measures could counteract these pricing strategies by the pharmaceutical industry is discussed below.
4.2 Possible drug-pricing measures
There are many ways to reduce spending on drugs. However, all are based on one of four general intervention options[ 36 ].
- Shift from expensive to cheap drugs, within the same class,
- Shift costs towards patients or insurers,
- Reduce drug prices,
- Reduce total drug uses.
It is not clear which mechanism is the most effective, but authorities in many countries often implement policies encompassing several of the abovementioned options. An overview of possible regulations and the mechanisms through which they reduce costs are given in Table 1 .
This table lists the policies that are in effect in various parts of the world, their effects and their unintended consequences. EU: European Union, USA: United States of America, DTCA: direct-to-consumer advertising, UK: United Kingdom, LMIC: Low- and Middle-Income Countries.
| Policy | Location | Mechanisms | Effects | Side effects |
|---|---|---|---|---|
| Patent laws | Worldwide | Increase innovation | Gives an incentive for innovation | Increased prices during patented period, reduced transparency in research |
| Orphan drugs | EU, USA | Increase innovation | Many new drugs have been developed | High prices, low quality evidence of effect |
| Biosimilars | EU, USA | Shift drugs | Availability of generic versions of biological drugs | Concerns about comparable effectivity and rare side effects due to fast market authorisation |
| Development cost reduction | EU, USA | Increase innovation, reduce price? | Faster entry of new drugs | Lower quality evidence |
| Limiting DTCA | EU | Reduce use, shift drugs | Reduced inappropriate prescriptions | Reduced awareness of new drugs for professionals and the public |
| Reference pricing | Several EU countries, Canada, Australia | Reduce price | Lower prices | Best payers get drugs first |
| Price ceilings | Several EU countries, Canada | Reduce price | Fewer price increases | Higher initial prices |
| Value-based pricing | Several EU countries | Reduce price, shift drugs | Decision making is more evidence-based, and treatments are rewarded for actual efficacy | Prices are set just below cost-effectiveness threshold, limitation of options |
| Preference policy, compulsory generic prescribing | Several EU countries | Shift drugs, shift payer, reduce price | Increased use of generic drugs | Shorter life cycle of patented drugs |
| Stimulate guideline adherence, pay for performance | EU, USA | Shift drugs, reduce price | Better prices and quality of healthcare | Limited options for treatment |
| Negotiation power through monopsony | New Zealand | Reduce price, shift payer | Reduced prices for population through increased negotiating power | Shift of costs to countries with less bargaining power |
| Voluntary out-licensing | LMIC | Reduce price, shift drugs | Lower prices in LMIC | Counterfeit parallel trade |
| Open tenders for exclusivity | Several EU countries, Russia | Reduce price, shift drugs | Reduces prices | Drug shortages due to less dynamic supply chains |
| Compulsory licensing | LMIC | Reduce price, shift drugs | Makes drugs affordable for LMIC | Counterfeit parallel trade |
| Incentivize physicians and pharmacists | Several EU countries, USA | Shift drugs, reduce price, reduce use | Directs towards prescribing cheaper drugs | Patients might lose access to more expensive brands |
| Profit limitation | UK | Reduce price | Lower profit margins, through lower prices or higher investments | Could incentivise companies to spend on less relevant causes |
Schemes to reduce drug prices are used most often to reduce overall healthcare spending. For example, according to one calculation setting prescription drug prices 20% lower than the current list price would increase the number of users who can afford the drug by 23%, while decreasing revenues for the drug company by only 1%.[ 33 ] More examples with comparable outcomes exist.[ 4 ] Of course, the outcome is completely dependent on specific market conditions, prices and regulations. The general consensus is that reducing prices increases the number of users, and this could at least partially offset losses due to lower pricing. A popular argument against paying less for drugs is that innovation would not be financially worthwhile, and society would not enjoy the possible benefits of new innovator drugs.[ 77 ] This argument will be discussed later on.
4.2.1 Biosimilar substitution regulation and resistance to substitution
Both the EMA (since 2003) and the FDA (since 2010) have regulations for accepting biosimilars to bring down the price of treatments by increasing competition without reducing safety.[ 65 ; 72 ; 159 ; 160 ] However, unlike the EMA, the FDA only allows for an interchangeability label if the manufacturer has shown that a biosimilar drug has the same effect and safety as the originator or for switching between them.[ 65 ] The regulatory framework for biosimilars is designed with similar intent as regulation for small molecule generic drugs, but its effect is thought to be less significant. This is due to a smaller price difference between the originator drug and generics,[ 143 ; 161 ] as previously mentioned. Research and production costs for designing a biosimilar are significantly higher than for designing small molecule generics.[ 65 ; 135 ; 161 ] Thus, innovators can prevent biosimilars market penetration by offering discounts on the original biological[ 135 ].
Furthermore, ongoing controversy on the interchangeability between biosimilars and originator drugs makes doctors and patients wary of using biosimilars.[ 159 ; 162 ] It requires significant effort on the part of reimbursement authorities to overcome this unexpected and rather persistent unease about and resistance to the use of biosimilars, that is exploited by marketers of the biological originator companies[ 159 ].
Pharmaceutical policy is often designed based on negotiations among various stakeholders, but doctors are not always invited to the table, according to critics.[ 163 ] If the medical community were more involved in creating biosimilar regulations and substitution programs, the effectiveness of regulatory and cost-reduction policies might improve significantly[ 159 ].
4.2.2 Engaging physicians and pharmacists in price reduction programs
As stated previously, physicians and pharmacists have a central role in determining which patient receives which medicine, and whether the use of expensive drugs is beneficial for specific patients.[ 133 ; 163 ; 164 ] Programs that provide financial incentives for prescribers to save on costs incentivize physicians to be cognizant of drug prices and have the potential to reduce pharmaceutical expenditure gradually and permanently, by either rewarding when expenses are low or enforcing penalties when expenses exceed indicative or earlier budgets.[ 34 ; 49 ; 165 ] After the implementation of such programs, doctors are more inclined to believe that medical costs are a relevant consideration in drug usage[ 166 ].
Just educating medical staff on drug pricing does not have a lasting effect. To change physicians’ attitudes, the prices of drugs must be considered, and constant reinforcement and easily available information is necessary. In one example, simply adding a sticker to indicate the prices of anesthetic drugs per hour in operating rooms significantly reduced their use and therefore, costs[ 167 ].
Another incentive to reduce spending is index pricing, which is similar to internal reference pricing. Drugs are classified in index groups of therapeutically interchangeable drugs. The prices for each group are determined based on the average drug used over the last period, which is frequently updated. The pharmacist is reimbursed for the price that is given for that index group, regardless of the drug that is actually dispensed. This incentivizes pharmacists to dispense the cheapest version of a drug, preferably below the index price so the difference can be kept as profit. This approach drives down the index price for the next period, thus creating a downward spiral.[ 36 ] Downsides of internal reference pricing are discussed in the next paragraph.
Finally, another way to reduce drug prices is for doctors to prescribe using the international non-proprietary names (INN) of drugs, and the brand names only when a brand is strictly necessary.[ 130 ] It is left to the pharmacist to dispense the drug based on the INN, so doctors have no financial incentives to prescribe more or specific brands of drugs[ 100 ; 164 ].
4.2.3 Reference pricing approaches
Reference pricing is a tool to set a benchmark for reimbursements.[ 36 ; 95 ; 168 ] Of all price control measures, this is the one with the most evidence for effectively reducing drug prices.[ 36 ; 99 ] Reference pricing can be done in two ways: internally or externally.
Internal, therapeutic or national reference pricing is based on comparing a drug to other drugs with the same active ingredient or with comparable clinical effects within a country. The maximum price of the new drug is then based on the average or lowest price in that cluster.[ 1 ; 55 ; 99 ; 163 ; 168 ] This incentivizes companies to develop drugs for indications with no competition, particularly no generic competition, because that would bring the price down.[ 1 ; 92 ] However, internal referencing discourages development in existing drug classes.[ 1 ] Late me-too drugs could be placed in a cheap class with therapeutic equivalents.[ 55 ; 169 ] This method of reference pricing only works by reducing prices and shifting patients towards cheaper drugs[ 36 ].
External, or international reference pricing is used by most EU member countries.[ 78 ] It is based on comparing a new drug’s price with other countries with a comparable economic status,[ 36 ; 59 ; 170 ] or with differing economic status after which the outcome is adjusted to purchasing power parity.[ 171 ] The mechanism of pricing used by a specific country, such as value-based pricing, can also be a reason to refer to that country[ 55 ].
For example, Norway reviews nine countries and takes the average price of the three lowest prices.[ 119 ] These prices are then regularly updated.[ 99 ] This approach often provides a skewed picture, because it does not take into account undisclosed prescription drug rebates and discounts that most purchasers receive for prices on the official list.[ 78 ; 99 ; 126 ; 172 ] This mechanism incentivizes companies to register in countries with the highest willingness to pay first.[ 155 ; 170 ; 172 ] Countries with a lower willingness to pay could see a drug launch delayed, or no launch at all, to prevent other countries from adjusting their prices downwards[ 92 ; 99 ; 170 ; 173 ].
4.2.4 Value-based pricing measures
The ideal pricing model should include the health and socio-economic benefits of a drug by deploying sophisticated out-come based compensation models.[ 105 ; 174 ] The price of a drug should be proportionate to the added value in terms of quality of life, life years saved or tumor shrinkage.[ 6 ; 24 ; 95 ] This would improve the value per monetary unit spent on health care, and increase innovation in relevant areas[ 90 ; 175 ].
Currently, cost-effectiveness analysis is used in many countries as a factor in the pricing of new drugs,[ 78 ; 176 ] but often drugs and treatments that are not cost-effective are still reimbursed, and sometimes by specific reimbursement funds like the Cancer Drug Fund in England.[ 177 ] Vice versa, cost-effective drugs are sometimes not reimbursed at all.[ 178 ; 179 ] A major reason for this is the lack of standardization in the practice of value-based pricing. Which factors are included and which are not varies, so value-based pricing is currently more of an art than a science.[ 24 ] Data about the effect of such schemes are contradictory.[ 99 ] One factor is that this policy has given a perverse incentive to drug companies to set high drug prices for the new generation of innovator medicines that are in line with the cost-effectiveness threshold (mostly in terms of quality-adjusted life year [QALY] and/or incremental cost-effectiveness ratio [ICER] terms) that a country is willing to pay. This also explains the differences in prices in individual countries, because cost-effectiveness thresholds differ across countries.
While not an official cut-off, the threshold for cost-effectiveness is set by the British regulators at approximately £30,000 per QALY,[ 155 ; 181 ; 182 ] and £50,000 for end-of-life drugs.[ 177 ; 179 ] Other European countries use cost-effectiveness thresholds that can vary between €10,000 and €50,000 per QALY.[ 6 ] After extensive analysis, the total British health care system was found to provide care at £13,000 per QALY, which was much cheaper than many drugs.[ 181 ; 182 ] This means that money would be spent more effectively on parts of the system other than high-priced innovator drugs.[ 177 ] Thus, the costs of high drug expenditures place a heavy burden on the health care system and may displace other high-quality healthcare services[ 178 ; 181 ; 183 ].
Another practical difficulty in value-based pricing is a drug’s indication. For example, Tarceva ® (erlotinib) is more effective for lung cancer (extends survival by 3.5 months longer than chemotherapy) than for pancreatic cancer (extended survival by two weeks versus placebo). Although the price is the same for both indications, Tarceva ® clearly creates less value for pancreatic cancer patients.[ 110 ; 180 ] Italy has an indication-specific pricing system to address this. Other EU member states and the US, struggle to implement a similar system[ 115 ; 180 ].
Some ways to ensure that a drug is priced according to its ‘true’ value are: risk-sharing pricing, pay-for-performance pricing or outcome-based pricing. These pricing schemes allow governments and companies to renegotiate a drug’s price based on its real-life performance in terms of effectiveness, sale volumes or cost-effectiveness.[ 56 ; 99 ; 185 ] In this way, companies have an interest in the real-life therapy outcomes (in terms of effectiveness), and not just the drug’s performance in clinical trials (in terms of efficacy). For example, companies could offer a drug at a reduced (or no) cost and receive annuity-style payments if a drug reduces hospitalizations after approval.[ 2 ; 169 ; 180 ; 186 ] Examples of outcome-based pricing exist, though only for specific indications and small populations.[ 169 ; 180 ] Tracking the results in the real world is also a difficult administrative assignment.[ 187 ] However, this scheme has recently been implemented for several larger indications, such as for the effectiveness of a new cholesterol-lowering drug[ 187 ; 188 ] and for the hepatitis drug Sovaldi ® in Japan based on volume[ 189 ].
4.2.5 Setting price and profit ceilings
Another method of controlling drug pricing is to set price ceilings in various forms. For example, to combat the high prices of generic drugs in Canada, the government has recently negotiated a fixed price ceiling for six of the most used generic drugs.[ 190 ] This one-size-fits-all approach might still result in overpricing for some of the six, and be too low to supply the entire market for others. A lower price could probably be negotiated through alternative tactics, like an open-tender invitation, but the several Canadian states failed to agree on an alliance for bulk purchasing[ 190 ].
In the UK, the government signed an agreement with the pharmaceutical industry that limits increases in spending on branded medicines to below 2% per year. If more is spent, the industry has to reimburse the government.[ 114 ; 119 ; 183 ] Companies that did not sign this agreement are subject to direct price control[ 114 ].
One more option is to introduce upward price rigidity, by prohibiting increases in drug prices.[ 93 ; 119 ] In the US, it is industry practice to increase the list prices of marketed drugs at least yearly by substantial amounts, synchronized with the competition.[ 146 ; 191 ; 192 ] Canada, however, only allows drug prices to rise with inflation.[ 119 ] This causes companies to set high initial prices, especially when the sales volume is uncertain[ 93 ].
A rather alternate approach is limiting companies’ ability to make high profits. An example of this is the Pharmaceutical Price Regulation Scheme (PPRS) in the UK.[ 36 ] If profits exceed a percentage agreed on after negotiations, a company must reduce prices, delay price increases or repay the excess to the Department of Health[ 36 ].
This approach is designed to be an incentive for pharmaceutical companies to either reduce the prices of their drugs, or invest more of their income in research. Conversely, it could also incentivize companies to spend more money on areas that are not relevant to reduce profit and avoid paying the Department of Health.
Partnerships between companies and charity foundations can help direct pharmaceutical research towards a needy cause. For example, the GlaxoSmithKline–PATH Malaria Vaccine Initiative partnership yielded Mosquirix, the first malaria vaccine.[ 193 ; 194 ] The partnership included a payment of $200m from the PATH Malaria Vaccine Initiative, backed by the Bill and Melinda Gates Foundation, to help fund pediatric trials.[ 195 ] The price of the drug was agreed to be the costs plus 5%, which is the same price as an insecticide-treated bed net, so that it would be available for those in need.[ 193 ; 194 ] The profit is then reinvested in more malaria research[ 194 ].
4.2.6 Lowering prices through open-tender invitation and negotiations
Many countries use open tenders to lower prices.[ 55 ] They invite manufacturers or wholesalers[ 55 ] to propose a confidential price for which they can guarantee to exclusively deliver a specific drug (e.g. simvastatin) or a drug in the same class (e.g. any statin) for the entire market for a term (often two years). The supplier or suppliers who offer the lowest price receive the contract, and secure income during the contract period[ 99 ].
This approach effectively reduces drug prices, since generic drug manufacturers are stimulated to offer the lowest, but still profitable price. However, open tenders require a market size that is significant enough for companies to be interested, enough generic producers to create competition[ 50 ] and an agreement on the contract conditions[ 190 ].
A downside to this market exclusivity for generics is the risk of shortages if the winning company fails to supply.[ 161 ; 190 ] For companies losing a bid, it could be difficult to offer a competitive price in the next round due to loss of capacity, which reduces competition and creates new monopolies[ 55 ].
Drug prices are often negotiated between national governments and companies, based on a reference price or a figure based on a drug’s cost-effectiveness and budget impact.[ 99 ] As for many schemes, increasing the number of patients for whom negotiations are held is thought to increase bargaining power and decrease prices, since companies are then less likely to deflect a low offer due to fears of losing market share.[ 84 ; 190 ; 196 ] New Zealand implemented a pharmaceutical management agency to have a single negotiator for the entire population, but not all countries are so organized.[ 78 ] For example, in the US, Medicare is not allowed to negotiate for drug prices[ 119 ; 197 ]; if a drug is approved and prescribed, Medicare has to cover it, so its bargaining power is limited.[ 119 ] Other players in the highly fragmented US market are small, thus reducing everyone’s negotiating power[ 98 ].
An important downside to negotiating lower prices on a health care provider level or national level is that this might indirectly increase prices for other providers and countries, who have smaller health care budgets, which results in a weaker position to negotiate[ 1 ; 145 ].
4.2.7 Transnational licensing and pricing frameworks
To increase access to drugs that are on patent and expensive, but necessary in LMIC, these countries’ authorities can choose to issue compulsory licences as allowed by the World Health Organization (WHO).[ 79 ; 198 ] This means that the authorities recognize the drug patents, but are allowed to have local generic manufacturers produce the same drugs, without fearing claims of patent infringement, or they can import the drug from another generic manufacturer.[ 7 ; 56 ; 79 ; 81 ] This reduces the costs of a new drug dramatically,[ 171 ] though other options like international procurement seem to offer a better discount. Unfortunately, this approach is also administratively cumbersome, since in general, it applies to one drug at a time,[ 56 ] and could result in other innovators withdrawing their drug from the market.[ 199 ] However, compulsory licensing can be used successfully as part of a strategy to reduce prices offered by the originator[ 56 ; 79 ; 81 ; 199 ].
International procurement is based on collective price negotiations between an innovative company and a union of LMIC. This approach leads to lower prices and more accessibility than compulsory licensing.[ 81 ] Lower prices can be achieved through voluntary out-licensing,[ 73 ] wherein the originator allows a generic manufacturer to produce the drug at reduced costs in exchange for a royalty. One example is the out-licensing of Harvoni ® (containing sofosbuvir and ledipasvir), which Gilead Sciences gave to an Indian manufacturer to produce for 91 LMIC, against a royalty of 7%[ 81 ].
High-income countries can also benefit from forming a union to increase bargaining power. For example, The Netherlands and Belgium recently signed an agreement to negotiate process for orphan drugs as a block. Several EU-countries have followed this example and joined the agreement, and some pharmaceutical companies have indicated their willingness to cooperate[ 150 ].
A more utopian option to regulate drug pricing is the proposal of a tiered pricing framework.[ 79 ; 80 ] This would put a global public body, such as the WHO, in charge of regulating prices of patented drugs worldwide. It would set prices for countries based on income, disease burden and possibly the rates of out-of-pocket payments. This would differentiate prices for rich and poor countries, and achieve fairer drug prices for consumers on a global scale.
Difficulties in the execution of this plan are to obtain international consent on the rules and calculations, and prevent leakage into other markets through parallel trade. Also patent rights would have to be equally respected and be applicable for the same terms, since it would be a void policy if patent-infringing cheaper generic drugs were available.[ 80 ] Analyses of existing systems with a comparable design in LMIC have shown increased profits for pharmaceutical companies and an increased availability of medical innovations[ 80 ].
A comparable idea is that a global fund financed by governments would reward companies, based on a share of the contribution they make to global health with all their products. In exchange, those companies would have to manufacture a drug at the lowest feasible price.[ 1 ; 200 ] Unfortunately, this approach would be extremely difficult to implement and measure.
5. Discussion
Taking into account the complex and interconnected dynamics of drug life cycles, pricing and intervention policies, several issues are worth mentioning and questioning. Recurring arguments in the drug pricing debate are now presented for discussion.
5.1. R&D through merger and acquisition
Companies are demonstrating a shift from in-house R&D to cheaper merger and acquisition-based development to fill the pipeline.[ 41 ; 42 ; 104 ; 201 ] This shift is due to the reduced efficiency of in-house research,[ 86 ] and transfers the risk of failing research from big companies to small startups. In this new format, small startups go bankrupt when the research does not yield a profitable product, so that large companies don’t have to suffer the losses. In the case of a successful start-up, larger companies simply buy the licence or the entire company. In the end risk is shifted from big manufacturers to investors and governments who have supported those startups. Although this business strategy is understandable in economic terms, it provokes perverse effects in the pharmaceutical marketplace that require new forms of regulation.
Valeant,[ 45 ; 114 ; 202 ] Turing and Amedra [ 197 ] are examples of companies that are mostly focused on buying profitable products, instead of performing in-house R&D. Another example is Gilead Sciences, which bought sofosbuvir (Sovaldi ® ) from Pharmasset, and marketed the drug at double the cost that Pharmasset had intended to charge.[ 203 ] We seriously doubt the long-term viability of investor-centered business strategy[ 147 ; 204 ].
Some generics are only produced by a couple of companies, so it is possible to buy all the drug rights and drastically raise prices.[ 16 ; 140 ; 184 ] This practice allows for a monopoly until competitors succeed in starting production as well. A major delay in this is the processing time for generic approvals, which is approximately ten months.[ 140 ] Many of the recent overnight increases in drug prices have been caused by this tactic that closely resembles a hedge fund strategy, rather than a pharmaceutical one.[ 16 ; 200 ] This strategy was used for Daraprim ® ,[ 15 ; 16 ] albendazole,[ 197 ] Aloquin,[ 20 ] doxycycline and thalidomide[ 140 ].
5.2. Patent law revision and stimulating public-private partnerships
The patent system is often blamed for high prices, because it limits competition and helps create monopolies. There certainly is an urgent need to revise the patent system in order to stimulate true innovation and prevent surrogate innovation as well as abuse of the patent system by a pharmaceutical industry that increasingly seeks to exploit the re-patenting loophole.[ 205 ] Unfortunately, removing the patent system completely would significantly reduce companies’ incentive to invest in research, so other solutions are required.[ 41 ] An alternative trajectory might be to have all drug research paid for by public money, in the same way that outcomes–prescription drugs–are currently paid for by public money. Academic institutions already perform a significant portion of new drug development, but currently lack the funds, capacity and incentives to develop a drug completely without support.[ 41 ; 206 ] Partnerships between companies, governments, research and charity organizations, referred to as public-private financing partnerships, with agreements on the drug availability and product price, seem most promising.[ 193 ; 194 ] This approach is what accelerated the development of the malaria vaccine, Mosquirix ® .
New initiatives to help reduce patents’ limiting accessibility and affordability effects are currently being developed.[ 32 ] GSK announced a graduated approach, stating that it would not defend patents in the poorest countries, and transfer licences to generic manufacturers in LMIC using the World Bank classification.[ 207 – 209 ] This approach would increase access to new drugs without limiting profitability in high-income countries.
Public-private partnerships could also stimulate commercially unattractive but essential therapeutic innovations in high-income countries. Exemplary is the clear societal need for new antibiotics, given the increasing prevalence of multi-drug-resistant bacteria. The possible gains for the industry are too low to make innovation economically viable.[ 200 ] The population base for a new antibiotic is small, because it would be the option of last resort. In addition, the length of antibiotic treatment is short, making the volume of the market very small.[ 210 ] Extremely high prices could compensate for this situation, but it is doubtful whether society would be willing to pay. Due to low incentives in the current market, governments are tempted to impose regulation to make antibiotic development more attractive,[ 206 ; 210 ; 211 ] as is in place for orphan drugs. We recommend that this policy also includes strict drug pricing conditions, and that it is accompanied by antimicrobial stewardship to prevent the overuse and misuse of the new antibiotic drug.
5.3. Me-too drugs, R&D resources
Me-too drugs or follow-on drugs are drugs with minor chemical variations relative to a drug already on the market within a given therapeutic class.[ 173 ] These drugs are highly controversial since they often cost roughly the same as the first-in-class drugs, but offer few relevant therapeutic improvements.[ 68 ; 69 ] Me-too drugs are seen as an ineffective consumption of R&D resources and diminishing incentives for innovation[ 24 ].
Another side of the argument is that me-too drugs increase choice, and make treatment available for certain groups, or help match a drug’s pharmacokinetics, effectiveness or side effects in specific populations.[ 24 ; 68 ] Me-too drugs are also a consequence of R&D races between multiple companies developing drugs for the same indication, so they are inherent to a competitive system.[ 173 ; 212 ] Given that a first-in-class drug has a significant advantage in market share and costs, this competition forces companies to speed up innovation[ 69 ].
Studies suggest that the price of me-too drugs only falls after the third introduction.[ 93 ; 173 ] Where the first movers compete over quality only, the drugs introduced later compete over price to overcome the disadvantage of being a new drug with no clinical record.[ 212 ] This holds for Gilead Sciences’ hepatitis C drugs as well, where MSD is competing with Zepatier ® with a price tag that is 40% lower than the first-in-class[ 115 ; 213 ].
5.4. Lack of systematic research into policy effectiveness
Various policies related to drug prices with roughly the same aim have been introduced around the world, but comparing the effectiveness across policies appears difficult.[ 41 ] Policies often lack a scientific basis, and an evaluation after the policy is introduced is not always performed, let alone in a standardized way.[ 41 ; 84 ; 165 ] Insights on the long-term policy effects of for instance value-based (QALY-guided) pricing are particularly scarce,[ 165 ] which causes some countries to pay unnecessarily high prices for medicines.[ 35 ; 163 ] Even regulatory guidelines are implemented without evaluation,[ 41 ] which causes some to ask for lowered regulation to speed up innovation[ 66 ] and others to ask for more regulation for additional safety. Unfortunately, both groups lack the evidence to back their case. Given the current trends in big data analytics, better monitoring of the effects of drugs with respect to health outcomes and the effects of policy on pricing should be possible.[ 89 ] Health Technology Assessment methodologies (HTA) could be used to strengthen evaluation of policy measures. Furthermore, the option of involving regulatory gatekeepers of safety and effectiveness like the FDA and the EMA in drug pricing policies should be considered more seriously. Like drugs, drug policy must be evidence-based[ 110 ].
5.5. Justification of drug prices
Life expectancy has gone up by 30 years in just a century in high-income countries.[ 77 ] It can be stated that innovation is always expensive, as seen in other new technologies, and once the price of innovation has been paid, generics make innovative treatments widely available. Revolutions in biotechnology, nanotechnology, biophysics and genome sequencing have moved precision medicine from the bench to the bedside,[ 53 ; 89 ; 214 ] yielding a burst of innovation in medicine.[ 30 ; 42 ; 75 ; 160 ] This helps to explain the large number of new and expensive targeted therapies that have come to the market recently. Cancer mortality has been reduced by 20% in the last two decades[ 77 ] through the introduction of new drugs, but that is also due to screening, prevention, vaccination and surgical improvements[ 58 ].
Steep pricing strategies are historically an oncological phenomenon,[ 91 ; 214 ] and recently prices are rising to even higher levels.[ 94 ] This is peculiar, since some oncological drugs offer only small benefits.[ 5 ] In addition to this low effect, oncological drugs are frequently priced so high that they are not cost-effective.[ 215 ] What makes the situation more financially challenging is that cancer drugs are often more effective in combinations,[ 75 ] thus making therapy cost the sum of the drugs.[ 98 ] Debates about this situation have usually ended in the consensus that patients cannot be denied new cancer treatments simply because of costs,[ 214 ] so eventually most cancer drugs have been reimbursed despite the high costs. But this is no longer sustainable given the steadily growing patient populations with cancer and stagnating health budgets.
As more treatments become possible, expectations rise.[ 58 ] Diseases that were a death sentence decades ago are now treatable, and leaving them untreated is not acceptable. With increasing possibilities come increased demands and increasing costs[ 216 ].
These innovations have allowed for more personalized diagnosis and cures, leading to precision or personal medicine, aimed at highly stratified patient populations. This gives better outcomes, but given the often smaller populations that qualify for a treatment (and thus smaller volumes of sales) prices are high to generate adequate revenue.[ 214 ] This shift from blockbuster to niche buster will make many untreatable diseases treatable, but costs for the healthcare sector will continue to rise[ 217 ].
5.6. Patient-assistance programs and list prices
Companies that raise prices often defend their actions by stating that patients who cannot afford the drugs are offered assistance in the form of patient assistance programs programmes in Western countries.[ 156 ] These programs allow patients to apply for the drugs at reduced or no cost, if they are uninsured and live below a certain income level.[ 114 ] The income level is set so that many patients on normal wages don’t qualify, so that drug prices can result in catastrophic spending.[ 114 ] Furthermore, patient-assistance programs increase the workload for general practitioners’ assistants, since they often require many forms.[ 140 ] The costs to the healthcare system are still unnecessarily large, and are shifted from patients to insurance companies.[ 141 ; 154 ; 218 ] These programs basically allow companies to sponsor the purchase of their own drugs[ 147 ].
Another argument for defending high prices is that pharmaceutical companies do not actually charge list prices for drugs. Hospitals and insurers often negotiate discounts on drug list prices,[ 142 ; 156 ; 219 ] and sometimes up to 50%.[ 141 ] However, drug prices worldwide remain at unrealistically high levels, since examples of annually raising prices (or prices rising after selling licences) to more than double the original price are plentiful[ 114 ; 220 ].
In some cases, companies provide free drugs for a small market simply because they feel they are morally obliged to do so. These are usually small companies who do not want to invest in regulatory approval procedures.[ 70 ] Larger companies see it as a moral obligation to maximize sales volumes and profits, reach patients and secure future investments in research, continue innovation,[ 94 ] and pay their stock holders.
Trying to defend high spending on pharmaceuticals, companies often point out that in the US only $300 billion is spent annually on prescription medicines compared to the $1 trillion that is spent on hospital care.[ 2 ; 184 ] Therefore, compared to total healthcare spending, US pharmaceutical costs are just 10% of the total health care budget, which is roughly the same throughout Europe.[ 184 ] This number has remained constant for over 50 years.[ 77 ; 221 ] Thus, all other healthcare costs, such as hospitals and general practitioners’ costs, have risen at the same rate as pharmaceutical costs.[ 39 ] It is simply easier for governments to reduce drug budgets than to reduce hospital staff wages or restructure inefficient healthcare infrastructures.[ 2 ] However, this still does not justify high drug prices. Just because other parts of the system are not functioning as efficiently as possible, does not mean pharmaceutical companies should be increasing prices at the same rate.
5.7 Methodological notes
This paper used both scientific publications and newspaper articles from several selected newspapers. The selection of those sources allowed for a comprehensive view on the subject of drug pricing. Clear differences in focus between the newspaper articles and scientific publications were observed.
The newspaper articles tended to focus on public opinion in one country based on one event, with examples and personal stories about the impact of high drug prices on patients’ lives. A thorough analysis on causes and policies of drug pricing was often missing, and a rather mono-dimensional conclusion was frequently offered that pharmaceutical companies choose to keep the prices high for their own exorbitant profits.
The journal articles offered more policy-focussed analyses of the causes of and solutions to high drug pricing. They more frequently covered in-depth discussions of single policy measures per country and their proposed or measured effect, but often left the patient perspective out.
In conclusion, the combination of both peer-reviewed scientific papers and newspaper articles allowed for a significantly contextualized evidence based conclusion. The mixed-method approach yielded deeper insights into the problem area of high drug pricing and sustainable drug markets than one of the two individual methods would have been able to achieve alone.
6. Conclusion
The current rise in drug prices worldwide is making healthcare unaffordable even in high-income countries. Apart from historic changes in the drug life cycle dynamics, price-volume proportions, and a transition from “one-size-fits-all” to more stratified precision medicine approaches, this problem is due to patent-induced monopoly positions, unintended consequences of drug and reimbursement policies and competitive market failure. This situation threatens to disturb the fragile compromise between the basic human right for affordable access to healthcare and the utilitarian protection of inventions to incentivize innovation. The current pricing spiral will only stop through well-designed regulatory interventions and measures around drug pricing on a national and transnational levels. Access to medicines needs to be central to any policy intervention discussion—something that can be overlooked when governments are arguing for lower health care costs to reduce spending, while the pharmaceutical industry is repeating the argument for competitive pricing to reimburse their R&D costs.
Many options to regulate the pharmaceutical market have been tried, some with better success than others. It is clear that reference pricing–both internal and external–and incentivizing physicians and pharmacists are the fastest, most effective and most reviewed options. Transnational cooperation, for example through the European Union, African Union, World Bank or WHO, would help reduce drug prices with increased bargaining power, and could potentially reduce administrative costs. Apart from creating collective negotiating power transnational cooperation would also stimulate exchanging trial data, sharing patient records and improving evaluation methods. A global framework for cooperation among drug regulatory authorities (e.g. FDA and EMA) would increase those benefits even more, and could be further amplified by reinforcing the existing WHO framework that already helps to reduce drug prices by means of an essential medicines list, which facilitates compulsory licensing.
Reduced healthcare spending is thought to reduce incentives for innovation, but given the current double-digit profit margins, industrial incomes could be lower without harming the industry’s outlooks. Public-private partnerships, in which charity funds are used to sponsor research in exchange for lower prices, could significantly help direct spending decisions on research away from primarily financial motives towards what is best for society.
Value-based pricing is a promising but also risky option that is already being used by some countries to reduce costs. The (inter-)national public debates about how much a QALY should cost and the regulatory and policy debates about whether and how to continue with the QALY appraisal tools still have to reach a conclusion. Until consensus is reached, drug companies will continue to strategically use the QALY and/or ICER thresholds to boost their profits. Governments should take this into account while continuing to deploy sophisticated ICER-based compensation models in the era of precision medicine.
In conclusion, the recent rise in drug prices is caused by uncontrolled market dynamics, changes in life-cycle dynamics and unanticipated policy side-effects. There is a wide range of policy tools to reduce drug prices available through various mechanisms. The most effective options are reference pricing and incentivizing physicians and pharmacists, whereas for the long term value-based and outcome-based pricing next to public-private partnerships are most promising developments. The challenge is, of course, how to strike a balance between rewarding investments in innovation, achieving reasonable drug pricing for governments and securing equitable access to medicines.
Supporting information
S1 prisma checklist, acknowledgments.
The authors would like to thank Cassandra Nemzoff, Nathalie Kuijpers and Julia Challinor for their English manuscript correction services. In addition, we would like to thank Frank-Jan van Lunteren for his graphics.
Funding Statement
This study was performed in the context of regular research of the Division of Pharmacoepidemiology and Clinical Pharmacology (Utrecht University), employing authors TvdG and TP, and of the Institute for Medical Technology Assessment, Department of Health Policy & Management, Erasmus University, Rotterdam, employing CU. The university had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability

COMMENTS
Global spending on prescription drugs in 2020 is expected to be ~$1.3 trillion; the United States alone will spend ~$350 billion 1. These high spending rates are expected to increase at a rate of 3-6% annually worldwide. The magnitude of increase is even more alarming for cancer treatments that account for a large proportion of prescription ...
The papers in this special issue include a sampling of the latest research on the epidemiology, clinical correlates, treatment, and public policy considerations of prescription drug abuse. Although much has been learned about prescription drug abuse in recent years, this research remains in early stages, particularly with respect to ...
Overview. Misuse of prescription drugs means taking a medication in a manner or dose other than prescribed; taking someone else's prescription, even if for a legitimate medical complaint such as pain; or taking a medication to feel euphoria (i.e., to get high). The term nonmedical use of prescription drugs also refers to these categories of ...
Global spending on prescription drugs in 2020 is expected to be ~$1.3 trillion; the United States alone will spend ~$350 billion 1. These high spending rates are expected to increase at a rate of ...
Introduction. Prescription drug use in the United States has reached record levels, rising to 6.3 billion prescriptions—approximately 19 prescriptions for every American—filled in 2020 alone (IQVIA Institute for Human Data Science 2021).Now "the most common therapeutic intervention" (World Health Organization [WHO] 2019), the modern prescription drug only emerged around the mid ...
Prescription drug misuse (PDM), or medication use without a prescription or in ways not intended by the prescriber, is a notable public health concern, especially in the United States. Accumulating research has characterized PDM prevalence and processes, but age-based or lifespan changes in PDM are understudied.
There is a scarcity of research on the effects of adverse social environments on adult brain function. 60, 61. ... Use of Prescription Drug Monitoring Programs (PDMPs) can help reduce doctor shopping and overdoses, but their use to date is inconsistent. This may be due in part to the voluntary nature of the programs in many states, ...
These findings are consistent with prior research indicating that the opioid epidemic is taking a significant toll on adolescents and young adults. ... McCabe SE. Prescription drug use, misuse and related substance use disorder symptoms vary by educational status and attainment in U.S. adolescents and young adults. Drug Alcohol Depend. 2018;189 ...
This report defines misuse of prescription drugs and describes the extent of prescription drug misuse in the United States. It also provides information about the safety of using prescription drugs in combination with other medicines, describes ways to prevent and treat prescription drug misuse and addiction, and lists resources that provide more information.
Importance: The increasing cost of prescription drugs in the United States has become a source of concern for patients, prescribers, payers, and policy makers. Objectives: To review the origins and effects of high drug prices in the US market and to consider policy options that could contain the cost of prescription drugs. Evidence: We reviewed the peer-reviewed medical and health policy ...
Macleod J, Steer C, Tilling K, et al. (2019) Prescription of benzodiazepines, z-drugs, and gabapentinoids and mortality risk in people receiving opioid agonist treatment: Observational study based on the UK clinical practice research datalink and office for national statistics death records.
Increasing spending and use of prescription drugs pose an important challenge to governments that seek to expand health insurance coverage to improve population health while controlling public expenditures. ... Health Services Research, and Medical Care Research and Review) and two working paper repositories (RePEc, Research Papers in Economics ...
Objective: Prescription opioid abuse and dependence have escalated rapidly in the United States over the past 20 years, leading to high rates of overdose deaths and a dramatic increase in the number of people seeking treatment for opioid dependence. The authors review the scope of the abuse and overdose epidemic, prescription practices, and the assessment, treatment, and prevention of ...
g and recogn. drug abuseSee page 10.from the director:The nonmedical use and abuse of prescription drugs is a ser. ous public health problem in this country. Although most people take prescription medications responsibly, an estimated 52 million people (20 percent of those aged 12 and older) have used prescription drugs for nonmedica.
Nonfederal hospitals accounted for $39.6 billion in prescription expenditures in 2020, an increase of 8.4% in 2021 after a historic decrease in 2020, whereas in clinics spending grew 7.7% to $105.0 billion. For 2022, we expect overall prescription drug spending to rise by 4.0% to 6.0%, whereas in clinics and hospitals we anticipate increases of ...
Misuse of Prescription Drugs Research Report | En español. June 2020 | Offers the latest research findings on prescription drug misuse ... Explores the relationship between prescription opioid abuse and heroin use, including prescription opioid use as a risk factor for heroin use, reasons why people progress from using ...
Prescription drug prices in the US are more than 2.5 times as high as those in other similar high-income nations and are a leading health care concern among US residents. Given these factors, and in response to President Biden's executive order promoting competition, the US Department of Health and Human Services (HHS) released a comprehensive plan to address drug prices in September 2021. 1 ...
Introduction. In recent years, drug abuse scenarios have evolved owing to the appearance of novel psychoactive substances (NPSs) and the recreational use of pharmaceuticals,,,Misuse of prescription drugs is a growing health problem, involving not only specific drug-related risks, but also the context in which they are consumed (e.g. the concomitant abuse of other substances with synergistic ...
Research. NBER working papers are circulated for discussion and comment purposes. They have not been peer- ... prescription drug expenditures.10 More recently, lay press reports, government investigations and published studies have documented massive price increases for certain very old drugs that are the
This paper first introduces important conceptual and practical distinctions among three key terms: substance "use," "misuse," and "disorders" (including addiction), and goes on to describe and quantify the important health and social problems associated with these terms. National survey data are presented to summarize the prevalence ...
CBO finds the cost of a prescription at gross Part D prices is 95% of the cost of a prescription at WAC prices for specialty brand-name drugs MFP dollar discount amount off price paid to ...
Introduction. Physicians' use of prescription drug samples has been debated. Some argue that samples decrease prescribing quality and increase overall prescription drug costs [Citation 1 - Citation 3]; while others argue that free drug samples are beneficial for patients [Citation 4, Citation 5].In fact, drug samples are a powerful marketing tool.
Most research designed to answer the "why" of the prescription opioid epidemic has relied on structured interviews, which rigidly attempt to capture the complex reasons people use opioids. In contrast this systematic literature review focuses on peer-reviewed studies that have used a qualitative approach to examine the development of an ...
The drug development process requires investments, estimated at between $60 million to $2.6 billion,[6;67;68;77] though most estimations are close to $800 million from bench research to prescription medicine.[33;66;72;84;85] The wide range of cost estimates is due to the lack of clear data and various methods of calculation, and depends on the ...