9 Things to Include in Your Cover Letter for Pharma Jobs

- Author Company: PharmiWeb.Jobs
- Author Name: Lucy Walters
- Author Email: [email protected]
- Author Website: https://www.pharmiweb.jobs/
When applying for your next pharmaceutical or life science job, your cover letter will be a big part of how you differentiate yourself from your peers. This is your opportunity to put together a compelling argument for why you are the ideal candidate, and what you could bring to the role that others couldn’t.
According to research , 47% of recruiters agree that including a cover letter in your application demonstrates motivation, with 30% saying that it provides additional information about a candidate and 23% saying it helps to demonstrate the candidate’s personality.
Unlike your CV which lists out your skills, qualifications, and experiences, your cover letter should highlight how you’ve put those to use, and the results you’ve produced from this. It should be tailored to each role you apply for, ensuring the achievements you mention match the essential requirements in the job description.
In this article, we’ve outlined everything you should include in your cover letter as well as a structure to follow to ensure your letter is clear, and concise, and communicates why you’re a strong candidate for the job..
Your Contact Details
Include your contact information at the top of the page, on the right-hand margin. Include your full name, telephone number, email address, and a link to your website/LinkedIn URL if relevant. You don’t need to include your full address unless requested, but you may want to include a county/city if the employer has stated a preference for candidates to be within a certain radius.
If you’re uploading your CV or cover letter online, please follow our advice on how to do so safely.
Employer Details
The contact details you include for your employer will also depend on how you’re submitting your application. If it’s an online application where you have a limited number of characters to play with, you don’t need to include their contact details. If you’re submitting a hard copy, or the application asks you to address the company in your letter, include their address along with the name of the hiring manager or HR department.
Again, if you’re submitting your application through an online portal, you don’t need to include the date within the body of your letter. But if you’re sending a prospective cover letter or are submitting a hard copy, include the date before the start of your letter.
Personalise the Salutation
Find out the name of the Hiring Manager/contact for this role. If a name hasn’t been given in the job description, reach out to the company to find out. If you still aren’t given a name, it’s best to use something like ‘Dear Hiring Manager’ rather than trying to figure out the person yourself – getting the name completely wrong won’t do you any favours!
Opening Paragraph
Begin your cover letter by introducing yourself using your elevator pitch , along with a line or two about how you learned about the position, and what drew you to apply. If you’re sending off a prospective cover letter and not applying for a specific role, outline the types of roles you’re looking for rather than being too vague.
Second Paragraph
Next, summarise your background as well as the key skills you have that make you qualified for the role. It’s important here to be as specific as you can, steering clear of making cliché statements and instead using statistics and specific anecdotes to contextualise your accomplishments.
Rather than trying to list every single relevant achievement, choose one or two that you feel reflect the key things the hiring manager is looking for in this position, using keywords from the job description to emphasise these.
Third Paragraph
In this paragraph, explain how your attributes make you a great fit for this role as well as for the company, drawing on their values and culture to highlight why you want to pursue a career with them over their competitors. This is your opportunity to show off the research you’ve done on the company, and to emphasise your cultural fit .
Closing Paragraph
In your final paragraph, reiterate your enthusiasm for the role and round up why you think you’d be a great fit, and what results you could bring to the company. Outline your availability, thank the person reading for their time and consideration, and indicate desire to be invited for an interview to learn even more about the role and the company.
How formally you sign off depends on how formal the company is, and the portal through which you’re submitting your application. Choose an appropriate sign off and consider also adding your signature as an extra touch. This can be handwritten if you’re submitting a physical copy or scanned onto the letter if you’re uploading it. It can look highly professional and adds a personal touch to your letter.
Find Your Next Pharma Job
Find your next pharmaceutical job on PharmiWeb.Jobs here.
You are leaving PharmiWeb.com

Disclaimer: You are now leaving PharmiWeb.com website and are going to a website that is not operated by us. We are not responsible for the content or availability of linked sites.
ABOUT THIRD PARTY LINKS ON OUR SITE
PharmiWeb.com offers links to other third party websites that may be of interest to our website visitors. The links provided in our website are provided solely for your convenience and may assist you in locating other useful information on the Internet. When you click on these links you will leave the PharmiWeb.com website and will be redirected to another site. These sites are not under the control of PharmiWeb.com.
PharmiWeb.com is not responsible for the content of linked third party websites. We are not an agent for these third parties nor do we endorse or guarantee their products. We make no representation or warranty regarding the accuracy of the information contained in the linked sites. We suggest that you always verify the information obtained from linked websites before acting upon this information.
Also, please be aware that the security and privacy policies on these sites may be different than PharmiWeb.com policies, so please read third party privacy and security policies closely.
If you have any questions or concerns about the products and services offered on linked third party websites, please contact the third party directly.

Resume Builder
- Resume Experts
- Search Jobs
- Search for Talent
- Employer Branding
- Outplacement
- Resume Samples
- Science and Biotech
Pharmacovigilance Scientist Resume Samples
The guide to resume tailoring.
Guide the recruiter to the conclusion that you are the best candidate for the pharmacovigilance scientist job. It’s actually very simple. Tailor your resume by picking relevant responsibilities from the examples below and then add your accomplishments. This way, you can position yourself in the best way to get hired.
Craft your perfect resume by picking job responsibilities written by professional recruiters
Pick from the thousands of curated job responsibilities used by the leading companies, tailor your resume & cover letter with wording that best fits for each job you apply.
Create a Resume in Minutes with Professional Resume Templates
- Perform aggregate safety analysis and case level review
- Represents PS on cross-functional project teams for developmental compounds and/or marketed products
- Leads the collaboration with GSP and Clinical representatives and authors the Reference Safety Information (RSI) for multiple or complex development products
- Leads and provides safety expertise to Investigator Brochures, Protocols, Informed Consents and Clinical Study Reports
- Perform literature searches and identify/analyze relevant publications
- Providing input into periodic reports (PSUR, BR, AR, ASR, DSUR)
- Develop report strategy with stakeholders
- Provide safety content review of clinical protocols, CSRs, and ICFs
- Support development and/or maintenance of core and local label for Kite products (e.g., CDS, USPI, SmPC, IB, etc)
- Contribute to development of signal detection strategy for post marketed products
- Support signal detection, evaluation and management according to Kite SOPs and guidelines
- Support preparation and maintenance of Risk Management plans
- Represents SABR and/or Pharmacovigilance on study teams, cross function product teams, etc
- This role can be based in Cambridge, Mass or Maidenhead, UK
- Performs duties as a Safety Strategy and Management Team (SSaMT) Leader for complex and/or multiple products
- Provides subject matter expertise in the therapeutic area and across multiple products
- Leads safety strategy for regulatory submissions of new products, formulations or indications (NDA/BLA, MAA), in partnership with the GSP and other functional experts
- Leads negotiations and provides expertise to the PS component of contracts/agreements with third parties to ensure quality and integrity of agreements
- As a Safety expert leads presentation of complex issues to Safety Information Review Committee (SIRC)
- Leads safety strategy for multiple or complex periodic regulatory documents (PBRERs, PSURs, DSURs) according to the agreed process and timelines
- Work with PV team on continuous process improvements in the field of safety surveillance, SOPs, guidance documents, templates, tools, upgrades
- Drives Accountability (creates cross-boundary accountability)
- Utilize technical skills and programs to analyze and organize data (eg, Excel pivot tables, SAS JMP)
- Partner with vendor to develop safety supporting documents/deliverables
- Works Collaboratively (establishes shared purpose across boundaries)
- Develops People and Organisation (invests in long-term development of others)
- Initiate/conduct searches of internal and external databases
- Commitment to Customers and Integrity (represents and shapes customer perspectives)
15 Pharmacovigilance Scientist resume templates

Read our complete resume writing guides
How to tailor your resume, how to make a resume, how to mention achievements, work experience in resume, 50+ skills to put on a resume, how and why put hobbies, top 22 fonts for your resume, 50 best resume tips, 200+ action words to use, internship resume, killer resume summary, write a resume objective, what to put on a resume, how long should a resume be, the best resume format, how to list education, cv vs. resume: the difference, include contact information, resume format pdf vs word, how to write a student resume, pharmacovigilance scientist lead resume examples & samples.
- 30% Product management/oversight. Oversight and assumes responsibility for assigned therapeutic products and PV activities. Supports an organizational structure that meets the evolving needs of Shire and the PVRM department. Fosters a collaborative culture within the product team and to external stakeholders. Ensures therapeutic product governance and communication in support of the PV Scientist Team Lead, Therapeutic Area Head, Head of Shire Pharmacovigilance and Risk Management and the Qualified Persons for Pharmacovigilance, including the EU QPPV
- 30% Aggregate report production. Responsible for the production of periodic and ad-hoc safety reporting for therapeutic team products, where Shire is the Global Safety Database holder. Establishes project management responsibility for aggregate reports (e.g. PBRER, DSUR, topic reports) within the therapeutic team to ensure effective planning such that stakeholder input is requested/received for applicable sections of the report and regulatory timelines are met. Collaborates with the PV physicians to ensure that all information is available to allow for informed medical review and benefit-risk assessment. Responsible for the quality of the final document and utilizes Shire’s EDMS system to provide quality control
- 20% Safety Review Team Coordination and signal detection. Responsible for the technical and scientific support for safety surveillance and signal detection activities for assigned Shire products. Responsible for efficiently planning routine and ad-hoc Safety Review Team (SRT) meetings and ensuring that stakeholders provide input from their respective areas of responsibility. Ensures that regulatory commitments and risks described in the product Risk Tracking Document are efficiently tracked and presented as part of the SRT process. Works with SRT Co-chairs to facilitate communication and support for decisions resulting from the SRT. (e.g. update of RMPs, labeling, regulatory notifications)
- 20% Support for investigational products and registration activities. Effectively collaborate with stakeholders outside of PVRM. Represent PVRM department in cross-functional clinical programs and registration activities and provide PVRM support for clinical developmental programs
- Life Science Degree or Healthcare Professional
- Experience in a pharmaceutical company (5+ years preferred) preparing, writing, authoring and submitting periodic safety reports
Pharmacovigilance Scientist Development Team Lead Resume Examples & Samples
- 30% - Product Management/Oversight. May act as a functional line manager to PV Scientists or a manager for a product or group of products. Oversight and assumes responsibility for assigned therapeutic products and PV activities. Supports an organizational structure that meets the evolving needs of Shire and the PVRM department. May be responsible to manage the development, objective setting and performance assessment of direct reports. Fosters a collaborative culture within the product team and to external stakeholders. Ensures therapeutic product governance and communication in support of the PV Scientist Team Lead, Therapeutic Area Head, Head of Shire Pharmacovigilance and Risk Management and the Qualified Persons for Pharmacovigilance, including the EU QPPV
- 20% - Development Aggregate Report Production.Responsible for the production of aggregate and ad-hoc safety reporting for therapeutic team products, where Shire is the Global Safety Database holder. Establishes project management responsibility for development aggregate reports (e.g. DSUR, topic reports) within the therapeutic team to ensure effective planning such that stakeholder input is requested/received for applicable sections of the report and regulatory timelines are met. Collaborates with the PV physicians to ensure that all information is available to allow for informed medical review and benefit-risk assessment. Responsible for the quality of the final document and utilizes Shire’s EDMS system to provide quality control
- 20% - Safety Review Team Coordination and Signal Detection. Responsible for the technical and scientific support for safety surveillance and signal detection activities for assigned Shire products. Responsible for efficiently planning routine and ad-hoc Safety Review Team (SRT) meetings and ensuring that stakeholders provide input from their respective areas of responsibility. Ensures that regulatory commitments and risks described in the product Risk Tracking Document are efficiently tracked and presented as part of the SRT process. Works with SRT Co-chairs to facilitate communication and support for decisions resulting from the SRT. (e.g. update of RMPs, labeling, regulatory notifications)
- 20% - Support for Investigational Products and Registration Activities. Effectively collaborate with stakeholders outside of PVRM. Represent PVRM department in cross-functional clinical programs and registration activities and provide PVRM support for clinical developmental programs
- 10% - Alliance Management for Outsourced PV Work. Provide guidance and oversight for CROs performing activities related to clinical trial SAEs
Pharmacovigilance Scientist Resume Examples & Samples
- Lead cross-functional Medical Surveillance Teams (MSTs) and assigned subteams, such as Clinical Data Review (CDR) Teams, and participate in related PV and product-development subteam(s). Appropriately elevate issues impacting key MST activities, milestones, documents to the MST Chair. Mentor individuals in aspects of project management, drug development and MST requirements, as appropriate to meet overall MST/subteam needs
- Create MST/assigned subteam meeting agendas and documentation of decisions, conclusions, timelines, milestones and action items in team minutes. Consistently ensures scientist verification of completion of action items to ensure that appropriate data, strategy, project milestones and individual member responsibilities are addressed and fully communicated. Influence the assigned team(s)
- Periodic review and summary of pertinent safety-related literature, analysis of pre-determined core signal data. Provides advice and mentoring to scientists of summaries, evaluations and conclusions
- Collaborate within and across client functions with appropriate disciplines to identify and ensure management of internal and external documentation and support when required
- Apply knowledge of product goals, strategy, drug development stage milestones, partnership agreement, HA commitments, and individual functional area responsibilities. Mentor individuals and teams on these applied learnings
- Coordinate and integrate scientific, medical, and regulatory input from a variety of scientific sources and functional groups, as needed to support responses to ad hoc queries and HA commitments. Contributing to and authoring regulatory documents
Principal Pharmacovigilance Scientist Resume Examples & Samples
- Lead Global DS&E initiatives to develop business solutions and meet emerging regulatory requirements, by directing cross-functional teams, managing progress, quality and timely completion of deliverables, and reporting progress to DS&E leadership
- Author along with Global Medical Safety Physicians, key regulatory periodic safety reports (Periodic Safety Update Reports, US Periodic Reports) for newly launched Novartis products: leading cross-functional expert team and collecting, organizing, analyzing and presenting the data by means of DS&E templates and proce-dures
- Lead cross functional expert teams to fulfill Periodic Safety Report accountabilities (DRA, Clinical, Marketing)
- Develop procedures to monitor Central DS&E and CPO compliance to regulatory requirements
- Drive the quality review/sampling of ICSRs and literature as part of DS&E quality management system
- Review global marketing programs and establish process for AEs collection with global marketing teams as required
- Review Risk Management Plans in coordination with Global Medical Safety groups and assess the operational feasibility and implications of pharmacovigilance commitments as required
- Proactively collaborate with licensing partners and Clinical Research Organizations to meet joint accountabil-ties
- Collaborate with Electronic Data Management team to reconcile Serious Adverse Events between the Clinical and Safety databases to meet joint accountabilities and enable locking of Clinical database
- Co-lead Safety Profiling Teams for newly launched products to ensure that case reports are accurately evalu-ated and databased, and authoring Product Specific Guidelines for assigned products
- Lead the preparation of Standard Operating Procedures and Argus processing conventions
- Develop and test safety systems/IT applications as business lead/co-lead and support the preparation of rele-vant manuals
- Lead sessions during Health Authorities inspections and audits as Subject Matter Expert, and lead the development and implementation of Corrective and Preventative Actions (CAPA) to address safety findings
- Act as Subject Matter Expert in cross-functional projects and external meetings
- Deputize for Team Leader/Group Head and assist with the recruitment of new staff
- Drive continuous DS&E business improvements by initiating & managing initiatives
- Number, timeliness and quality of deliverables according to established directives
- Role model of company values & behavior
Senior Pharmacovigilance Scientist Resume Examples & Samples
- Assists with assessments of potential safety signals for safety physician review; involves synthesis of data from multiple sources and critical thinking skills as well as authoring monthly signaling reports
- Prepare and author reports of aggregate safety data such as PSURs, DSURs, Pharmacovigilance Plans, Risk Evaluation and Mitigation Strategy Plans (REMS), and EU Risk Management Plans
- Key member of the Product Surveillance Team, including setting agenda, producing necessary data outputs, facilitating discussions, documenting conclusions
- Works closely with SABR Clinical Trial Physicians and Global Safety Officers on data analysis, signal detection, ad hoc requests and other product activities, as assigned
- Coordinates and authors responses to regulatory agencies in collaboration with safety physician; includes proposing a strategy for the response, review of relevant data, and authoring responses
- Supports safety activities for clinical trials, including protocol review, study team representation, document authoring, etc
- This role can be based in Cambridge, Mass or Maidenhead, UK**
- Ability to understand, interpret, analyze, and clearly present scientific and medical data in verbal and written format (including intermediate understanding and application of medical concepts and terminology)
- Able to interact collaboratively and effectively in a team environment (including Safety, Clinical Development, Medical Affairs, Clinical Operations, and Regulatory), as well as with external colleagues
- Able to develops and conduct, independently and/or collaboratively, all aspects of substantive projects such as signaling, authoring of aggregate data reports, and responses to regulatory agency requests
- Able to apply clinical judgment to interpret case information
- Strong Pharmacovigilance and drug development foundation, including knowledge of applicable clinical trial safety regulations and post-marketing safety regulations. Includes basic knowledge of case processing, expedited reporting rules, and safety database concepts
- Basic knowledge of common data processing software (EXCEL, PowerPoint, Microsoft Word, Business Objects). Knowledge of common safety database systems
- Accountable for efficient and accurate maintenance and monitoring of data and processes to support the management of individual case safety reports
- Responsible for developing and maintaining up to date knowledge for assigned drug responsibilities, regulatory authority regulations and internal processes and procedures. To use said knowledge to provide input to topics as a Subject Matter Expert and to advise members of PDS/other departments on safety and pharmacovigilance related topics as needed
- Responsible for supporting ICSR Team Manager and Senior PV Scientists with improvements and /or remediation’s to processes and procedures as applicable
- Responsible for training and mentoring team members on the team deliverables to effectively deliver high quality, compliant and medically cohesive ICSRs
- Responsible for engaging with Stakeholders within and outside PDS, to develop better working relationships, move forward with prioritised process improvements and ensure smooth, well defined handoffs
- Ensuring readiness for Audits & Inspections
- Supporting/leading process improvement and simplification activities following continuous improvement principals across the department by collaborating with stakeholders and other teams within PDSO to provide expertise and input into ICSR-M related areas, as well as effective CAPA development and management
- Lead Pharmacovigilance projects to develop business solutions and meet regulatory requirements, by directing the team, manage progress, quality and timely completion of deliverables, and reporting progress to DS&E management
- Author along with Global Medical Safety Physicians, regulatory periodic safety reports (Periodic Safety Update Reports, US Periodic Reports) for Novartis in-patent products: collecting, organizing, analyzing and presenting the data by means of DS&E templates and procedures
- Review emerging regulatory guidelines and legislations and identification of impact to DS&E processes
- Collaborate with licensing partners and Clinical Research Organizations to meet joint accountabilities
- Review global marketing programs and establish process for AEs collection with global marketing teams
- Review Risk Management Plans in coordination with Global Medical Safety groups and assess the operational feasibility and implications of Pharmacovigilance commitments
- Assess seriousness, causality and labeling of serious adverse events and quality check ICSRs to ensure accurate and consistent Argus data entry from source documents
- Key contributor to Safety Profiling Teams to ensure that case reports are accurately evaluated and database, and authoring Product Specific Guidelines for assigned products
- Contribute to the preparation of Standard Operating Procedures
- Participate in Health Authorities inspections and audits as Subject Matter Expert, and develop and implement Corrective and preventative Actions (CAPA) to address safety findings
- Act as Subject Matter Expert in cross-functional meetings
- Train and mentor new DS&E associates and associates from other line functions
- Ability to lead cross-functional teams
- Alert manager to potential safety signals based on incoming case reports
- Work with Novartis country safety departments, Clinical Safety Scientists and Pharmacovigilance Leaders to ensure that reports are accurately collected, evaluated and database
- Assist with related administrative and procedural activities as required or requested
- Assists in the training of other Safety Processing Experts as necessary
- Support DS&E Projects or database validation activities as required
- Collaborate with Electronic Data Management team to reconcile Serious Adverse Events be- tween the Clinical and Safety databases to meet joint accountabilities and enable locking of Clinical database
- Alert the Medical Safety Physicians of potential safety issues and assist the Medical Safety Physicians in monitoring the safety profile of products
- Member of Safety Profiling Teams to ensure that case reports are accurately evaluated and database, authoring or contributing to Product Specific Guidelines for assigned products
- Contribute to the preparation of Standard Operating Procedures and Argus processing conventions (MAP)
- Support the development and testing of safety systems/IT applications and in the preparation of relevant manuals
- Support Health Authorities inspections and audits, and development of Corrective & Preventative Actions (CAPA) to address safety findings
- Train and mentor new DS&E associates
- Ability to learn quickly
- Consistent demonstration of company values & behavior
- Collaborate with the Medical Safety Physicians and other line functions to monitor the safety profile of newly launched products, by analyzing large data sets, reviewing clinical study protocols, responding to inquiries from Health Authorities, drafting communications to Health Care Professionals, and responding to CPO requests
- Author along with Global Medical Safety Physicians, key regulatory periodic safety reports (Periodic Safety Update Reports, US Periodic Reports) for newly launched Novartis products: leading cross-functional expert team and collecting, organizing, analyzing and presenting the data by means of DS&E templates and procedures
- Review Risk Management Plans in coordination with Global Medical Safety groups and assess the operational feasibility and implications of Pharmacovigilance commitments as required
- Proactively collaborate with licensing partners and Clinical Research Organizations to meet joint accountabilities
- Co-lead Safety Profiling Teams for newly launched products to ensure that case reports are accurately evaluated and database, and authoring Product Specific Guidelines for assigned products
- Lead the preparation of Standard Operating Procedures
- Develop and test safety systems/IT applications as business lead/co-lead and support the preparation of relevant manuals
- Train and mentor new associates
- Assist with the recruitment of new staff
- Ability to lead cross-functional teams and represent DS&E at external meetings
- Supports the process for preparing and authoring aggregate safety reports for assigned products, such as PSURs, DSURs, Pharmacovigilance Plans, Risk Evaluation and Mitigation Strategy Plans (REMS), and Risk Management Plans (RMPs). Interacts with vendor contracted to author such reports for specific products, as needed
- Assists with signal management process for assigned products (i.e., signal tracking, leading review meetings, etc.) and in collaboration with SABR MDs and Sr. PV Scientist / AD, evaluates safety data and signals as part of ongoing pharmacovigilance activities. May include authoring signal evaluation reports, or sections of signal evaluation reports. May include support of literature review for safety information
- With Sr. PV Scientist / AD oversight, supports Clinical Trial Physicians and Global Safety Officers on assigned investigational programs including protocol review, safety committee management, data analysis, signal detection, ad hoc requests and other product activities, as assigned
- In collaboration with Sr. PV Scientist / AD, contribute to and help coordinate responses to safety questions from regulatory authorities for assigned products
- Contributes to initiatives for process improvement and consistency regarding aggregate reporting, clinical trial safety oversight, signal management and responding to ad hoc safety questions
- Understands, interprets, analyzes, and clearly presents scientific and medical data in verbal and written format (including intermediate understanding and application of medical concepts and terminology)
- Interacts collaboratively and effectively in a team environment (including Safety, Clinical Development, Medical Affairs, Clinical Operations, and Regulatory), as well as with external colleagues
- Applies clinical judgment to interpret case information
- Leads and/or conducts proactive pharmacovigilance and risk management planning for designated products, including preparation of safety aspects of Global Risk Management Plans, in partnership with the GSP and others as appropriate
- Has the ability to present safety information at external meetings
- Has the ability to perform duties as a Safety Strategy and Management Team (SSaMT) leader for smaller or less complex projects
- Presents issues to Safety Information Review Committee (SIRC) and has the capacity to take the lead role in data evaluation and discussion of the results with the SIRC Chair, GSP and other key stakeholders
- Produces accurate and fit for purpose evaluation documents with clear conclusions in response to internal or regulatory authority requests for safety data
- Collaborates with GSP and Clinical representatives and authors the Reference Safety Information (RSI) for assigned development products; coordinates meetings and tracks timelines to ensure completion
- Proactively evaluates the clinical implications of safety data from pre-clinical studies, clinical studies, literature and other information sources to establish the safety profile of drugs and manage the risk to patients
- Authors/provides strategic input or oversight for periodic regulatory documents (PBRERs, PSURs, DSURs) according to the agreed process and timelines
- Authors/provides strategic input to regulatory submissions for new products, formulations or indications (NDA/BLA, MAA), in partnership with the GSP and other functional experts
- Contributes to the PS component of contracts/agreements with third parties to ensure quality and integrity of agreement
- Trains junior members of the team in PS tools and systems
- Leads and/or conducts proactive pharmacovigilance and risk management planning for more complex products, including preparation of the safety aspects of Global Risk Management Plans and Risk Evaluation and Mitigation Strategies (REMS) in partnership with the GSP and others as appropriate
- Leads PS activities of cross-functional project teams for developmental compounds and/or marketed products
- Has the ability to perform duties as a Safety Strategy and Management Team (SSaMT) Leader for larger or more complex projects
- Presents complex issues to Safety Information Review Committee (SIRC) and takes the lead role in data evaluation and discussion of the results with the SIRC Chair, GSP and other key stakeholders
- Authors/provides strategic input or oversight for multiple or complex periodic regulatory documents (PBRERs, PSURs, DSURs) according to the agreed process and timelines
- Authors/provides strategic leadership to regulatory submissions for new products, formulations or indications (NDA/BLA, MAA), in partnership with the GSP and other functional experts
- Participates in negotiations and provides expertise to the PS component of contracts/agreements with third parties to ensure quality and integrity of agreements
- Trains and mentors junior members of the team, e.g. in approved PV processes, analytic methodologies, etc
- A life sciences/pharmacy/nursing degree, and demonstrated Patient Safety and/or Clinical/ Drug Development experience. This should include aggregate reporting, safety surveillance and analytics
- Intermediate (PV Sci) to advanced (Sr PV Sci) knowledge of PV regulations
- Fluent in written and verbal English
- MD, MSc/PhD in scientific discipline, preferred
- Basic (PV Sci) to intermediate (Sr PV Sci) understanding of epidemiology data, preferred
- Leads the strategy for proactive pharmacovigilance and risk management planning of complex or multiple products, including preparation of the safety aspects of Global Risk Management Plans and Risk Evaluation and Mitigation Strategies (REMS) in partnership with the GSP and others as appropriate
- Has the ability to lead cross-functional process improvement or other initiatives on behalf of the Patient Safety organization
- Takes accountability and leads resolution of complex safety issues and mediates cross-functional agreement
- Has the ability to participate in due diligence activities
- Work in conjunction with Clinical Safety Associates, Clinical Safety Scientists from other processing sites and Medical Safety Physicians (Pharmacovigilance Leader and Brand Safety Leader) to ensure that reports are accurately evaluated and databased
- Triage literature cases for databasing or pass them on to the Team Leader for approval of rejection
- Co-author, together with the Medical Safety Physicians, all required regulatory periodic re-ports, collecting, organizing and presenting the available data
- Work with external partner groups, e.g. co-licensing partners and Clinical Research Organisations to meet joint accountabilities
- Represent DS&E at internal and external meetings
- Assist the Medical Safety Physicians with project activities in specific therapeutic areas, compatibly with the timely processing/production of individual case reports and regulatory periodic reports
- Assist the Medical Safety Physicians in monitoring the safety profile of product 8. Be involved in development and testing of safety systems/IT applications and in the preparation of relevant manuals
- Participate in cross-functional teams on safety matters/DS&E special projects relating to investigational and marketed drugs Impact on the organization
- 13 Ensure that Serious Adverse Event / Post Marketing Adverse Event are evaluated accurately and within the required timeframes to meet regulatory requirements
- Alert the Medical Safety Physicians to potential safety issues
- Good knowledge of pharmacovigilance practices
- Good knowledge of US and EU pharmacovigilance regulatory requirements
- Ability to present and critically discuss safety data in both internal and external discussions
- Ability to evaluate, interpret and synthesize scientific data (analytical thinking)
- Team player with ability to function in a cross-functional environment
- Self–motivated, able to prioritize and plan effectively, and independently, with minimal supervision
- Fluent in English (verbal and written)
- Good moderator skills
- Ability to navigate in databases and pull information correctly
- Good organizational skills and attention to detail
- Evaluation of safety data for assigned products
- Interpretation of reviewed data
- Drafting of reports and writing and/or review of key documents in the context of safety andbenefit risk for assigned products (RMP, Health Hazard Evaluation, IB, NDA submissions, adhoc expert reports, supporting documents for the assignment of frequency categories toADRs)
- Overview of key safety information and draft summary of documents supporting responsesto Health Authorities and Affilitate queries, Ethics committees, revision of company coresafety data sheets; drafting of (sections of) and/or assisting GSO with Clinical Overviews,Summary of Clinical Safety, Investigator’s Brochures, etc
- Initiating and reviewing searches of safety data in respective databases
- Acquire and maintain therapeutic area knowledge and ongoing assessment of therapeutic areas, competitive products and therapies
- 4 Work with external partner groups, e.g. co-licensing partners and Clinical Research Organisations to meet joint accountabilities
- Assist the Medical Safety Physicians in monitoring the safety profile of product
- Be involved in development and testing of safety systems/IT applications and in the preparation of relevant manuals
- Ensure that Serious Adverse Event / Post Marketing Adverse Event are evaluated accurately and within the required timeframes to meet regulatory requirements
- Explore complex safety data from clinical studies and post-marketing sources and present at internal, external and professional meetings
- Signal detection and evaluation in collaboration with the Safety Physicians
- Monitor identified areas of interest – produce dashboards
- Prepare, compile, author, or collaborate in various aggregate reports such as PADERs, annual IND reports, PSURs/PBRERs, DSUR, Clinical Overviews, Safety Summaries
- Represent DS PV in functional project teams, with business partners and CROs
- Perform periodic scientific literature review for safety content
- Minimum BS or Health Care Professional (RN, BSN, PA, etc.)
- 5 years relevant experience in PV and risk management
- Experience in all safety regulatory document types (e.g., IND annual report, ASR, DSUR, PSUR, PADER, EU RMP, ISS, and ad hoc safety responses for Regulatory Authorities)
- Knowledge of FDA, EU, and ICH guidelines and regulations
- Proficiency with Safety database management and design
- Knowledge of clinical trials and drug development
- Coding dictionaries (e.g., WHO ATC drug dictionary, and MedDRA)
- Advanced Bioscience degree (MS, PhD, Pharm D, MD, …)
- Preferred Experience with quantitative methods is a plus
- Experience in project management are desired
- Safety database management and design in Argus
- Proficiency with Microsoft Word, Microsoft Excel, Microsoft PowerPoint, Microsoft Outlook, Project, Visio and Adobe Acrobat
- Familiarity with analytics tools (e.g. Cognos, Spotfire, etc.)
- Excellent oral communication
- Excellent writing skills
- Planning, coordination, and organizing
- Decision making, judgment, and problem solving
- People skills, influencing skills
- Attention to detail combined with the ability to summarize
- Work well in team
- Ability to work in tight timelines while maintaining accuracy
- Ability to work proactively with minimal supervision
- Appropriately experienced to undertake all usual Pharmacovigilance Scientist activities, including case report QC, review, follow-up reportability assessments and input into aggregate safety reports globally, with the flexibility of mindset this requires
- Work with other relevant functional areas both within and outside of global PV to ensure efficient and timely attainment of compliant and patient focused safety data
- Represent pharmacovigilance department in cross-functional teams and/or meetings at a regional and global level as required, alongside the commensurate communication skills required
- Build on knowledge and understanding of designated products/studies
- Regular interaction with scientist and physician colleagues under the auspices of the safety Therapeutic Area Lead (TAL), particularly when the responsible GSL
- Help mentor new entrants/less experienced PV scientist colleagues
- Appropriate involvement in ongoing professional education/training to ensure contemporary skill set
- Involvement in facilitating safety procedures for developmental programs
- Bachelors required; Advanced degree in scientific/medical field preferred
- Detailed knowledge of clinical trial methodology, pharmacovigilance regulations, safety profile and risk/benefit analysis
- Critical thinking and decision making skills
- Commitment to a global organization
- Excellent communication and presentational skills
- Collaborate with the Medical Safety Physicians and other line functions to monitor the safety profile of newly launched products, by analyzing large data sets, reviewing clinical study protocols, responding to inquiries from Health Authorities, drafting communications to Health Care Professionals, and responding to CPO requests. -Lead Global DS&E initiatives to develop business solutions and meet emerging regulatory requirements, by directing cross-functional teams, managing progress, quality and timely completion of deliverables, and reporting progress to DS&E leadership. -Develop procedures to monitor Central DS&E and CPO compliance to regulatory requirements. -Drive the quality review/sampling of ICSRs and literature as part of DS&E quality management system. -Review global marketing programs and establish process for AEs collection with global marketing teams as required. -Review Risk Management Plans in coordination with Global Medical Safety groups and assess the operational feasibility and implications of pharmacovigilance commitments as required. -Proactively collaborate with licensing partners and Clinical Research Organizations to meet joint accountabil-ties. -Collaborate with Electronic Data Management team to reconcile Serious Adverse Events between the Clinical and Safety databases to meet joint accountabilities and enable locking of Clinical database. -Co-lead Safety Profiling Teams for newly launched products to ensure that case reports are accurately evalu-ated and databased, and authoring Product Specific Guidelines for assigned products. -Lead the preparation of Standard Operating Procedures and Argus processing conventions. -Develop and test safety systems/IT applications as business lead/co-lead and support the preparation of rele-vant manuals. -Review emerging regulatory guidelines and legislations and identification of impact to DS&E processes. -Lead sessions during Health Authorities inspections and audits as Subject Matter Expert, and lead the development and implementation of Corrective and Preventative Actions (CAPA) to address safety findings. -Act as Subject Matter Expert in cross-functional projects and external meetings. -Train and mentor new DS&E associates and associates from other line functions. -Deputize for Team Leader/Group Head and assist with the recruitment of new staff
- 4 to 7 years experience in Drug Development or closely re- lated areas of responsibility, with a minimum of 3 to 5 years’ experience in drug safety
- Excellent understanding of drug development process, GCP and medical terminology
- Strong negotiation, presentation and communication skills, and ability to operate effectively in an international environment and across functions and sites
- Strong organizational and project management skills
- Ability to lead global and cross-functional work groups
- Ability to mentor and coach
Pharmacovigilance Scientist Phd-research & Development Resume Examples & Samples
- An advanced degree in either a health care profession (e.g. Pharm. D., M.D.) or life science (PhD)
- Good collaboration skills and experience in establishing and maintaining working relationships
- Excellent written and oral communication skills in English with an emphasis on communication of human health relevance, safety and clinical information. Experience with external communications to regulatory authorities and industry groups would be beneficial but is not essential
- Excellent organization skills, an attention to detail and an ability to manage complex systems
- Experience with databases, data reporting tools and statistical analysis would be beneficial
- Leads the signal management process (i.e., signal tracking, leading review meetings, etc.) for assigned product(s) and evaluates safety data and signals as part of ongoing pharmacovigilance activities. Includes synthesis of data from multiple sources and authoring signal evaluation reports. Leads signaling review process and product Safety Signaling Team meetings. Manages literature review for safety information
- Collaborates with Global Safety Officers and other SABR MDs for assigned investigational programs including clinical trial activities (protocol review, ICF review, etc.) safety committee management, data analysis, signal detection, ad hoc requests and other product activities, as assigned
- Leads process for responding to safety questions from regulatory authorities
- Contributes to and leads initiatives for process improvement and consistency regarding aggregate reporting, clinical trial safety oversight, signal management and responding to ad hoc safety questions
- Leads and collaborates with Aggregate Reports on strategy, review and finalization of aggregate safety reports for assigned products, such as PSURs, DSURs, Pharmacovigilance Plans, Risk Evaluation and Mitigation Strategy Plans (REMS), and Risk Management Plans (RMPs)
- Demonstrates leadership and interacts collaboratively and effectively in a team environment (including Safety, Clinical Development, Medical Affairs, Clinical Operations, and Regulatory), as well as with external colleagues
- Leads and conducts, independently and/or collaboratively, all aspects of substantive projects such as signaling, authoring of aggregate data reports, and responses to regulatory agency requests. Oversees and mentors less experienced PV Scientist staff
- Strong Pharmacovigilance and drug development foundation, including knowledge of applicable clinical trial safety regulations and post-marketing safety regulations. Includes knowledge of case processing, expedited reporting rules, and safety database concepts
- Participate in cross-functional Medical Surveillance Teams (MSTs) and assigned subteams, such as Safety Data Review (SDR) Teams, and participate in related PV and product-development subteam(s). Appropriately elevate issues impacting key MST activities, milestones, documents to the MST Chair
- Periodic review of pertinent safety-related literature, analysis of pre-determined core signal data. Provides advice and summaries, evaluations and conclusions of safety data reviewed
- Collaborate within and across client's functions with appropriate disciplines to identify and ensure management of internal and external documentation and support when required
- Assist in oversight of Pv vendors/CROs as applicable
- Contribute to preparing safety assessments reports
- Participate in preparing responses to safety related requests from health authorities
- Previous experience in preparing periodic safety reports and risk management plans
- Demonstrated persuasion, influencing and negotiation skills
- Effective team player, with proven ability to effectively lead projects and teams to successful conclusion
- Present results to safety physician or cross-functional team
- Author/contribute to the preparation of safety supporting documents
- Comply with processes and ensure appropriate documentation
- Adhere to report timelines and escalate issues to management as appropriate
- Bachelor’s degree in health or biomedical science (6+ years industry experience or equivalent) or
- Advanced degree preferred in health or biomedical science (4+ years industry experience or equivalent)
- Clinical/medical writing and/or PV experience
- Aggregate safety report writing and aggregate safety analysis
- Knowledge of MedDRA hierarchy
- Searching and analysis of the literature
- Responsible in leading scientific, technical or drug safety input for a project, group of products, systems or processes across the CDS Regions
- You will represent the Region, externally where required. You may lead a group of Clinical Drug Safety colleagues to deliver a project and project manage across a number of groups and/or projects and use in depth knowledge of specific products, therapeutic or technical areas. Senior Patient Safety Scientists are also mapped to this job capsule
- As a Pharmacovigilance Scientist you will participate in the triage process for incoming documents to insure timely and effective medical and scientific evaluation of adverse event information
- Request follow-up information from consumers, and health care professionals and requests clarification from foreign affiliates according to specific report types using both global and local SOP timeframes
- Responsible to review adverse event documents prepared by the Patient Safety personnel for accuracy, completeness, and validity prior to submission to the FDA
- Lead teams in preparing, organizing, and reviewing tabulations for Regulatory reports. You assume responsibility for completing special projects (i.e. IND Annual Reports, PSUR table preparation)
- Support Safety Surveillance, Safety Support - Compliance and Marketing Company and the US Clinical Teams
- Serve as a knowledge resource for departmental personnel regarding medical, scientific and Operational issues
- Responsible for reflecting the Patient Safety philosophy of being a team player and supports the development of those individuals within the department
- Initiate special projects and demonstrate leadership capabilities at target level or above
- Personal responsibility for creating a culture of courageous leadership, creativity and collaboration
Related Job Titles
Drug Safety Associate Resume Sample
The resume builder.
Create a Resume in Minutes with Professional Resume Templates
Work Experience
- Knowledge and experience with safety reporting and regulatory compliance, and experience of international safety reporting/regulations are required
- Highly organized, analytical, and logical in approach to all assigned tasks
- Two- three years’ experience in drug safety in the pharmaceutical industry or other comparable experience
- Manage workflow of all SAEs received into the department to facilitate routing, review/approval, and timely case finalization and archiving
- Intake and documentation of receipt of SAE reports, follow-up information and source documents
- Perform quality review of ICSR which includes review of source documents and ensuring that the case is accurate and that corrections to the case, if applicable, are incorporated
- Liaison with Case Receipt and/or Safety Surveillance Physicians (SSP) staff as appropriate to clarify appropriate information required for case processing
- Other activities relating to case processing as appropriate per case, including but not limited to
- Broad knowledge of domestic and international drug safety regulations, industry practices and standards
- Proven ability to influence and collaborate with business stakeholders effectively and in a positive manner
- Great project management skills and ability to execute and manage multiple projects and deadlines with shifting priorities and resources in a fast-paced working environment
- Maintaining an excellent knowledge of case processing conventions and guidelines, client’s procedures and international drug safety regulations
- External – TGA, MedSafe, Consumers, health care professionals, Medicines Australia, and others
- Internal – personnel from various departments/functions (i.e. Regulatory Affairs, Clinical Operations, Sales, Marketing, Medical Affairs, Health Care Compliance, Finance, etc.)
- Write and send SAE queries to Investigative sites, CRAs, and CTM’s utilizing Query templates and Query Activity tracking within ARGUS
- BS/BA: 1-2 years of relevant experience
- Relevant experience includes experience in the pharmaceutical, biotechnology, or CRO industry or in related areas such as Medical Affairs, Clinical Data Entry and Clinical Data Management, Clinical Data Monitor, Regulatory Affairs, or Quality Assurance
- Knowledge of medical and dmg terminology desirable
- Familiarity of Good Clinical Practice (GCP) related to clinical safety documentation
- Familiarity with ICH Guidelines
- Familiarity of worldwide regulatory requirements and reporting of adverse event for both marketed and investigation products
- Familiarity of worldwide regulatory requirements and reporting of adverse event for both marketed and investigational products
- Submission of safety reports to investigators via SIS (Safety Information System)
- Assist with measuring investigative site performance in conducting required tasks in SIS
- Assist with unblinding of SUSARs, as required
- Support collection and review of metrics for measuring reporting compliance Support Global Pharmacovigilance Information Office (GPIO) in the collection and organization of global PV requirements
Professional Skills
- Excellent organizational skills, demonstrated ability to prioritize multiple projects
- Proven experience and report writing skills in accordance with agency requirements for content, format and timelines
- Excellent communication and interpersonal skills with the ability to effectively influence others
- Strong computer skills with experience in Word, Excel and Outlook
- Analytical and organizational ability, able to prioritize with problem solving skills under minimal supervision
- Effective communicator with excellent verbal and written skills
- Strong organizational skills, detail oriented, ability to adapt to change
How to write Drug Safety Associate Resume
Drug Safety Associate role is responsible for organizational, medical, research, reporting, excel, manufacturing, training, database, oncology, insurance. To write great resume for drug safety associate job, your resume must include:
- Your contact information
- Work experience
- Skill listing
Contact Information For Drug Safety Associate Resume
The section contact information is important in your drug safety associate resume. The recruiter has to be able to contact you ASAP if they like to offer you the job. This is why you need to provide your:
- First and last name
- Telephone number
Work Experience in Your Drug Safety Associate Resume
The section work experience is an essential part of your drug safety associate resume. It’s the one thing the recruiter really cares about and pays the most attention to. This section, however, is not just a list of your previous drug safety associate responsibilities. It's meant to present you as a wholesome candidate by showcasing your relevant accomplishments and should be tailored specifically to the particular drug safety associate position you're applying to. The work experience section should be the detailed summary of your latest 3 or 4 positions.
Representative Drug Safety Associate resume experience can include:
- Provide technical and process-related support to drug safety management (clinical trial and post-marketed) and medical monitoring activities, ensuring compliance with relevant regulations and Standard Operating Procedures (SOPs)
- Ensure accurate transfer of information from initial and follow-up source documents or E2B inputs for adverse event reports to appropriate fields within Argus; code adverse events using MedDRA, create narrative of the adverse events, and manage case reports within the Argus workflow to meet local and global regulatory timelines
- Process case-related information including interpretation of medical conditions, lab results, and procedures; ensuring proper coding of data into the global safety database
- Prior work experience with Pharmacovigilance database is an asset
- Understanding and application of good documentation
- Solid understanding of GMPs, FDA regulations and ICH guidance pertaining to drug and/or biologic regulations
Education on a Drug Safety Associate Resume
Make sure to make education a priority on your drug safety associate resume. If you’ve been working for a few years and have a few solid positions to show, put your education after your drug safety associate experience. For example, if you have a Ph.D in Neuroscience and a Master's in the same sphere, just list your Ph.D. Besides the doctorate, Master’s degrees go next, followed by Bachelor’s and finally, Associate’s degree.
Additional details to include:
- School you graduated from
- Major/ minor
- Year of graduation
- Location of school
These are the four additional pieces of information you should mention when listing your education on your resume.
Professional Skills in Drug Safety Associate Resume
When listing skills on your drug safety associate resume, remember always to be honest about your level of ability. Include the Skills section after experience.
Present the most important skills in your resume, there's a list of typical drug safety associate skills:
- Demonstrated problem solving skills including successful resolution and proactivity
- Attention to detail along with strong scientific, analytical and conceptual skills and the ability to reach reasoned conclusions
- Excellent written, oral communication, personal organizational skills and resourcefulness
- Excellent verbal/communications skills
- Prior experience with adverse event reporting
- Effectively troubleshoot and recommend solutions as issues arise
List of Typical Experience For a Drug Safety Associate Resume
Experience for senior drug safety associate resume.
- Demonstrate strong organizational skills, including the ability to prioritize work
- Collect, document and evaluate adverse event information from Healthcare professionals using medical knowledge, experience, and communication skills
- Prior experience with Drug Safety database entry and narrative writing
- Experience working with data extracted from a database
- Maintains 100% compliance with regulatory reporting. Maintains Good Clinical Practices and complies with FDA regulations
- Demonstrate knowledge of applicable clinical trial regulations
Experience For Drug Safety Associate, Case Processing Resume
- Supporting the training of new personnel on reporting of adverse events
- Preparing metric reports pertaining to Drug Safety department for management
- Participating in the creation and compliance of FP policies and department standard operating procedures
- Contributing to inspection readiness planning, as needed
- Using medical / pharmacological expertise to assist with data review, literature review and signal detection processes
- Working knowledge of pharmaceutical development process
- Primary client contact in partnering with the sponsor, their sites, etc. as necessary, regarding safety issues in support of the case processing team
Experience For Drug Safety Associate / Specialist Resume
- Assist in project specific safety database setup, development of data entry guidelines, user acceptance testing, policies and training materials
- Assists in the review and implementation of training and cases processing procedural documents
- Support the preparation and conduct of internal audits or PV inspections as required and contribute to inspection readiness on an ongoing basis
- Proactive and timely communication and escalation of issues or deviations identified relating to any aspect of PV processes
- Assists in obtaining additional report information from clinical sites, CPMs/CRAs and service providers
- Compile the safety data into the relevant sections of periodic reports such as PBRER, PADER, and DSUR according to the client
- Work with the Aggregate Reporting lead to escalate issues or tasks outside the normal scope of work
- Review and evaluate AE case information to determine required action based on and following internal policies and procedures
Experience For Medical Information / Drug Safety Associate Resume
- Process all incoming cases in order to meet timelines
- Apply clinical judgment when interacting with healthcare professionals and consumers to obtain and follow up on reports of possible adverse events
- Liaise with other Safety and Compliance Associates to ensure timely processing
- Healthcare professional background including but not limited to, pharmacist, nurse, medical doctor, and dentist
- General working knowledge of document management systems
- Supports Drug Safety Management in ensuring PV compliance with global company and local regulatory authority PV requirements
Experience For UBC Senior Drug Safety Associate Resume
- Perform safety review of clinical and diagnostic data as part of case processing
- Generation of project specific procedures/safety management plan/Sae forms templates using the departmental guidance templates
- Act as Pharmacovigilance lead for global and local US projects assigned supporting your line manager and Director
- Liaise with other ICON departments and/or other Sponsor vendors such as data management and clinical project management, medical writing, etc
- Extensive knowledge of regulatory reporting obligations in both US and ROW
- Generate medical narratives derived from the collection of adverse event information and verify medical coding in the safety database system
- Ensure case receives appropriate medical review and prepare follow-up correspondence consulting medical staff accordingly
- Assist in the review of cumulative safety data for submission to Drug Safety Monitoring Boards (DSMBs), regulatory authorities or clients
Experience For Drug Safety Associate, Taiwan Resume
- Assist with SAE reconciliation activities with internal departments and external partners as needed
- Ensure timely preparation & submission of reports to regulatory agencies in accordance with applicable regulations - including United States DSUR and global PBRER reports
- Generate aggregate safety data output from the Safety database for activities related to compliance, metrics, monitoring, KPIs or internal stakeholder or Partner related activity
- Participate in audit preparedness and support Partner and/or Regulatory audit
- Vendor and CRO oversight
- Conduct regular reconciliation with health authorities, medical information (spontaneous),
Experience For Drug Safety Associate, QC Focus Resume
- Assess the nature of the business activities and determine the applicable PV requirements
- Liaise with other departments to ensure processes and systems are established and maintained to enable oversight and compliance of all affiliate business activities with PV implications
- Development of PV clauses for third party agreements
- Maintain local DS procedures with ongoing review for efficiency and review against updates to global Roche requirements and applicable local regulations and laws
- To ensure the accurate, timely and complete receipt, evaluation and follow- up of domestic adverse event reports
Experience For Drug Safety Associate Resume
- To maintain the local archive of safety reports
- To maintain current awareness of local and global safety regulations
- To ensure compliance with local expedited and periodic regulatory reporting requirements for marketed products and those under development, utilizing ARISg and E2B
- To contribute to the development and maintenance of corporate policies, standard operating procedures and associated documents on safety data handling
- Receipt and allocation of case reports and other safety relevant information in accordance with relevant procedures and within applicable business timelines
- Follow-up activities on case reports as required and in accordance with relevant procedures and within applicable business timelines
- IRT/OST workflow checks to ensure compliance with regulatory and internal business timelines for submission of case reports to TGA
- Support the Group Drug Safety Manager in the review and update of case submission rules for Australia
- Training of third party vendors conducting PV activities on behalf of Roche Australia and of Roche employees/contractors regarding assessment and processing of case reports and other PV activities as required
- Other activities relating to case processing as appropriate per case, including but not limited to single case unblinding, Serious Adverse Event (SAE) /Adverse Event (AE) reconciliation, deviation memo preparation, deletion/admin edit requests, review protocol update request forms for accuracy
- Proficient skill level in Adobe Acrobat, Microsoft Word and Microsoft Excel
- Demonstrates a practical and innovative approach to continual improvement, implementation of change and inspection readiness within Drug Safety
- Completion of all assigned training on company and GMSO procedural documents relating to case processing
- Oriented to quality, attention to detail and accuracy
- Interacts with partners and service providers in the receipt of safety information, notification to other parties, reconciliation and compliance review
- Assesses adverse event reports for accuracy and completeness
- Performs quality control review of individual case safety information and aggregate data outputs
- Generates safety reports and monthly/summary report outputs from the safety database
- Works closely with other Drug Safety personnel and the clinical teams to communicate safety information internally
List of Typical Skills For a Drug Safety Associate Resume
Skills for senior drug safety associate resume.
- Knowledge and experience with safety reporting and regulatory compliance, and experience of international safety reporting/regulations is required
- Experience in obtaining, analyzing, disseminating, and reporting safety information in compliance with global regulations
- Demonstrated understanding of the assessment and processing of safety reports
- Proven ability to analyze and interpret aggregate patient safety data relating to drug products
- Maintaining an excellent knowledge of data capture conventions and guidelines, client’s procedures and international drug safety regulations
- Proven ability to work well with others in a proactive, positive, and constructive manner. A positive attitude for driving ambiguous situations
- Work in a dynamic environment and manage competing priorities
- Have direct hands-on experience with post-marketing regulatory activities (i.e. AR, CBE-30, PAS, Variations) for biologic and/or drug products
- Regulatory, Manufacturing, QA/QC experience
Skills For Drug Safety Associate, Case Processing Resume
- Experience in using drug safety database (ARISg or Argus)
- Experience in using MedDRA and WHO-Drug dictionaries
- Experience with exchanging safety data with business partners or affiliates
- Proven proficiency using MS Word, Excel, Power Point and Outlook
- Experience working in a pharmacovigilance department at a pharmaceutical company
Skills For Drug Safety Associate / Specialist Resume
- Shares of ideas and suggestions with team members ensuring effective communication
- Experience using or familiarity with ARGUS or other safety database applications
- Experience using or familiarity with MedDRA
- Demonstrated excellence in the processing of clinical safety data for investigational products in the biopharmaceutical industry
- Relevant drug safety experience
- Related experience gained in healthcare environment is an advantage
- Prepare or QC consolidated clinical trial case narratives
- Receive and process all reports of adverse drug experiences in accordance with established Local
- Manages own work: ability to prioritize, plan and organize work assignments, and able to work under strict timelines
Skills For Medical Information / Drug Safety Associate Resume
- Good understanding of safety regulations
- Ensuring timely reporting of SAEs/AEs to Regulatory Authorities, and cross-reporting to pharmaceutical partners
- Tracking and filing of submission cases as required
- Working knowledge of US regulations and guidances pertaining to post-marketed Human Drug products
- Conducting periodic reconciliation of SAEs between drug safety and clinical trial databases for ongoing clinical studies with little or no supervision
Skills For UBC Senior Drug Safety Associate Resume
- Understanding of medical terminology and ability to summarize medical information
- Coding all AEs in MedDRA independently
- Participating in the preparation of aggregate safety reports, such as biannual safety reports to Ethics Committees and annual reports to regulatory authorities
- High level of computer literacy, particularly in the use and management of Drug Safety databases including ICSR data entry and regulatory reporting
- In-depth understanding of the ICSR assessment and reporting process
- Collect and review metrics for measuring reporting compliance
- Perform coding review for adverse events, medical history, and concomitant medications from ongoing clinical trials
- Case processing: completion of full case information on the database, including quality review to ensure accuracy and completeness
Skills For Drug Safety Associate, Taiwan Resume
- Completion of training relating to relevant PV Agreements for assigned products
- Assists in the development of safety surveillance processes and writing corresponding SOPs
- Determine expectedness/listedness against appropriate label and identify clinically significant information missing from initial reports ensuring collection
- In-depth knowledge of regulatory requirements for biopharmaceutical development and manufacturing
- Proven ability to interpret and follow the patient safety guidelines of the FDA and comparable international regulatory organizations such as the International Conference on Harmonization
- Working knowledge of FDA and international pharmacovigilance and clinical safety regulations and guidelines
- Extensive working knowledge of FDA regulations, FDA guidance and ICH guidance; some familiarity exposure to EMEA regulations
Skills For Drug Safety Associate, QC Focus Resume
- Line listing and tabulation generation for safety reports i.e. periodic safety reports, ad hoc safety reports etc
- Maintain local drug safety reporting requirements
- Knowledge of worldwide regulatory requirements and the reporting of adverse events for both marketed and investigational products
- Measure investigative site performance in conducting required tasks in ISIS
- Un-blinding of SUSARs, as required
Skills For Drug Safety Associate Resume
- BS, MS or PhD in Biology, Chemistry, Engineering or related field
- Work well in cross-functional teams in a fast-paced challenging environment
- Support of Medical Directors/Safety Physicians, as needed, in medical monitoring activities
- Attend internal, drug safety and project specific training sessions
- Participate in interdepartmental activities and assist management with weekly and monthly project status reports including quality review findings and metrics
- Correspond and interface with CRO’s regarding collection of safety information for development products
- Participate in clinical team meetings supporting the Ironwood development portfolio as representative of Drug Safety, when needed
- Vendor training and oversight
- Quality review of case reports processed by third party vendors or in-house ensuring compliance with applicable procedures
- Participate in designated activities to support revision/creation of case processing procedural documents
- Participation in inspections and audits as identified, including interviews and provision of requested data
- Assists with the processing of adverse event reports from clinical sites, partner companies and marketed product use
- MedDRA and WHO-DD coding
- Collect data regarding adverse events
- Maintains up- to-date information on local Regulatory Authority out of hour’s contacts
- Reviewing ancillary documentation accompanying ICSRs and identifying the relevant information for processing and electronic capture on the client’s safety database
- Prepares the respective submission documents to Health Authorities
- Supervision of proper flow of confirmations of receipt of documentation sent to the Health
- Preparation and everyday distribution of respective DS&E documents externally
- Participation in the verification process of data correctness in the global safety database
- Verification of correctness and compliance of documentation sent to CPO DS&E Department
- Quality control of case reports, line listings, and tabulations
List of Typical Responsibilities For a Drug Safety Associate Resume
Responsibilities for senior drug safety associate resume.
- Experience preparing and finalizing documents in Word and Adobe
- Training of third party vendors conducting PV activities on behalf of Roche Australia
- Ensure all ICON, Sponsor, and regulatory timeframes are met for the processing andreporting of safety information
- Participate on cross functional, multidisciplinary teams and contribute to defining aggregate reporting guidelines
- Partner with Case Processing, Quality/Compliance and Safety Systems staff to ensure efficient and compliance aggregate reporting processes
- In-depth understanding of the aggregate reporting process
Responsibilities For Drug Safety Associate, Case Processing Resume
- Holds the training for GRA project team, Investigators and other departments on regulatory reporting activities. Generation of project specific procedures
- Act as safety reporting lead for multiple projects providing management/project management support
- Perform domestic and foreign case assessment for Health Canada reporting
- Request product complaint investigation (e.g. PQMS number) on appropriate cases according to the SOP
- Provide mentoring and oversight of Drug Safety Associate staff
- Support the preparation and conduct of internal audits or inspections as required and contribute to inspection readiness on an ongoing basis
Responsibilities For Drug Safety Associate / Specialist Resume
- Receives direction and guidance from more senior colleagues and managers
- Interacts with different members of the clinical departments and regulatory affairs
- Interact with a variety of Array departments, service providers and Array partners
- Lead/Co Lead cross functional teams contributing to periodic safety reports in accordance with Global Pharmacovigilance and Risk Management (GPRM) business rules, standard operating procedures (SOPs), and global regulatory requirements
- Support roles involved in creation of Aggregate Safety Reports (ASRs) (i.e. PSURs, PADER, DSURs, PBRER’s, Addendum to Clinical Overview’s, Addendum to PSUR’s, Summary Bridging Report’s and RMP Updates)
- Review aggregate safety data from the database and generate line listings (LL) and summary tabulations (ST) and include the LL & ST in the appropriate template
- Create all documents in accordance to all applicable SOPs, and convention of the client
- Plan, organize, and manage daily work to meet service level timelines and deliverables
- Provide word and PDF versions of the final signed documents to the client
Responsibilities For Medical Information / Drug Safety Associate Resume
- Participate in internal and external audits and inspections by clients and health authorities
- Responsible for data entry of individual case safety reports into the safety database
- Follow-up on reconciliation of discrepancies
- Perform submission activities when trained and assigned
- Responsible for case intake, duplicate check, and registration
- Maintain log of source documents and other communications
- Perform literature review activities when trained and assigned
Responsibilities For UBC Senior Drug Safety Associate Resume
- Timely process or QC clinical trial cases in ARISg safety database
- Prepare, track, and follow up case queries
- Perform routine SAE reconciliations
- Familiarity with key safety regulations such as FDA, ICH, EMA
- Review and determine regulatory reporting requirements based on assessment of seriousness, causality and expectedness/listedness in accordance with regulatory guidelines and product labeling
- Prepare adverse events reports for submission to Health Canada within the appropriate reporting timeframes. Verify accuracy, completeness and validity of report information
- Evaluate adverse event reports, assesses regulatory status (seriousness and expectedness/relatedness)
- Process adverse event reports from spontaneous sources, clinical trials, studies and marketing activities (including healthcare professionals and consumers) in accordance with company standard operating procedures (SOP) and guidelines for maintaining regulatory compliance. The sources include mail, voicemail, phone, fax and other electronic means
- Determine and conduct follow-up actions as required with appropriate communication methods (e.g. fax, email, telephone)
Responsibilities For Drug Safety Associate, Taiwan Resume
- Provide coverage to all products/devices as assigned
- Respond to GMS (Global Medical Safety) and international affiliates as required for operational (e.g. queries), compliance and/or safety functions
- Provide safety reporting training to third-party vendors (market research, patient assistance providers) with manager’s oversight. Train DSS team members as assigned
- Relevant clinical practice or pharmaceutical industry
- Mentor and guide the activities of the Drug Safety Associate
- Perform case processing for serious adverse events, serious and non-serious adversedrug reactions, and other medically-related project information such as adverse events ofspecial interest and clinical endpoints
- Assist in ensuring the completion of all departmental project activities accurately inaccordance with ICON standards, regulatory requirements, and contractual obligations toSponsors
- Min 1-2 yrs relevant exp in pharmacovigilance
- Proactively maintains a current awareness of the internal and external environments and seeks opportunities to apply this knowledge and upskill the Drug Safety department

Responsibilities For Drug Safety Associate, QC Focus Resume
- Performing active follow-up via telephone contact with consumers and health care professionals
- Monitors status of ICSR follow-up letters to ascertain they are being sent out in the required timeframe
- Leads specific projects as nominated by the Group Drug Safety Manager
- Attend study meetings with the clinical team and participate in client meetings related to safety oversight and management, as well as lead PV project team meetings internally and with the client
- Assist with identifying out of scope activities in conjunction with the PV Project lead (as applicable)
- Preparing clinical narrative summaries independently for AE reports from clinical studies and spontaneous post-marketing reports and formulates follow-up information requests
- Working with Clinical Research Department and Contract Research Organizations, sometimes in a lead capacity, regarding information exchange and safety exchange agreements
Responsibilities For Drug Safety Associate Resume
- Triages assigned adverse event reports using medical and regulatory expertise. Enters and maintains the events in the electronic drug safety database
- Responsible for obtaining follow-up information from healthcare professionals and consumers
- Scan, import and QC all adverse event source documentation into the Individual Case Safety Report (ICSR)
- Mentors and provides safety support to Drug Safety Associates
- Monitors DSA case workload on a weekly basis and escalates issues/concerns to Data Manager
- Performs weekly reconciliation of adverse event and product complaint reports with Quality Assurance and Medical Information departments respectively. Maintains verification of reconciliation and email correspondence in Drug Safety shared drive
- Work closely with Pharmacovigilance Data Manager and Quality Manager and provide back-up in their absence
- Develops follow-up processes (letters, questionnaires and phone contact) to obtain relevant medical information pertinent to case analysis and signal detection
- Co-author / co-develop data entry process instructions and update accordingly when processes and procedures change
- High level of computer literacy, particularly with safety database functionality
- Report endpoints to clients, regulatory authorities, ethics committees, investigators and Covance project personnel, if required, within study specified timelines
- Prepare timely pharmacovigilance reports for products and safety issues, including Individual Case Summary Reports (ICSRs) of Serious Adverse Events (SAEs), Annual IND reports, European Annual Safety Reports (ASRs), Development Safety Update Report (DSUR) Periodic Reports (PRs) line listings, and aggregate reports
- Representing Drug Safety Department in study team meetings
- Facilitating the request for listings of similar events from the drug safety vendor, for the analysis of similar events as needed
- Supporting the identification of new data management tools and innovative approaches
- Ensures filing of SAE reports, SUSARS, and safety documents
- Communicates and updates Team of any process related issues/ concerns Assists with special projects and other ongoing safety activities and programs as needed
- Manage scheduling and workflow of aggregate reports through established company processes
- Monitor workflow for assigned studies and programs to ensure all deadlines are met and actively participate in project team and client meetings
- Draft, modify, and deliver safety presentations
- Begin to prepare Safety Management Plans (SMPs), Reconciliation Plans and other safety-specific plans under supervision
Related to Drug Safety Associate Resume Samples
Drug safety resume sample, drug safety specialist resume sample, epidemiologist resume sample, science resume sample, research science resume sample, life science resume sample, resume builder.

45,000+ students realised their study abroad dream with us. Take the first step today
Here’s your new year gift, one app for all your, study abroad needs, start your journey, track your progress, grow with the community and so much more.

Verification Code
An OTP has been sent to your registered mobile no. Please verify

Thanks for your comment !
Our team will review it before it's shown to our readers.

The Best Cover Letter for Fresher (Download Free PDF)
- Updated on
- Nov 16, 2023

Whenever we are applying for a particular job or role at an organisation, we are required to submit an application, a cover letter . This situation is a little challenging for freshers as they have zero experience about things to add in their cover letter, its right format, writing style, etc. A cover letter is a document that provides information to the prospective employer about your educational qualifications, career goals, projects, and other key details in a very crisp manner. It is a document which quickly and directly communicates the position you are looking for in the organization so that the recruiter does not confuse two candidates who have the same qualifications. Also termed as a ‘ Job Application Letter ’, a cover letter for freshers must highlight one’s exceptional academic records and educational qualifications. Read this blog to find the best cover letter for job freshers, sample cover letters, format as well as free templates.
5 Best Resume Designs for 2023
This Blog Includes:
How to write a cover letter for fresher, cover letter for fresher: sample #1, cover letter for fresher: sample #2, cover letter for fresher: sample #3, cover letter for an engineering fresher: sample #4 , cover letter for a teacher: sample #5.
- Cover Letter for an MBA Fresher: Sample #6
Cover Letter for Job Application for Fresher: Sample #6
Cover letter for job application for fresher: sample #7, email cover letter for fresher: sample #8, cover letter for data analyst fresher: sample #9, tips and tricks to write the perfect job application letter.
Also Read: How to Write a Cover Letter?
While building a perfect cover letter , the candidate should follow the chronology to make it systematic and clear for the employer to have a glance at. Your potential recruiter will take around 5 to 6 seconds to scan through your resume, which means you need to sincerely work on your cover letter to serve as the opening act. Given below are the different components of a cover letter for fresher:
- Name of the employer/organization and the person to be contacted should be mentioned at the top-left corner of the page.
- Date of the application.
- Now comes the reference line or the subject of the cover letter which must contain the position you are applying for. For example, “Application for the position of Public Relations Officer .”
- Greet the employer directly by writing their name (for example Dear Mr X) instead of writing, “To whomsoever, it may concern”.
- After the greeting, start the body of the cover letter by introducing yourself to the reader.
- The second paragraph should highlight your educational background and your skills and qualities in a very crisp manner.
- Conclude the body of the cover letter by talking about “why should you be hired.”
- Lastly, thank the employer and mention your name and contact details.
Here’s our exclusive guide on Resume Format for Fresher !
A well-written cover letter for freshers can help employers pick out the most suitable candidate for the job. Thus, we have come up with a sample to help you write that perfect cover letter and get the recruiter’s attention.
Download Cover Letter for Fresher Sample PDF here!

Mr. Kapoor Brainside Link Road, Andheri, Mumbai,-400053 (xxx)xxxxxxx [email protected]
Subject: Application for the Counselor Position at Brainside
Dear Recruiter’s Name, I am excited to write this letter about the job opening as a counsellor at Brainside. I chanced upon your company’s opening at LinkedIn and upon reading the required qualities, I certainly believe I meet those requisite skills.
I completed my M. A in Psychology from KC College, Mumbai, and have also completed various certificate courses in REBT and CBT which add to my field of expertise. I hold a CGPA of 7.0 and have maintained above-average scores consistently throughout my master’s. Being passionate about my subject, I also invested considerable time in assisting senior psychologists and learning from them at every step.
I have also attached my resume and certainly believe that my skills and education qualifications make me fit for the position of counsellor. I assure you that I’ll put my best foot forward and conform with the team.
I’m grateful for this opportunity and waiting in anticipation to discuss the position in detail. My contact details are mentioned above for your reference.
Thank you. Respectfully, Rishi Sharma
Aarushi Jain [email protected] 98765432129876543210
June 24th, 2021
Mr. Shinde Planet Research C-14 Gurugram, Haryana, 110038 (xxx)xxxxxxx [email protected]
Subject: Application for the Engineer Position at Planet Research
Dear Mr. Shinde,
I am very eager to apply for the Engineer post at Planet Research. This particular position fully encapsulates everything I aspired to achieve as an engineer when I began my career. Your company’s work is intriguing, and I’ve read extensively about the cutting-edge technology that’s being used. This employment would put me on the proper track to achieving my professional objectives.
I learned to work in multidisciplinary teams that encompassed both technical and non-technical fields during my prior position as an intern at ABC Tech. Here, I was able to thrive as an excellent communicator, ensuring that all stakeholders’ demands were both properly conveyed to the team and satisfied to the greatest degree of customer satisfaction.
I was able to adopt a new testing method that reduced our beta testing period by up to 18%, allowing our clients to see a completed prototype weeks before our competition. In addition, I am presently preparing for my Fundamentals of Engineering test, which will put me on the route to becoming a Professional Engineer. I aim to improve as a Planet Research employee and as a professional in this job. In my new position, I am eager to work hard to fulfil the demands of the firm and to become a valuable member of the team as soon as possible.
Thank you for your thoughts and time. I’m excited to learn more about the Engineer role and Planet Research. My experience qualifies me for this role, and I want to be allowed to demonstrate to the team personally what a valuable contribution I can be. I’m looking forward to hearing from you.
Thank You Best Regards, Aarushi JainAarushi Jain
Mrs Ram, The Indian School C-77 Green Park Extension New Delhi, 110016 (xxx)xxxxxxx [email protected]
Subject: Application for 7th grade Mathematics Teacher Position at The Indian School.
Dear Mrs Ram,
I am writing to apply for the post of 7th grade Mathematics teacher at The Indian School. I acquired considerable student teaching experience with students while studying elementary education at the University of Delhi. I think that my commitment to teaching in urban settings, as well as my enthusiasm for assisting students who do not find Mathematics enjoyable, uniquely qualifies me to fill this role and contribute to The Indian School’s mission of shaping the leaders of tomorrow.
My nearly five years of studying Mathematics and 6 months of student teaching experience as an intern have properly equipped me to teach Mathematics to students with a distinct mentality and approach. I have considerable expertise with major digital teaching platforms, and I am dedicated to assisting students in mastering Mathematics to better prepare them for future and other competitive tests. I am also fluent in English and Hindi, so I can interact successfully with the bulk of the school’s student population.
As a teacher, I am deeply devoted to helping children learn new skills and expand on their unique abilities. I have a Master’s degree in Mathematics as well as a Bachelor’s degree in Education . I’m certified in bilingual education, technological education , and mathematics. I am now pursuing further certifications in Vedic Mathematics.
I would welcome the chance to meet with you in person to discuss this 7th grade Mathematics teacher vacancy. Thank you for taking the time to read this. I’m looking forward to conversing with you more.
Thank You Best Regards, Aarushi Jain
Cover Letter for an MBA Fresher: Sample # 6
Aarushi Jain [email protected] 9876543210
Mr Ahuja, ABS Consultancy Services A/77 Malviya Nagar New Delhi 110017 (xxx)xxxxxxx [email protected]
Subject: Application for the position of Recruitment Assistant.
Dear Mr Ahuja,
I’m writing to convey my enthusiasm for the post of Recruitment Assistant at your prestigious organisation. I have just completed my Master’s Degree in Business Administration with a major in Human Resource Management from ZXY University and would like to contribute my knowledge, talents, and dedication to excellence to your company’s creative atmosphere.
Throughout my schooling, I’ve gained the information required for the role, such as manpower recruiting, workforce organisation, personnel training and pay, as well as legal provisions and other labour problems.
My internship at JK Brothers & Company also provided me with valuable experience working with some of the top experts in the recruiting and human resources industries. During my four-month internship, I acquired an excitement and love for human resources, and the experience convinced me that human resource management is my genuine calling.
Among my accomplishments during my internship was serving as the project lead for the company’s sports engagement programme. I also collaborated with other interns, and we successfully organised an outing for 150 employees, for which we earned a 90 per cent ‘good to outstanding’ grade from the post-event survey comments.
Please see my résumé, which is attached to this letter, for further information on my qualifications and experience. Thank you for considering this application; I look forward to hearing from you.
Subject: Application for ________ position
Dear Mr/Ms/Mrs (Manager’s Name)
My name is _______. I came across the recent opening for _______(job position) on LinkedIn and I believe that the role describes me perfectly. I have always been interested in the workspace that your organisation is known to have in the market. I am seeking a challenging, competitive yet friendly environment and I truly believe that working at your organisation will be an enriching experience.
I have studied ________ (course) at _______ (university/college name) and I have recently completed a 3-month internship at _______ where I was a part of _______ team. It helped me learn about the type of roles and responsibilities in the field of ______, the work ethics that need to be followed as well as deadlines that need to be adhered to. I possess excellent communication and teamwork skills.
I have attached my resume along with the cover letter which contains my educational and professional qualifications. I will give a call to your office the next week to know more about the job profile. You can also reach out to me at the contact details mentioned in the resume.
Sincerely,
| Subject: Application for ________ position Dear Mr/Ms/Mrs (Manager’s Name) I am ________ (your name) currently pursuing _______ (course name). As I am seeking opportunities in _______ (field), I came across the job opening for the position of _______. I am very interested in working for ________ (company name). I believe that I meet the required qualifications and skills for this position. I have strong analytical and problem-solving skills (mention any other skills related to the position) and have no problem working under tight deadlines as and when required of me. Along with being at the top of my class during my undergraduate degree, I have also been extensively involved in extracurricular activities. More details are present in my resume which is enclosed with this letter. I await your call for a personal interview with regard to this position. Thank you for your consideration. Sincerely, Name |
A job application e-mail is quite similar to a cover letter since you have to elaborate on your educational and professional qualifications, skills as well as how well you fit the job role. Here is a job application email sample for freshers:
Subject: Application for the Role of Junior Editor- Content (Job Role) at Headspace (Company Name)
Dear Mr./Miss ______ (Name of the Recruiter or Manager),
I recently came across the opening of Junior Editor – Content at Headspace on LinkedIn and I wanted to apply for this role. The job role fits my qualifications and skills thus making me a perfect fit for this position.
I recently graduated with a first-class honours degree in English Literature from the University of Delhi and have also worked as a magazine editor for my college magazine during my undergraduate studies. Further, I have also worked as an editorial intern at Rupa Publications assisting the team of head editors and managing editors for over 6 months. I believe that I have the requisite qualifications and skills required for this role.
Please find attached my CV and cover letter with this email. I look forward to hearing back from you.
Thanks and Regards, Ritu
Subject: Application for the role of Associate Data Analyst at Infoysis
Dear Sir/ Ma’am ________ (Name of Recruiter or Manager)
I am writing to express my strong interest in the Entry-Level Data Analyst position at [Company Name], as advertised on your website. As a recent graduate with a degree in [Your Degree] from [Your University], I am eager to contribute my analytical skills and passion for data to your dynamic team.
During my academic studies, I developed a solid foundation in statistical analysis, data interpretation, and database management. My coursework included hands-on experience with tools such as Python, R, and SQL, and I successfully completed projects that involved collecting, cleaning, and analyzing data to derive actionable insights.
In my internship at [Previous Company/Institution], I had the opportunity to apply my skills in a real-world setting. What excites me about [Company Name] is your commitment to innovation and the use of cutting-edge technologies.
I am confident that my academic achievements, coupled with my practical experience, make me a strong candidate for this position. I am excited about the opportunity to contribute to [Company Name] and grow as a professional in the field of data analysis.
Thank you for considering my application. I look forward to the possibility of discussing how my skills and enthusiasm align with the goals of [Company Name].
Writing a cover letter is not as easy as you think it to be. It takes a great deal of patience and self-introspection to formulate a cover letter which proves your worthiness and makes your job application outshine other applications. Let us have an insightful look into the different tips to keep in mind while creating a cover letter for freshers:
- Never restate anything that you have already mentioned in your resume.
- Since you are a fresher, all you have as an asset is your education and your skills. Highlight these two factors most gracefully and cleverly possible.
- After you draft your cover letter , make sure there are no grammatical errors.
- Avoid making your cover letter too long and keep it limited to one page.
- Never be dishonest at any point or brag about your qualities.
- Think of a good conclusion and sign off with words such as, “Thanking You,” “Best Regards,” “Sincerely,” etc.
- When writing a cover letter for fresher, be specific about the job role and description.
Explore more interesting reads below
In the 1st Paragraph- Introduce yourself. In the 2nd Paragraph- highlight your relevant skills, work experiences, achievements, and accomplishments. In the 3rd Paragraph- Highlight your best qualities and explain why you’re a good fit for an XYZ company or organization. In the 4th Paragraph- Conclude with a call to action.
Examine the job posting carefully and do some research on the company’s website. At the start of the paper, include your contact information. Introduce yourself and greet the reader. Explain your relevant abilities and achievements for the role. Remind them why you’re the ideal candidate for the job.
A well-written cover letter for a fresher can assist companies in selecting the best applicant for the position.
Your cover letter should accomplish four goals: Introduce yourself as a strong prospect to the possible employer. Include your reasoning for why the employer should hire you. Make a pleasant and lasting first impression of the company as a candidate.
There are 3 types of cover letters: the application cover letter, the prospecting cover letter, and the networking cover letter.
As a fresher, job hunting can be a tedious task and creating a resume or a cover letter for a fresher are equally important. There are certain things we tend to miss out – when we lack work experience. But do not worry, our study abroad experts at Leverage Edu will assist you in developing a job-winning resume with perfection. Give us a follow on Facebook , Instagram , and LinkedIn . Call us immediately at 1800 57 2000 for a free 30-minute counselling session.
Team Leverage Edu
Leave a Reply Cancel reply
Save my name, email, and website in this browser for the next time I comment.
Contact no. *

Leaving already?
8 Universities with higher ROI than IITs and IIMs
Grab this one-time opportunity to download this ebook
Connect With Us
45,000+ students realised their study abroad dream with us. take the first step today..

Resend OTP in

Need help with?
Study abroad.
UK, Canada, US & More
IELTS, GRE, GMAT & More
Scholarship, Loans & Forex
Country Preference
New Zealand
Which English test are you planning to take?
Which academic test are you planning to take.
Not Sure yet
When are you planning to take the exam?
Already booked my exam slot
Within 2 Months
Want to learn about the test
Which Degree do you wish to pursue?
When do you want to start studying abroad.
January 2025
September 2025
What is your budget to study abroad?

How would you describe this article ?
Please rate this article
We would like to hear more.
How to write a successful pharmacy cover letter
Writing a cover letter that helps you stand out from other applicants can be challenging. Pharmacy career consultant Amy Zydzienowski, from Vertica Career Consultants, explains how it is done.

Shutterstock.com
Also read: How to write a successful pharmacy CV
Do I really need to write a cover letter? In my role as a pharmacy recruiter and career coach, I am often asked whether a cover letter is a necessary accompaniment to a CV as part of a job application. Some people believe that cover letters just repeat information from their CVs — others are concerned that recruiters never even read them. However, if you can write a cover letter that explains why you are the right candidate for the job you want, you can ensure that your application stands out for the right reasons.
Cover letters — what’s the point?
Traditionally, you would send a CV and cover letter by post in response to a job application or as a speculative approach. Your cover letter would introduce you in a professional sense, explain why you were applying for the position and provide some evidence of your competency for the role.
Nowadays, applications are less frequently sent by post — they are usually emailed or completed online. Despite this, presenting a professional cover letter is still part of the established protocol. If you are responding to a job advertisement in today’s competitive pharmacy jobs market, it is likely you will be one of many applicants (sometimes one of hundreds). Therefore, you need to do everything you can to ensure your application stands out, which includes an excellent cover letter.
Consider the hiring manager’s point of view
Before you start writing your CV and cover letter, you need to ask yourself: “What would the hiring manager want to see in the application?”
One way to find out is to simply call and ask the hiring manager or recruiter exactly what they would like to see. A recent survey of US employers by Saddleback College in America has shown that they can have differing views on whether a cover letter is important, how long it should be and what information it should contain. By asking the employer directly, you can find out their specific views and tailor your cover letter accordingly.
Laying out your letter
There are no set rules for your cover letter, but a good structure is important. The cover letter is telling a story about you and, like all good stories, it should have a beginning, middle and end.
The beginning
If the application is being posted then use a standard letter format, with your own address and date on the right and the organisation’s contact name and address on the left. For email applications, put your cover letter in the main body of the email and add your CV as an attachment. Your cover letter can be ignored more easily if you attach it as a separate document.
You should always try to address your cover letter to a specific person when possible. This will be easier if you have already called the hiring manager. Research by Forum3 (now called Charity People), a not-for-profit recruitment company for the third sector, suggests you are 10–15% more likely to receive a reply if you address your application to a person and 5% more likely to get an interview [1] . If you do not know the name of the person, then use a professional address such as “Dear Sir or Madam”.
Immediately after addressing the reader, you should state the purpose of the application, so that the reader can quickly understand the reason for the email. For example, “Reference: Application for Band 6 hospital pharmacist role”. This could also be included as the subject line of the email.
The first paragraph should describe what your current professional situation is and why you are applying for the position. This paragraph should also include any research you have done into the role or organisation, including anyone you have spoken to, any site visits you have undertaken and the name of anyone who may have referred you. If you have taken the time to research the organisation and the role, this could be a key differentiator for your application. However, you should avoid making generic statements, such as “I want to join your esteemed company”. Make sure anything you say about an organisation is relevant to them and based on the research you have undertaken.
Your cover letter should demonstrate to the reader that you have the key skills and experience relevant to the particular role. You can do this by providing specific examples, tailored to the requirements listed in the job description, of when you have demonstrated these from your own experience to date. Choose three or four of these relevant examples that each tell a story about your skills, experience or traits and provided a positive outcome for the stakeholders involved in the situation.
These examples could come from any part of your life, as long as they are relevant. Newly qualified pharmacists will likely use examples from their pharmacy placements, academia, part-time work and also extra-curricular activities, in order to demonstrate a range of skills. A more experienced pharmacist candidate would generally choose examples from their work history because it is the most relevant. However, sometimes it is appropriate to bring in other examples, such as voluntary work.
Always try to use an active voice when explaining your achievements, because this serves to make the reader feel that you were in control in these situations. Additionally, try to avoid making vague or generic statements that could apply to any applicant.
If an achievement is strong enough to be included in your cover letter, it should be repeated on your CV. Try not to repeat examples word-for-word on both documents — instead, try to interpret them differently. Sometimes, due to time constraints, the hiring manager may bypass your cover letter and go straight to your CV, which could mean they miss your best examples. In addition, repetition will serve to reinforce these key messages like a sales brochure would, which is, in essence, what your CV and cover letter are.
In your final paragraph, thank the reader for taking the time to read your application and summarise why you feel you are a good fit for the role, based on your skills and experience. State how and when you can be contacted with regards to arranging an interview and then make sure you are available when you say you will be.
Sign off the letter professionally with “Yours sincerely” (to a specific person) or “Yours faithfully” (to an unnamed person), followed by your name.
Formatting your letter
In terms of format, a cover letter is usually written as a traditional letter, laid out in paragraphs. It is different to your CV, which is an abbreviated document that uses various techniques to draw the eye to the most important parts quickly and make the document as succinct as possible. In your cover letter, keep your language concise and purposeful. To achieve this, you may need to redraft your letter several times.
The grammar, spelling and formatting of your cover letter is just as important as the content of the document, so make sure it is perfect. Particularly, ensure you have spelt names and company names correctly and there are no typing errors. Research from student recruitment website StudentGems.com suggests half of employers discard job applications that contain spelling or formatting errors.
Choose a standard, well known and professional font, such as Arial, Verdana, Calibri, Times New Roman or Trebuchet. This will make the letter easier to read and will also support applicant tracking systems that may not be able to pick up lesser-known fonts. Keep your font size between 10 and 12 for ease of reading.
Through my own experience of recruiting pharmacists, I have observed that the standard of today’s pharmacy job applications is generally quite poor. If you spend time putting together a strong application, which includes an excellent cover letter, then it will stand out to an employer and increase your chances of securing the role.
[1] The company has since rebranded as www.charitypeople.co.uk and the research is no longer available online.
You may also be interested in

How to enhance a pharmacy CV

How to write a successful pharmacy CV

Pharmacy regulator to require ‘two-way communication’ in new draft guidance
Pharmacist cover letter examples
If you’re hoping to secure your next pharmacist role, but you’re struggling with the application process, let us dispense some helpful advice.
You need to tailor your application and highlight your relevant skills, and experience.
Find out how to do just that using our pharmacist cover letter examples and top tips in the detailed guide below.
CV templates
Pharmacist cover letter example 1

Build your CV now
Pharmacist cover letter example 2

Pharmacist cover letter example 3

The Pharmacist cover letter examples above should give you a good idea of the type of content you need to include in your own cover letter, and how it should be structured.
But if you’re really looking to wow recruiters and get your CV in front of the very best employers, then check out our guidance on how to write your own effective cover letter below.
How to write a Pharmacist cover letter
Here’s how to write your own winning Pharmacist cover letter
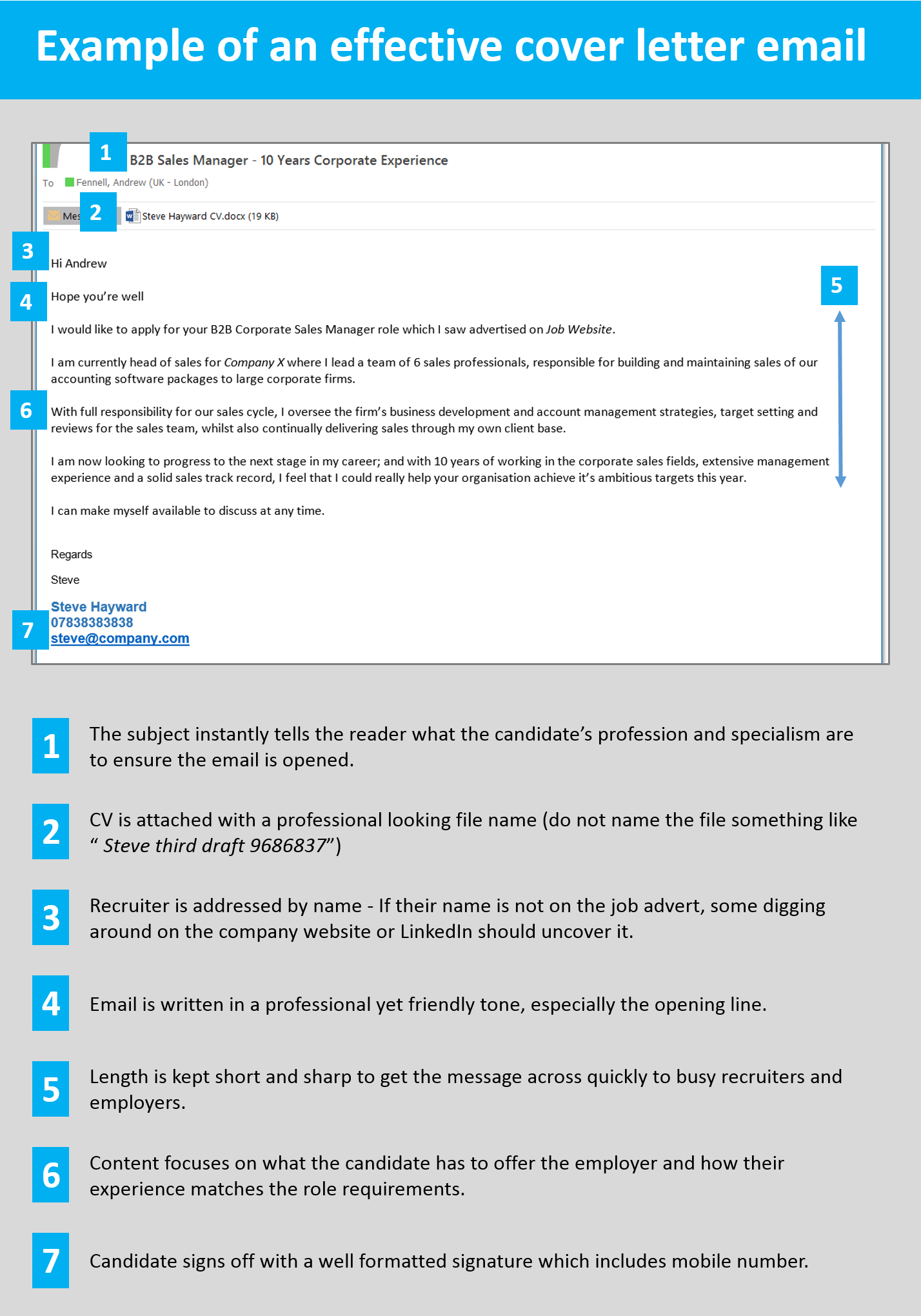
Write your cover letter in the body of an email/message
When writing your Pharmacist cover letter, it’s best to type the content into the body of your email (or the job site messaging system) and not to attach the cover letter as a separate document.
This ensures that your cover letter gets seen as soon as a recruiter or employer opens your message.
If you attach the cover letter as a document, you’re making the reader go through an unnecessary step of opening the document before reading it.
If it’s in the body of the message itself, it will be seen instantly, which hugely increases the chances of it being read.
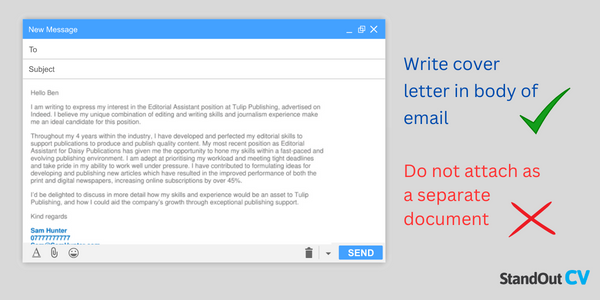
Start with a friendly greeting

Start you cover letter with a greeting that is professional but friendly.
This will build rapport with the recruiter whilst showing your professionalism.
- Hi, hope you’re well
- Hi [insert recruiter name]
- Hi [insert department/team name]
Avoid overly formal greetings like “Dear sir/madam ” unless applying to very traditional companies.
How to find the contact’s name?
Addressing the recruitment contact by name is an excellent way to start building a strong relationship. If it is not listed in the job advert, try these methods to find it.
- Check out the company website and look at their About page. If you see a hiring manager, HR person or internal recruiter, use their name. You could also try to figure out who would be your manager in the role and use their name.
- Head to LinkedIn , search for the company and scan through the list of employees. Most professionals are on LinkedIn these days, so this is a good bet.
Identify the role you are applying for
Now that you have warmed the recruiter up with a friendly greeting, firstly you need to let them know which role you are applying for.
Sometimes a recruitment consultant will be juggling 10 or 10 vacancies, so it’s important to specify which one you are applying to.
Give us much detail as possible (team/department, role title etc.) and paste in the reference number if you have one.
Here are some examples you can use.
- I am interested in applying for the role of Pharmacist with your company.
- I would like to apply for the role of Sales assistant (Ref: 40f57393)
- I would like to express my interest in the customer service vacancy within your retail department
- I saw your advert for an IT project manager on Reed and would like to apply for the role.
See also: CV examples – how to write a CV – CV profiles
Highlight your suitability
The sole objective of your cover letter is to motivate recruiters into to opening your CV. And you achieve this by quickly explaining your suitability to the roles you are applying for.
Take a look at the job descriptions you are applying to, and make note of the most important skills and qualifications being asked for.
Then, when crafting your cover letter, make your suitability the central focus.
Explain why you are the best qualified candidate, and why you are so well suited to carry out the job.
This will give recruiters all the encouragement they need to open your CV and consider you for the job.

Keep it short and sharp
When sending a job application to a recruiter or hiring manager, it is important to remember that they will normally be very busy and pushed for time.
Therefore, you need to get you message across to them quickly (in a matter of seconds ideally). So, keep your cover letter short and to-the-point. A long waffling cover letter will overwhelm recruiters when they are running through hundreds of emails in there inbox, but a concise one will get their attention.
So, keep your cover letter to just a few sentences long, and save the extensive detail for your CV.
Sign off professionally
To round of your cover letter, add a professional signature to the bottom, giving recruiters your vital contact information.
This not only gives various means of contacting you, it also looks really professional and shows that you know how to communicate in the workplace.
Include the following points;
- A friendly sign off – e.g. “Warm regards”
- Your full name
- Phone number (one you can answer quickly)
- Email address
- Profession title
- Professional social network – e.g. LinkedIn
Here is an example signature;
Warm regards,
Gerald Baker Senior Accountant 07887500404 [email protected] LinkedIn
Quick tip : To save yourself from having to write your signature every time you send a job application email, you can save it within your email drafts, or on a separate document that you could copy in.

What to include in your Pharmacist cover letter
So, what type of information should you write about in your Pharmacist cover letter?
The specifics will obviously depend on your profession and the jobs you are applying to, but these are the key areas you should be covering.
- Your industry experience – Tell recruiters the types of companies you have been working for and the roles you have held in the past.
- Your qualifications – Highlight your most important relevant qualifications to show employers you are qualified to do the roles you are applying for.
- The impact you have made – Demonstrate the positive impact you have made for employers in previous jobs. Have you saved money? Improved processes? Made customers happy?
- Your reasons for moving – Employers will want to know why you are leaving your current/previous role, so provide them with a brief explanation here.
- Your availability – When will you be able to start a new job ? Check your current contract to find out your notice period if you are in a position already.
Pharmacist cover letter templates
Copy and paste these Pharmacist cover letter templates to get a head start on your own.
I am writing to express my interest in the Junior Pharmacist position at Pertemps Medical. As a recent Pharmacy Graduate with a GPhC registration, strong academic background, and a passion for delivering quality patient care, I am eager to begin my career and contribute to the success of your team.
During my education at the University of Manchester, I gained a solid foundation in pharmacology, medication management, and patient counselling. My coursework included hands-on experience in compounding, drug interactions, and pharmaceutical calculations, which have prepared me to provide accurate and safe services.
I have completed a clinical rotation at the NHS, where I had the opportunity to work closely with experienced pharmacists and medical professionals. I participated in medication reconciliation, consultations, and prescription verification, which demonstrated my attention to detail and commitment to ensuring optimal health outcomes, as well as assisted in reducing overstock by 20%, minimising waste, and attaining £1K cost-savings though effectively maintaining inventory.
Thank you for taking the time to consider my application, and I am available for an interview ASAP.
Kind regards
Grace Peters
Good day David
I am writing to you concerning the advertised Pharmacist position at Trust Primary Care Ltd on LinkedIn. With 8 years of experience in pharmacy practice and a proven track record of leadership and process improvement, I am eager to contribute my competencies and expertise to your dynamic department.
Throughout the duration of my time working for Hampshire Hospitals, I have successfully implemented process optimisation strategies that have positively impacted workflow efficiency and patient care. I successfully introduced a medication synchronisation programme that reduced patient wait times by 30% and increased medication adherence rates by 50% and helped boost health outcomes by 10% through organising and facilitating 25+ education workshops for patients and caregivers, where topics such as diabetes management and cardiovascular health were addressed.
Another aspect I can bring is my ability to lead and mentor junior team members due to fostering a culture of continuous learning and growth. I have also collaborated with interdisciplinary healthcare personnel to ensure seamless patient transitions and coordinated care throughout all interactions.
I am immediately available for an interview to discuss my qualifications/licences and additional skills.
Troy Simmonds
Good morning, Marc
As a seasoned healthcare professional, I am excited to apply for the Senior Pharmacist vacancy at Airedale NHS Foundation Trust. With a strong track record of process optimisation, quality assurance, and operational leadership, I am eager to contribute towards your team’s success.
Over the past 13 years working as a Pharmacist for Tesco, I have been instrumental in streamlining medication dispensing procedures, implementing inventory control measures, and enhancing workflow. Here, I spearheaded initiatives that resulted in a 30% reduction in prescription processing times, improved patient satisfaction scores by 20%, and achieved a 95% adherence to QA protocols. Furthermore, my experience in supervising cross-functional teams and conducting regular performance audits has contributed to maintaining a culture of excellence and continuous improvement.
I am proud to consistently bridge the gap between pharmacists and doctors/nurses to ensure successful treatment plans and enhanced patient and safety outcomes.
Please feel free to contact me to schedule for an interview as I am available ASAP, or if you require any additional information concerning my qualifications and skills.
Rachel Meadows
Writing an impressive cover letter is a crucial step in landing a Pharmacist job, so taking the time to perfect it is well worth while.
By following the tips and examples above you will be able to create an eye-catching cover letter that will wow recruiters and ensure your CV gets read – leading to more job interviews for you.
Good luck with your job search!

IMAGES
VIDEO
COMMENTS
Domestic Violence Advocate Cover Letter Example. Drug and Alcohol Counselor. Drug Safety Associate Cover Letter Example. Family Advocate Cover Letter Example. Family Support Worker Cover Letter Example. Fundraising Manager Cover Letter Example. Gender Specialist Example. Hospital Social Worker Cover Letter Example.
Here is the Thorough Drug Safety Associate Cover Letter Example: Dear Ms. Monique Downey, I am writing to apply for the Drug Safety Associate position with Onyx Pharmaceuticals, Inc. I have a bachelor's degree in pharmacy and the ability to work with the physicians in drug safety to make sure all medical assessments and reviews are reported ...
Example of Pharmacovigilance Scientist Cover Letter. 2196 Runolfsson Flat. North Blairmouth, TN 73689. Dear Campbell Franecki, I am excited to be applying for the position of pharmacovigilance scientist. Please accept this letter and the attached resume as my interest in this position.
2. Add your contact information. Add your contact details at the top of your resume. Include your full name, phone number, email address, city, state or union territory. This makes it easier for an interviewer to contact you for follow-up discussions or to schedule meetings.
Claire. [email protected]. (555) 432-1000. Montgomery Street, San Francisco, CA 94105. Professional Summary. Enthusiastic Industrial Pharmacist with a long track record of maintaining positive relationships with customers, pharmaceutical representatives, medical liaisons, opinion leaders, and healthcare organizations.
Again, if you're submitting your application through an online portal, you don't need to include the date within the body of your letter. But if you're sending a prospective cover letter or are submitting a hard copy, include the date before the start of your letter. Personalise the Salutation. Find out the name of the Hiring Manager ...
The Guide To Resume Tailoring. Guide the recruiter to the conclusion that you are the best candidate for the pharmacovigilance scientist job. It's actually very simple. Tailor your resume by picking relevant responsibilities from the examples below and then add your accomplishments. This way, you can position yourself in the best way to get ...
Senior Pharmacovigilance Scientist. Leads the signal management process (i.e., signal tracking, leading review meetings, etc.) for assigned product (s) and evaluates safety data and signals as part of ongoing pharmacovigilance activities. This includes synthesis of data from multiple sources and authoring signal evaluation reports.
If you don't have the name of a contact, address the letter to "Dear Hiring Manager," or "Dear Human Resources Director.". Don't address the letter to "To whom it may concern," or "Dear Sir/Madam". Limit the letter to 3-4 paragraphs, and 1 page. Expand on your resume; do not repeat it verbatim. Don not copy exact words ...
2. Create a header. Begin your clinical research cover letter with a header that includes your personal information, such as your full name, current location, email address and phone number. Also, include the date you plan to submit the cover letter. Below your information, list the hiring manager's name, the company name and the location of ...
Senior Drug Safety Associate. 10/2011 - 09/2017. Boston, MA. Proven ability to influence and collaborate with business stakeholders effectively and in a positive manner. Great project management skills and ability to execute and manage multiple projects and deadlines with shifting priorities and resources in a fast-paced working environment.
Shayna Booker. City, State, Zip Code. Home: 000-000-0000 | Cell: 000-000-0000. [email protected]. Summary. Analytical and results-oriented Drug Safety Associate with over 10 years' experience collaborating as a strong team player to uncover and address pharmaceutical safety issues. Great communication organization and time management abilities.
14. Guidance for Industry. • Information to include in the cover letter can also be found: -in specific guidances for submission types or -in a specific MAPP (Manual of Policies and ...
Email Cover Letter for Fresher: Sample #8. A job application e-mail is quite similar to a cover letter since you have to elaborate on your educational and professional qualifications, skills as well as how well you fit the job role. Here is a job application email sample for freshers: Subject: Application for the Role of Junior Editor- Content ...
The beginning. If the application is being posted then use a standard letter format, with your own address and date on the right and the organisation's contact name and address on the left. For email applications, put your cover letter in the main body of the email and add your CV as an attachment. Your cover letter can be ignored more easily ...
Write your cover letter in the body of an email/message. When writing your Pharmacist cover letter, it's best to type the content into the body of your email (or the job site messaging system) and not to attach the cover letter as a separate document.. This ensures that your cover letter gets seen as soon as a recruiter or employer opens your message.
Here's how to format a pharmacist cover letter: Use a 3-paragraph layout, with or without bullet points. Set 1-inch margins and single line-spacing. Left-align all your cover letter parts. Set your length at a page or less. Use the cover letter font from your CV.
Pharmacovigilance Specialist , 03/2013 to 06/2015. Grifols Inc. - Rexburg, ID. Responsible for instituting and maintaining the key aspects of drug safety. Collecting, monitoring, processing and documenting of adverse reactions to medical products. Communication of relevant safety information to national and regional regulatory authorities ...
But she really wants this awesome pharmacist job she found. It's got a fat 401K and full benefits. This sample cover letter for a pharmacist position will put her in the lab coat: Example #2: Junior Pharmacist Cover Letter. Example #2: Junior Pharmacist Cover Letter—Text Sample. Anna Zuber.
Regulatory Affairs and Pharmacovigilance Manager, 05/2021 - 08/2022. Anthem, Inc. - Middletown, NY. Worked with governance committees to make and evaluate plans. Always in contact with every client for answering and also requiring necessary documentation or service as I have been working closely with all departments.