Development of apple wine from Golden Delicious cultivar using a local yeast isolate
- Original Article
- Published: 11 April 2019
- Volume 56 , pages 2959–2969, ( 2019 )

Cite this article

- Sukhvir Sukhvir 1 &
- G. S. Kocher ORCID: orcid.org/0000-0003-0189-8871 1
596 Accesses
8 Citations
Explore all metrics
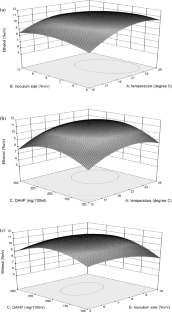
The present study was conducted to optimize fermentation parameters for apple wine production using Golden Delicious apples. Physicochemical analysis of the cultivar revealed a °Brix-acid ratio of 24.61 with ample amount of total and reducing sugars (9.6 and 6.03% w/v); making it a suitable substrate to produce ethanol. Microbiological analysis lead to isolation of a yeast strain (namely A2) which was molecularly identified and accessed at GenBank as S. cerevisiae KY069279. Ethanol fermentation optimization using response surface methodology revealed that a temperature of 20 °C, an inoculum size of 7.08 (%v/v) and diammonium hydrogen phosphate supplementation @ 154.4 mg/100 mL as optimum for apple wine production which lead to 10.73% (v/v) ethanol production with a desirability of 86.9%. Fresh wine having malic acid content of 1.87 (mg/100 mL) was subjected to malolactic fermentation (MLF) for 8 days using Leuconostoc oenos NCIM 2219 resulting in apple wine having 0.4 (mg/100 mL) malic acid. Sensory analysis of MLF and non-MLF apple wines categorised them as superior quality with average scores of 69.5 and 74.5, respectively. Gas chromatography–mass spectrometric analysis of apple wine revealed the presence of 38 volatile compounds including higher alcohols, acids, esters etc. The study thus revealed a process for apple wine preparation using an indigenous yeast and also optimized and compared malolactic and non-malolactic fermented ciders.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Similar content being viewed by others

Optimization of a Process for Preparation of Base Wine for Cider Vinegar Production

The profile of organic acids and polyphenols in apple wines fermented with different yeast strains
Table wine from tropical fruits utilizing natural yeast isolates, explore related subjects.
- Environmental Chemistry
Alberti A, Vieira RG, Drilleau JF, Wosiacki G, Nogueira A (2011) Apple wine processing with different nitrogen contents. Braz Arch Biol Technol 54(3):551–558
Article Google Scholar
Amerine MA, Roessler EB (1976) Wines: their sensory evaluation. Freeman, San Francisco
Google Scholar
Cabranes C, Mangas JJ, Blanco D (1997) Selection and biochemical characterisation of Saccharomyces cerevisiae and Kloeckera Apiculata strains isolated from Spanish cider. J Inst Brew 103:165–169
Article CAS Google Scholar
Campo GD, Berregi IA, Santos JI, Duen M, Irastorza A (2007) Development of alcoholic and malolactic fermentations in highly acidic and phenolic apple musts. Biores Technol 99:2857–2863
Cheema HS, Singh B (1991) Software Statistical Package CPCS-1. Developed at Department of Statistics, Punjab Agricultural University, Ludhiana
Cruz SH, Cilli EM, Ernandes JR (2002) Structural complexity of the nitrogen source and influence on the yeast growth and fermentation. J Inst Brew 108:54–61
Davis CR, Wibowo DJ, Eschenbruch R, Lee TH, Fleet GH (1985) Practical implications of malolactic fermentation: a review. Am J Enol Vitic 36:290–301
CAS Google Scholar
Downing DL (1989) Apple cider. In: Downing DL (ed) Processed apple products, 1st edn. Van Nostrand Reinhold, New York, pp 169–188
Chapter Google Scholar
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
GenBank (2016) GenBank Submissions Handbook www.ncbi.nlm.nih.gov/books/NBK51157 . Accessed 2 Nov 2016
Goodban AE, Stark JB (1957) Rapid method for determination of malic acid. Anal Chem 29:283–287
Henick-Kling T (1993) Malolactic fermentation. In: Fleet GH (ed) Wine microbiology and biotechnology, 1st edn. Harwood Academic Publishers, Switzerland, pp 289–326
Henick-Kling T, Acree TE, Krieger SA, Laurent MH, Edinger WD (1994) Modification of wine flavour by malolactic fermentation. Wine East 4:8–15
Herrero M, Garcial LA, Diaz M (2006) Volatile compounds in cider: inoculation time and fermentation temperature effects. J Inst Brew 112(3):210–214
Jarvls B, Forster MJ, Kinsella WP (1995) Factors affecting the development of cider flavour. J Appl Bacter Symp Sup 79:5S–18S
Jolicoeur C (2013) The new cider maker’s handbook: a comprehensive guide for craft producers. Chelsea Green Publishing, Hartford
Joshi VK, Attri D (2005) Panorma of research and development of wines in India. J Sci Nat Res 64:9–18
Joshi VK, Kumar V (2017) Influence of different sugar sources, nitrogen sources and inocula on the quality characteristics of apple tea wine. J Inst Brew 123(2):268–276
Kahle K, Kraus M, Richling E (2005) Polyphenol profiles of apple juices. Mol Nut Food Res 49:797–806
Laurent MH, Henick-Kling T, Acree T (1994) Changes in the aroma and odor of Chardonnay wine due to malolactic fermentation. Wein Wissenschaft 49:3–10
Liu SQ (2002) Malolactic fermentation in wine- beyond deacidification: a review. J Appl Microbiol 92:589–601
Article CAS PubMed Google Scholar
Lorrenzini M, Zapparoli G (2019) Assessment of yeasts for apple juice fermentation and production of cider volatile compounds. LWT Food Sci Tech 99:224–230
Malik CB, Singh MB (1991) Plant enzymology and histoenzymology. Kalyani Publishers, New Delhi, pp 274–279
Miller GL (1959) Use of Dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Musyimi SM, Sila DN, Okoth EM, Onyango CA, Mathooko FM (2013) The influence of process optimization on the fermentation profile of mango wine prepared from the Apple mango variety. J Animal Plant Sci 17(3):2600–2607
NHB (2015) World Wide Web source for National Horticulture Board data. http://nhb.gov.in/area-pro/NHB_Database_2015.pdf . Published 11 Feb 2015. Accessed 5 Apr 2016
Nikhanj P, Kocher GS, Boora RS (2017) Fermentative production of guava wine from pectinase treated and untreated juice of ‘punjab pink’ cultivar of Psidium guajava L. Agric Res J 54(2):244–247
Novak A, Wilk MK, Pogorzelski E, Czyowska A (2013) Effect of nitrogen sources on fermentation process and formation of H 2 S and ethyl carbamate by wine yeast. Biotech Food Sci 77(1):11–23
Oyeleke O (2007) Extraction of juice from some tropical fruits using a small scale fruit-juice extractor. Afr Crop Sci 8:1803–1808
Peng B, Lei Y, Zhao H, Cui L (2015) Response surface methodology for optimization of fermentation process parameters for improving apple wine quality. J Food Sci Technol 52(11):7513–7518
Polshettiwar SA, Ganjiwale RO (2007) Spectrophotometric estimation of total tennins in some ayurvedic eye drops. Ind J Pharm Sci 69(4):574–576
Pooja (2016) Optimization of enological practices for production of quality wines from grapes. Ph.D., Dissertation. Punjab Agricultural University, Ludhiana, India
Santos JP, Arroyo T, Aleixandre M, Lozano J, Sayago I, García M, Fernándeza MJ, Arésa L, Gutiérreza J, Cabellosb JM, Gilb M, Horrilloa MC (2004) A comparative study of sensor array and GC–MS: application to Madrid wines characterization. Sens Actuators B Chem 102:299–307
Sauvageot F, Vivier P (1997) Effects of malolactic fermentation on sensory properties of four Burgundy wines. Am J Enol Vitic 48(2):187–192
Shankar S, Babu JD, Reddy YN (2006) Fermentation of guava pulp with grape grown yeast (Saccharomyces cerevisiae var. ellipsoideus) for wine production. Ind J Hort 63(2):171–173
Sukhvir (2016) Evaluation of apple cultivars for fermentative production of cider. M.Sc. thesis, Punjab Agricultural University, Ludhiana, India
Volschenk H, Van Vuuren H, Viljoen-Bloom M (2006) Malic acid in wine: origin, function and metabolism during vinification. S Afr J Enol Vitic 27(2):123–136
Wang D, Xu Y, Hu J, Zhao G (2004) Fermentation kinetics of different sugars by Apple wine Yeast Saccharomyces cerevisiae . J Inst Brew 110:340–346
Williams AA (1974) Flavour research and the cider industry. J Inst Brew 80:455–470
Zhao H, Zhou F, Dziugan P, Yao Y, Zhang J, Lv Z, Zhang B (2014) Development of organic acids and volatile compounds in cider during malolactic fermentation. Czech J Food Sci 32:69–76
Download references
Author information
Authors and affiliations.
Department of Microbiology, Punjab Agricultural University, Ludhiana, 141001, India
Sukhvir Sukhvir & G. S. Kocher
You can also search for this author in PubMed Google Scholar
Corresponding author
Correspondence to G. S. Kocher .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Reprints and permissions
About this article
Sukhvir, S., Kocher, G.S. Development of apple wine from Golden Delicious cultivar using a local yeast isolate. J Food Sci Technol 56 , 2959–2969 (2019). https://doi.org/10.1007/s13197-019-03771-0
Download citation
Revised : 19 March 2019
Accepted : 04 April 2019
Published : 11 April 2019
Issue Date : 01 June 2019
DOI : https://doi.org/10.1007/s13197-019-03771-0
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Fermentation
- Response surface methodology
- Find a journal
- Publish with us
- Track your research
Wine Production from Apple (Malus Pumila) Using Yeast Isolated from Palmwine
Current Journal of Applied Science and Technology, 41(3): 1-6, 2022, Article no.CJAST.57683, ISSN: 2457-1024
6 Pages Posted: 12 May 2022
Constance Chinyere Ezemba
Chukwuemeka Odumegwu Ojukwu University; Chychy Gilgal Limited
Vivian Nonye Anakwenze
Nnamdi Azikiwe University - Department of Applied Microbiology and Brewing
A. S. Ezemba
Nnamdi azikiwe university.
Date Written: 2022
The study was aimed at the production of apple (Malus pumila) fruit wine with the use of yeast Saccharomyces cerevisiae isolated from palm wine. Both primary and secondary fermentation of the apple lasted 28 days. Aliquot samples were removed and used daily from the fermentation tank for analysis of alcohol content, specific gravity, pH, titratable acidity, and reducing sugar using standard procedures. During fermentation, pH of the fruit must range from 5.0 to 3.2. There was an increase in alcohol content, which was observed with time. Finally at the end of the 28th day’s fermentation, the alcohol concentration in the fruit wine was observed to be 3.2%. Also titratable acidity concentration of the wine shows steady increase with time throughout the fermentation period. This study has revealed that much acceptable wine with quality could be produced from apple with Saccharomyces cerevisiae isolated from palm wine. Sensory evaluation results showed there were no significant differences (p > 0.05) in flavor, taste, clarity and overall acceptability between apple wine and a reference wine. The apple wine was generally accepted.
Keywords: Apple; fermentation; saccharomyces cerevisiae; wine; fruit
Suggested Citation: Suggested Citation
Chinyere Constance Ezemba (Contact Author)
Chukwuemeka odumegwu ojukwu university ( email ).
Nigeria +2348037444096 (Phone)
Chychy Gilgal Limited ( email )
Locust RIVERSIDE, CA CA 92501-3121 United States 4156055932 (Phone)
Nnamdi Azikiwe University - Department of Applied Microbiology and Brewing ( email )
Enugu-Onitsha Expressway PMB 5025 Awka, DE Anambra State 234 Nigeria
Do you have a job opening that you would like to promote on SSRN?
Paper statistics, related ejournals, biology & sustainability ejournal.
Subscribe to this fee journal for more curated articles on this topic
Life Sciences Education eJournal
Chemical engineering ejournal, food microbiology ejournal.
Information
- Author Services
Initiatives
You are accessing a machine-readable page. In order to be human-readable, please install an RSS reader.
All articles published by MDPI are made immediately available worldwide under an open access license. No special permission is required to reuse all or part of the article published by MDPI, including figures and tables. For articles published under an open access Creative Common CC BY license, any part of the article may be reused without permission provided that the original article is clearly cited. For more information, please refer to https://www.mdpi.com/openaccess .
Feature papers represent the most advanced research with significant potential for high impact in the field. A Feature Paper should be a substantial original Article that involves several techniques or approaches, provides an outlook for future research directions and describes possible research applications.
Feature papers are submitted upon individual invitation or recommendation by the scientific editors and must receive positive feedback from the reviewers.
Editor’s Choice articles are based on recommendations by the scientific editors of MDPI journals from around the world. Editors select a small number of articles recently published in the journal that they believe will be particularly interesting to readers, or important in the respective research area. The aim is to provide a snapshot of some of the most exciting work published in the various research areas of the journal.
Original Submission Date Received: .
- Active Journals
- Find a Journal
- Proceedings Series
- For Authors
- For Reviewers
- For Editors
- For Librarians
- For Publishers
- For Societies
- For Conference Organizers
- Open Access Policy
- Institutional Open Access Program
- Special Issues Guidelines
- Editorial Process
- Research and Publication Ethics
- Article Processing Charges
- Testimonials
- Preprints.org
- SciProfiles
- Encyclopedia

Article Menu

- Subscribe SciFeed
- Recommended Articles
- PubMed/Medline
- Google Scholar
- on Google Scholar
- Table of Contents
Find support for a specific problem in the support section of our website.
Please let us know what you think of our products and services.
Visit our dedicated information section to learn more about MDPI.
JSmol Viewer
The impact of the fermentation strategy on the flavour formation of ilzer rose ( malus domestica borkh.) apple wine.

1. Introduction
2. materials and methods, 2.1. fruit material, 2.2. preparation of juice samples, 2.3. preparation of the apple wines, 2.4. analysis of basic fruit wine parameters, 2.4.1. sugar concentration, 2.4.2. acidity, 2.4.3. sample preparation and gc-ms analysis, 2.4.4. sensory evaluation, 2.4.5. statistical analysis, 3. results and discussion, 3.1. volatile compounds obtained from apple juice and dependence on the maceration time, 3.2. investigation of the apple wines, 3.2.1. fermentation and basic apple wine parameters, 3.2.2. volatile compounds from apple wines, 3.2.3. sensory analysis, 4. conclusions, author contributions, institutional review board statement, informed consent statement, data availability statement, acknowledgments, conflicts of interest.
- AICV. The European Cider & Fruit Wine Association, European Cider Trends. 2020. Available online: https://aicv.org/en/news/aicv-european-cider-trends-2020-now-published (accessed on 2 July 2021).
- Picinelli Lobo, A.; Antón-Díaz, M.J.; Mangas Alonso, J.J.; Suárez Valles, B. Characterization of Spanish ciders by means of chemical and olfactometric profiles and chemometrics. Food Chem. 2016 , 213 , 505–513. [ Google Scholar ] [ CrossRef ]
- Qin, Z.; Petersen, M.A.; Bredie, W.L.P. Flavor profiling of apple ciders from the UK and Scandinavian region. Food Res. Int. 2019 , 105 , 713–723. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Nespor, J.; Karabin, M.; Stuulikova, K.; Dostalek, P. An HS-SPME-GC-MS Method for Profiling Volatile Compounds as Related to Technology Used in Cider Production. Molecules 2019 , 24 , 2117. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Wilson, A.; Johnson, J.B.; Batley, R.; Lal, P.; Wakeling, L.; Naiker, M. Authentication Using Volatile Composition: A Proof-of-Concept Study on the Volatile Profiles of Fourteen Queensland Ciders. Beverages 2021 , 7 , 28. [ Google Scholar ] [ CrossRef ]
- Alberti, A.; Machado dos Santos, T.P.; Ferreira Zielinski, A.A.; Eleuterio dos Santos, C.M.; Braga, C.M.; Demiate, I.M.; Nogueira, A. Impact on chemical profile in apple juice and cider made from unripe, ripe and senescent dessert varieties. LWT Food Sci. Technol. 2016 , 65 , e436–e443. [ Google Scholar ] [ CrossRef ]
- Vidrih, R.; Hribar, J. Synthesis of higher alcohols during cider processing. Food Chem. 1999 , 67 , 287–294. [ Google Scholar ] [ CrossRef ]
- Laaksonen, O.; Kuldjärv, R.; Paalme, T.; Virkki, M.; Yang, B. Impact of apple cultivar, ripening stage, fermentation type and yeast strain on phenolic composition of apple ciders. Food Chem. 2017 , 233 , 29–37. [ Google Scholar ] [ CrossRef ]
- Antón-Díaz, M.J.; Suárez Valles, B.; Mangas-Alonso, J.J.; Fernández-García, O.; Picinelli-Lobo, A. Impact of different techniques involving contact with lees on the volatile composition of cider. Food Chem. 2016 , 190 , 1116–1122. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Lukic, I.; Horvat, I.; Radeka, S.; Damijanic, K.; Staver, M. Effect of different levels of skin disruption and contact with oxygen during grape processing on phenols, volatile aromas, and sensory characteristics of white wine. J. Food Process. Preserv. 2019 , 43 , e13960. [ Google Scholar ] [ CrossRef ]
- Rosend, J.; Kuldjarv, R.; Arju, G.; Nisamedtinov, I. Yeast performance characterisation in different cider fermentation matrices. Agron. Res. 2019 , 17 , 2040–2053. [ Google Scholar ] [ CrossRef ]
- Wicklund, T.; Skotthei, E.R.; Rember, S.F. Various Factors Affect Product Properties in Apple Cider Production. Int. J. Food Stud. 2020 , 9 , SI84–SI96. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Calugar, P.C.; Coldea, T.E.; Salanta, L.C.; Pop, C.R.; Pasqualone, A.; Burja-Udrea, C.; Zhao, H.; Mudura, E. An Overview of the Factors Influencing Apple Cider Sensory and Microbial Quality from Raw Materials to Emerging Processing Technologies. Processes 2021 , 9 , 502. [ Google Scholar ] [ CrossRef ]
- Wurm, L.; Wendelin, S.; Gössinger, M.; Kieler, M.; Sigl, K.; Patzl, W.; Kickenweiz, M.; Rühmer, T.; Klöckl, V.; Brandes, W.; et al. Ertrag, Fruchtqualität, Inhaltsstoffe und Geschmacksqualität alter Apfelsorten unter biologischen und integrierten Anbaubedingungen. Mitt. Klosterneubg. 2014 , 64 , 63–81. [ Google Scholar ]
- Bingman, M.T.; Stellick, C.E.; Pelkey, J.P.; Scot, J.M.; Cole, C.A. Monitoring Cider Aroma Development throughout the Fermentation Process by Headspace Solid Phase Microextraction (HS-SPME) Gas Chromatography–Mass Spectrometry (GC-MS) Analysis. Beverages 2020 , 6 , 40. [ Google Scholar ] [ CrossRef ]
- Rühmer, T.; Voit, J. Most und Saft—Die veredelte Form von Äpfeln. Haidegger Perspekt. 2020 , 3 , 6–9. [ Google Scholar ]
- Siegmund, B.; Leitner, E. Characterisation of the Flavour of the old Austrian Apple variety ‘Ilzer Rose’. In Flavour Science: Proceedings of the XV Weurman Flavour Research Symposium ; Siegmund, B.; Leitner, E. Technischen Universität Graz: Graz, Austria, 2018; pp. 135–138. [ Google Scholar ] [ CrossRef ]
- Hjelmeland, K.; Ebeler, S.E. Glycosidically Bound Volatile Aroma Compounds in Grapes and Wine: A Review. Am. J. Enol. Vitic. 2015 , 66 , 1–11. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Ruppert, V.; Innerhofer, G.; Voit, J.; Hiden, P.; Siegmund, B. Enzymatic Release of Flavour Compounds from Heritage Apple Varieties. In Flavour Science—Proceeding of the 16th Weurman Flavour Research Symposium ; Le Quéré, J.L., Guichard, E., Eds.; INRAE: Dijon, France, 2021; under review. [ Google Scholar ]
- Galati, A.; Schifani, G.; Crescimanno, M.; Migliore, G. “Natural wine” consumers and interest in label information: An analysis of willingness to pay in a new Italian wine market segment. J. Clean. Prod. 2019 , 227 , e405–e413. [ Google Scholar ] [ CrossRef ]
- Urdapilleta, I.; Demarchi, S.; Parr, W.V. Influence of culture on social representation of wines produced by various methods: Natural, organic and conventional. Food Qual. Prefer. 2021 , 87 , 104034. [ Google Scholar ] [ CrossRef ]
- Ancient Georgian Traditional Qvevri Wine-Making Method. Available online: https://ich.unesco.org/en/RL/ancient-georgian-traditional-qvevri-wine-making-method-00870 (accessed on 12 July 2021).
- Gonzalez, G.A.; Parga-Dans, E. Natural wine: Do consumers know what it is, and how natural it really is? J. Clean. Prod. 2020 , 251 , 119635. [ Google Scholar ] [ CrossRef ]
- Sancho-Galán, P.; Amores-Arrocha, A.; Jiménez-Cantizano, A.; Palacios, V. Influence of the Presence of Grape Skins during White Wine Alcoholic Fermentation. Agronomy 2021 , 11 , 452. [ Google Scholar ] [ CrossRef ]
- OIV. Resolution OIV/OENO 390/2010, Guidelines on Infrared Analysers in Oenology. 2010. Available online: https://www.oiv.int/public/medias/1239/oiv-oeno-390-2010-en.pdf (accessed on 27 September 2021).
- Johnson, L.G.; Skou, P.B.; Khakimov, B.; Bro, R. Gas chromatography—Mass spectrometry data processing made easy. J. Chromatogr. A 2017 , 1503 , 57–64. [ Google Scholar ] [ CrossRef ]
- Risum, A.B.; Bro, R. Using deep learning to evaluate peaks in chromatographic data. Talanta 2019 , 204 , 255–260. [ Google Scholar ] [ CrossRef ]
- DIN EN ISO 8586: 2014-05. Sensory Analysis—General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors ; DIN Deutsches Institut für Normung. e.V., Beuth Verlags GmbH: Berlin, Germany, 2014. [ Google Scholar ]
- Elmore, J.S. Aroma extract analysis. In Flavour Development, Analysis and Perception in Food and Beverages ; Parker, J.K., Elmore, J.S., Methven, M., Eds.; Woodhead Publishing: Cambridge, UK, 2015; pp. 47–62. [ Google Scholar ] [ CrossRef ]
- Schwab, W.; Wüst, M. Understanding the Constitutive and Induced Biosynthesis of Mono- and Sesquiterpenes in Grapes ( Vitis vinifera ): A Key to Unlocking the Biochemical Secrets of Unique Grape Aroma Profiles. J. Agric. Food Chem. 2015 , 63 , 10591–10603. [ Google Scholar ] [ CrossRef ]
- Alexandre, H.; Costello, P.J.; Remize, F.; Guzzo, J.; Guilloux-Benatier, M. Saccharomyces cerevisiae–Oenococcus oeni interactions in wine: Current knowledge and perspectives. Int. J. Food Microbiol. 2004 , 93 , 141–154. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Antón, M.J.; Valle, B.S.; Hevia, A.G.; Lobo, A.P. Aromatic Profile of Ciders by Chemical Quantitative, Gas Chromatography-Olfactometry, and Sensory Analysis. J. Food Sci. 2014 , 79 , S92–S99. [ Google Scholar ] [ CrossRef ] [ PubMed ]
- Hirst, M.B.; Richter, C.L. Review of Aroma Formation through Metabolic Pathways of Saccharomyces cerevisiae in Beverage Fermentations. Am. J. Enol. Vitic. 2016 , 67 , 4. [ Google Scholar ] [ CrossRef ] [ Green Version ]
- Marullo, P.; Trujillo, M.; Viannais, R.; Hercman, L.; Guillaumie, S.; Colonna-Ceccaldi, B.; Albertin, W.; Barbe, J.C. Metabolic, Organoleptic and Transcriptomic Impact of Saccharomyces cerevisiae Genes Involved in the Biosynthesis of Linear and Substituted Esters. Int. J. Mol. Sci. 2021 , 22 , 4026. [ Google Scholar ] [ CrossRef ]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae . Microb. Biotechnol. 2010 , 3 , 165–177. [ Google Scholar ] [ CrossRef ] [ PubMed ] [ Green Version ]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.M.; Dequin, S.; Mouret, J.R. Combined effects of nutrients and temperature on the production of fermentative aromas by Saccharomyces cerevisiae during wine fermentation. Appl. Microbiol. Biotechnol. 2015 , 99 , 2291–2304. [ Google Scholar ] [ CrossRef ]
- Campo, E.; Ballester, J.; Langlois, J.; Dacremont, C.; Valentin, D. Comparison of conventional descriptive analysis and a citation frequency-based descriptive method for odor profiling: An application to Burgundy Pinot noir wines. Food Qual. Prefer. 2010 , 21 , 44–55. [ Google Scholar ] [ CrossRef ]
- Buck, D.; Kemp, S.E. Check-All-That-Apply and Free Choice Description. In Descriptive Analysis in Sensory Evaluation ; Kemp, S.E., Hort, J., Hollowood, T., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2018; pp. 579–607. [ Google Scholar ]
Click here to enlarge figure
| Compound | RI [DB5] | RI [DB5] | Juice 0 h Maceration Mean ± SD [µg L ] | Juice 2 h Maceration Mean ± SD [µg L ] | Juice 5 h Maceration Mean ± SD [µg L ] | Juice 8 h Maceration Mean ± SD [µg L ] | Significance |
|---|---|---|---|---|---|---|---|
| acetic acid | 612 | 625 | 1.53 ± 0.3 | 0.72 ± 0.6 | 2.56 ± 0.2 | 1.37 ± 1.2 | No |
| 2-methyl butanoic acid | 842 | 856 | 25.3 ± 1.3 | 15.9 ± 1.3 | 17.7 ± 1.5 | 20.4 ± 0.2 | * |
| hexanoic acid | 967 | 980 | 3.0 ± 0.0 | 7.1 ± 0.1 | 7.0 ± 0.2 | 10.3 ± 0.3 | ** |
| 1-propanol | <600 | 548 | 1.94 ± 0.1 | 2.00 ± 0.7 | 2.96 ± 0.0 | 2.36 ± 0.6 | No |
| 2-butanol | 601 | 605 | 0.91 ± 0.0 | 0.81 ± 0.3 | 1.25 ± 0.0 | 1.17 ± 0.3 | No |
| 2-methyl Propanol | 628 | 654 | 2.92 ± 0.1 | 0.71 ± 0.8 | 1.20 ± 0.3 | 1.03 ± 1.1 | No |
| 1-butanol | 665 | 660 | 40.3 ± 3.1 | 51.4 ± 2.7 | 60.9 ± 1.6 | 61.9 ± 2.7 | * |
| 1-penten-3-ol | 684 | 686 | 0.34 ± 0.0 | 0.92 ± 0.1 | 0.95 ± 0.0 | 1.14 ± 0.1 | ** |
| 2-methyl-1-butanol | 739 | 743 | 67.5 ± 3.8 | 84.1 ± 4.5 | 78.0 ± 0.6 | 93.6 ± 3.3 | * |
| 1-pentanol | 767 | 766 | 0.36 ± 1.9 | 3.35 ± 0.5 | 3.68 ± 0.1 | 5.07 ± 0.2 | No |
| 2,3-butanediol | 780 | 773 | 1.38 ± 0.4 | 3.37 ± 1.7 | 4.31 ± 1.1 | 4.02 ± 1.1 | No |
| 3-hexen-1-ol (Z)- | 851 | 858 | 0.89 ± 0.5 | 20.5 ± 0.5 | 21.0 ± 0.1 | 34.1 ± 2.5 | ** |
| 2-hexen-1-ol (E)- | 866 | 887 | 12.6 ± 2.1 | 356 ± 13.9 | 317 ± 11.3 | 394 ± 19.6 | ** |
| 1-hexanol | 868 | 867 | 106.9 ± 0.2 | 623 ± 43.4 | 617 ± 23.1 | 821 ± 60.6 | ** |
| 1-heptanol | 967 | 970 | 8.02 ± 1.0 | 24.0 ± 4.7 | 27.3 ± 0.4 | 29.6 ± 0.7 | * |
| 1-octen-3-ol | 979 | 979 | 0.55 ± 0.0 | 1.93 ± 0.1 | 1.72 ± 0.0 | 2.07 ± 0.1 | ** |
| methionol | 982 | 980 | 5.46 ± 0.2 | 3.91 ± 0.2 | 3.97 ± 0.3 | 5.12 ± 0.3 | * |
| 6-methyl-5-hepten-2-ol | 993 | 994 | 15.6 ± 1.0 | 29.2 ± 1.5 | 33.9 ± 0.9 | 40.0 ± 2.2 | ** |
| 2-ethyl hexanol | 1029 | 1028 | 2.01 ± 0.1 | 3.58 ± 0.1 | 3.00 ± 0.0 | 3.74 ± 0.0 | ** |
| benzyl alcohol | 1042 | 1032 | 3.04 ± 0.3 | 8.06 ± 0.2 | 10.4 ± 0.3 | 21.5 ± 0.2 | ** |
| 1-octanol | 1069 | 1069 | 1.14 ± 0.1 | 5.14 ± 0.1 | 7.70 ± 0.1 | 13.06 ± 0.3 | ** |
| 3-octen-1-ol (Z)- | 1071 | 1047 | 1.85 ± 0.2 | 5.96 ± 0.1 | 6.30 ± 0.2 | 8.03 ± 0.9 | * |
| phenylethyl alcohol | 1127 | 1113 | 2.29 ± 0.3 | 3.12 ± 0.1 | 3.69 ± 0.2 | 6.04 ± 0.1 | ** |
| 1,3-octanediol | 1264 | 1275 | 451 ± 24.3 | 544 ± 20.3 | 688 ± 24.4 | 703 ± 71.1 | * |
| 1-dodecanol | 1477 | 1475 | 6.60 ± 2.8 | 7.69 ± 1.6 | 6.18 ± 1.4 | 5.82 ± 1.0 | No |
| 2,3-butanedione | 615 | 623 | 0.15 ± 0.0 | 0.25 ± 0.0 | 0.37 ± 0.0 | 0.39 ± 0.0 | ** |
| butanal | 602 | 601 | 51.2 ± 0.0 | 1.45 ± 0.1 | 1.13 ± 0.0 | 1.33 ± 0.1 | No |
| 2-butanone | 600 | 600 | 2.79 ± 0.4 | 3.47 ± 0.4 | 3.54 ± 0.1 | 4.41 ± 0.7 | No |
| 1-penten-3-one | 687 | 687 | 0.55 ± 0.1 | 0.74 ± 0.1 | 0.65 ± 0.0 | 0.50 ± 0.0 | No |
| 2-pentanone | 688 | 695 | 2.76 ± 0.1 | 2.52 ± 0.3 | 2.69 ± 0.1 | 2.70 ± 0.3 | No |
| 2-pentenal (E)- | 756 | 748 | 0.62 ± 0.0 | 1.10 ± 0.1 | 0.61 ± 0.0 | 0.51 ± 0.1 | * |
| 3-hexenal (Z) | 797 | 795 | 10.3 ± 0.5 | 6.44 ± 0.5 | 2.78 ± 0.1 | 2.67 ± 0.3 | ** |
| hexanal | 799 | 801 | 51.4 ± 2.2 | 49.2 ± 3.0 | 18.6 ± 0.1 | 18.3 ± 1.2 | ** |
| 2,4-hexadienal (E,E)- | 911 | 916 | 12.03 ± 0.8 | 11.3 ± 1.1 | 5.28 ± 0.3 | 5.24 ± 0.3 | ** |
| 2-heptenal (E)- | 960 | 964 | 1.47 ± 0.2 | 3.40 ± 0.1 | 3.16 ± 0.0 | 2.83 ± 0.1 | ** |
| benzaldehyde | 972 | 961 | 5.03 ± 0.2 | 25.5 ± 0.4 | 13.7 ± 0.6 | 22.0 ± 0.3 | ** |
| 6-methyl-5-hepten-2-one | 988 | 988 | 4.03 ± 0.1 | 10.7 ± 0.3 | 13.3 ± 0.5 | 14.8 ± 0.8 | ** |
| phenylacetaldehyde | 1055 | 1047 | 0.29 ± 0.1 | 1.01 ± 0.1 | 1.14 ± 0.0 | 1.05 ± 0.0 | * |
| 2-octenal (E)- | 1063 | 1063 | 5.69 ± 0.2 c | 9.54 ± 0.0 | 9.88 ± 0.2 | 10.7 ± 0.1 | ** |
| nonanal | 1107 | 1102 | 3.63 ± 1.8 | 6.45 ± 0.6 | 4.69 ± 0.4 | 4.98 ± 0.4 | No |
| methyl acetate | <600 | 522 | 0.22 ± 0.0 | 0.21 ± 0.0 | 0.34 ± 0.0 | 0.28 ± 0.1 | No |
| ethyl acetate | 615 | 628 | 15.2 ± 0.4 | 15.9 ± 1.5 | 20.9 ± 0.4 | 23.3 ± 1.4 | * |
| propyl acetate | 715 | 695 | 0.50 ± 0.0 | 0.59 ± 0.1 | 0.64 ± 0.0 | 1.02 ± 0.1 | * |
| methyl butanoate | 724 | 710 | 1.00 ± 0.1 | 0.81 ± 0.1 | 0.88 ± 0.0 | 0.84 ± 0.1 | No |
| butyl acetate | 812 | 802 | 8.02 ± 0.1 | 9.52 ± 0.9 | 12.43 ± 0.4 | 17.63 ± 1.2 | * |
| 2-methyl butyl acetate | 877 | 880 | 24.5 ± 0.6 | 36.7 ± 2.7 | 40.8 ± 1.2 | 49.3 ± 3.3 | * |
| pentyl acetate | 911 | 926 | 1.04 ± 0.1 | 3.16 ± 0.1 | 5.72 ± 0.0 | 7.22 ± 0.3 | ** |
| ethyl-3-hydroxy-butanoate | 935 | 945 | 2.75 ± 0.1 | 2.93 ± 0.1 | 3.20 ± 0.3 | 1.58 ± 0.1 | * |
| butyl butanoate | 995 | 1002 | 2.03 ± 0.1 | 8.17 ± 0.8 | 7.15 ± 0.1 | 7.59 ± 0.7 | * |
| 3-hexen-1-yl acetate (Z) | 1001 | 996 | 1.04 ± 0.4 | 11.5 ± 0.5 | 22.6 ± 0.4 | 29.8 ± 2.2 | ** |
| 2-hexen-1-yl acetate (E) | 1013 | 1017 | 5.83 ± 2.1 | 195 ± 9.6 | 244 ± 2.7 | 237 ± 12.4 | ** |
| hexyl acetate | 1011 | 1011 | 18.5 ± 1.4 | 153 ± 9.3 | 279 ± 5.7 | 315 ± 20.7 | ** |
| 2-methyl butyl butanoate | 1042 | 1041 | 3.88 ± 0.6 | 11.0 ± 0.9 | 12.3 ± 0.3 | 10.8 ± 1.1 | * |
| methyl octanoate | 1123 | 1129 | 0.18 ± 0.1 | 2.10 ± 0.6 | 0.57 ± 0.1 | 0.41 ± 0.0 | * |
| hexyl-2-methylpropanoate | 1147 | 1138 | 0.30 ± 0.0 | 1.92 ± 0.1 | 2.23 ± 0.0 | 2.54 ± 0.3 | ** |
| hexyl-2-methyl butanoate | 1238 | 1236 | 9.27 ± 0.8 | 65.4 ± 0.1 | 104 ± 0.4 | 101 ± 5.9 | ** |
| pentyl hexanoate | 1287 | 1282 | 0.29 ± 0.0 | 3.01 ± 0.0 | 3.53 ± 0.1 | 3.20 ± 0.3 | ** |
| butyl octanoate | 1387 | 1393 | 0.63 ± 0.1 | 3.06 ± 0.1 | 4.45 ± 0.1 | 4.21 ± 0.0 | ** |
| hexyl hexanoate | 1386 | 1386 | 4.19 ± 0.3 | 28.7 ± 1.5 | 48.7 ± 1.7 | 48.5 ± 0.6 | ** |
| 3-methylbutyl octanoate | 1451 | 1450 | 0.16 ± 0.1 | 0.67 ± 0.0 | 1.43 ± 0.2 | 1.11 ± 0.1 | * |
| methyljasmonate | 1672 | 1647 | 1.29 ± 0.2 | 0.89 ± 0.1 | 1.38 ± 0.2 | 1.15 ± 0.0 | No |
| 2-methyl 1,3-pentadiene (E)- | 669 | n/a | 0.12 ± 0.0 | 0.88 ± 0.1 | 0.87 ± 0.1 | 1.11 ± 0.1 | * |
| linalool oxide isomer I | 1084 | n/a | 11.6 ± 0.6 | 22.2 ± 1.6 | 26.8 ± 0.0 | 26.9 ± 1.3 | * |
| linalool oxide isomer II | 1099 | n/a | 11.5 ± 0.5 | 19.9 ± 0.5 | 23.4 ± 0.6 | 23.2 ± 0.6 | ** |
| β-damascenone | 1408 | 1400 | 4.06 ± 0.2 | 4.25 ± 0.8 | 4.50 ± 0.9 | 5.72 ± 0.3 | No |
| β-ionone | 1511 | 1493 | 4.74 ± 0.4 | 7.25 ± 0.1 | 6.27 ± 0.1 | 7.32 ± 0.1 | * |
| α-farnesene | 1517 | 1508 | 21.4 ± 13.5 | 32.6 ± 4.5 | 60.0 ± 9.6 | 48.0 ± 4.4 | No |
| 2-ethylfuran | 703 | 702 | 1.52 ± 0.2 | 1.28 ± 0.1 | 0.84 ± 0.1 | 0.73 ± 0.0 | * |
| 2-pentylfuran | 995 | 991 | 0.67 ± 0.1 | 1.86 ± 0.5 | 1.59 ± 0.4 | 1.11 ± 0.3 | No |
| hexanal dimethyl acetal | 978 | 980 | 6.95 ± 0.9 | 7.02 ± 0.4 | 1.81 ± 0.3 | 1.90 ± 0.3 | * |
| Ethanol [%v/v] | Residual Sugar [g L ] | Titratable Acid [g L ] | Malic Acid [g L ] | Lactic Acid [g L ] | |
|---|---|---|---|---|---|
| AW1 | 7.4 | <1.7 | 6.2 | 7.0 | n.d. |
| AW2 | 7.3 | 2.5 | 6.8 | 7.4 | n.d. |
| AW3 | 7.4 | 8.3 | 5.9 | 2.2 | 2.3 |
| AW4 | 7.3 | 3.0 | 6.8 | 7.2 | n.d. |
| AW5 | 7.2 | 8.1 | 5.8 | 2.1 | 2.3 |
| Compound | RI [DB5] | RI [DB5] | AW1 Mean ± SD [µg L ] | AW2 Mean ± SD [µg L ] | AW3 Mean ± SD [µg L ] | AW4 mean ± SD [µg L ] | AW5 mean ± SD [µg L ] | Significance |
|---|---|---|---|---|---|---|---|---|
| 3-methyl butanoic acid | 823 | 835 | 8.0 ± 1.2 | 12.1 ± 0.8 | 13.6 ± 0.6 | 11.25 ± 0.2 | 13.2 ± 1.1 | ** |
| 2-methyl butanoic acid | 835 | 846 | 10.1 ± 2.1 | 18.6 ± 0.3 | 30.7 ± 2.5 | 17.30 ± 1.7 | 27.4 ± 2.5 a | ** |
| hexanoic acid | 969 | 980 | 403 ± 45.7 | 357 ± 22 | 105 ± 8.7 | 375.47 ± 12.4 | 102 ± 12 | ** |
| octanoic acid | 1164 | 1178 | 721 ± 158 | 889 ± 177 | 169 ± 10.3 | 977.1 ± 211.4 | 152 ± 15 | ** |
| 2-methyl-1-butanol | 719 | 730 | 437 ± 38.3 | 580 ± 16 | 800 ± 84 | 569 ± 22 | 777 ± 15 | ** |
| 3-methyl-1-butanol | 722 | 743 | 3526 ± 230 | 3 812 ± 417 | 3 648 ± 315 | 3 936 ± 87 | 3 864 ± 343 | no |
| 1-pentanol | 753 | 766 | 4.0 ± 0.2 | 6.9 ± 0.3 | 11.0 ± 1.2 | 6.27 ± 0.6 | 10.5 ± 0.3 | ** |
| 2,3-butanediol | 779 | 773 | 54.0 ± 18.7 | 51.1 ± 27.9 | 24.7 ± 4.5 | 42.77 ± 13.5 | 18.3 ± 3.8 | no |
| 3-ethoxy-1-propanol | 836 | 816 | 58.7 ± 2.8 | 27.7 ± 1.7 | 5.9 ± 3.2 | 18.73 ± 1.5 | 4.1 ± 1.6 | ** |
| 3-hexen-1-ol (E) | 848 | 858 | 3.8 ± 0.7 | 17.2 ± 0.8 | 22.0 ± 0.7 | 16.14 ± 0.6 | 21.3 ± 0.8 | ** |
| 1-hexanol | 865 | 867 | 434 ± 31.4 | 1 158 ± 48 | 1 388 ± 78 | 1 097 ± 23.8 | 1 388 ± 59 | ** |
| 1-heptanol | 968 | 970 | 0.9 ± 0.2 | 1.5 ± 0.2 | 19.6 ± 0.3 | 1.38 ± 0.1 | 18.9 ± 0.6 | ** |
| 2-methyl-6-hepten-1-ol | 992 | 994 | 49.6 ± 2.0 | 54.6 ± 2.3 | 79.4 ± 5.0 | 50.8 ± 2.3 | 71.6 ± 1.8 | ** |
| 3-ethyl-4-methylpentan-1-ol | 1024 | 1020 | 0.3 ± 0.1 | 3.5 ± 0.1 | 4.2 ± 0.3 | 3.47 ± 0.1 | 4.1 ± 0.1 | ** |
| 2-ethylhexanol | 1029 | 1029 | 4.8 ± 0.4 | 5.8 ± 1.0 | 5.6 ± 0.2 | 5.98 ± 0.5 | 5.8 ± 0.2 | no |
| benzyl alcohol | 1041 | 1032 | 1.2 ± 0.2 | 5.1 ± 0.6 | 76.4 ± 3.4 | 4.84 ± 0.4 | 67.7 ± 2.8 | ** |
| 1-octanol | 1069 | 1069 | 4.3 ± 0.5 | 18.1 ± 0.7 | 33.9 ± 0.8 | 17.19 ± 0.8 | 30.9 ± 1.3 | ** |
| phenylethyl alcohol | 1127 | 1113 | 629 ± 48 | 727 ± 55 | 618 ± 17 | 611 ± 39 | 558 ± 20 | * |
| benzaldehyde | 971 | 961 | 6.8 ± 0.9 | 11.1 ±1.3 | 19.5 ±1.6 | 10.3 ±0.3 | 18.5 ± 0.8 | ** |
| nonanal | 1106 | 1101 | 8.5 ± 3.4 | 7.3 ± 3.2 | 6.6 ± 1.3 | 7.0 ± 5.8 | 5.8 ± 1.2 | no |
| 2-butanone | 601 | 600 | 9.5 ± 1.3 | 8.3 ±1.3 | 7.5 ±1.1 | 9.8 ±0.9 | 9.1 ±1.2 | No |
| ethyl acetate | 614 | 628 | 315 ± 63 | 311 ± 52 | 143 ± 32 | 309 ± 20 | 168 ± 20 | ** |
| ethyl propanoate | 692 | 706 | 1.4 ± 0.4 | 2.5 ± 0.3 | 3.3 ± 0.8 | 2.0 ± 0.2 | 3.12 ± 0.3 | ** |
| propyl acetate | 694 | 695 | 4.5 ± 1.0 | 3.9 ± 0.4 | 1.2 ± 0.2 | 3.7 ± 0.4 | 1.2 ± 0.2 | ** |
| ethyl butanoate | 791 | 808 | 31.3 ± 8.6 | 36.5 ± 3.1 | 6.4 ± 1.6 | 30.5 ± 2.2 | 5.5 ± 2.2 | ** |
| butyl acetate | 805 | 802 | 29.4 ± 5.4 | 26.0 ± 1.7 | 9.9 ± 0.5 | 23.2 ± 1.0 | 11.4 ± 0.4 | ** |
| ethyl 2-methyl butanoate | 846 | 850 | 0.9 ± 0.3 | 2.1 ± 0.2 | 5.9 ± 1.3 | 1.5 ± 0.2 | 5.6 ± 0.9 | ** |
| 2-methylbutyl acetate | 876 | 880 | 114 ± 33 | 95.4 ± 6.3 | 13.1 ± 1.2 | 81.7 ± 7.2 | 15.7 ± 1.9 | ** |
| 3-methylbutyl acetate | 873 | 876 | 1028 ± 254 | 770 ± 46 | 106 ± 9.5 | 670 ± 48 | 121 ± 12 | ** |
| methyl hexanoate | 923 | 936 | 7.5 ± 2.6 | 18.7 ± 2.1 | 12.8 ± 1.9 | 19.7 ± 3.3 | 13.4 ± 1.6 | ** |
| ethyl-3-hydroxy butanoate | 934 | 945 | 1.0 ± 0.1 | 2.1 ± 0.2 | 2.4 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.2 | ** |
| ethyl hexanoate | 997 | 999 | 956 ± 258 | 980 ± 47 | 587 ± 15 | 846 ± 46 | 275 ± 37 | ** |
| hexyl acetate | 1010 | 1011 | 1072 ± 290 | 795 ± 44 | 47.0 ± 1.5 | 657 ± 32 | 55.4 ± 6.3 | ** |
| isoamyl lactate | 1071 | 1065 | 0.3 ± 0.1 | 0.2 ± 0.1 | 23.8 ± 1.4 | 0.3 ± 0.1 | 20.5 ± 1.1 | ** |
| methyl octanoate | 1123 | 1129 | 216 ± 78 | 233 ± 63 | 69.9 ± 17.2 | 241 ± 69 | 66.8 ± 15.6 | * |
| ethyl benzoate | 1183 | 1170 | 0.7 ± 0.1 | 1.4 ± 0.0 | 12.8 ± 0.3 | 1.6 ± 0.1 | 10.8 ± 0.7 | ** |
| ethyl octanoate | 1196 | 1194 | 2083 ± 479 | 2148 ± 123 | 561 ± 46 | 1 994 ± 101 | 483 ± 77 | ** |
| hexyl-2-methylbutanoate | 1238 | 1234 | 0.5 ± 0.1 | 3.1 ± 0.1 | 36.9 ± 2.8 | 2.2 ± 0.2 | 32.4 ± 4.9 | ** |
| ethylphenyl acetate | 1255 | n/a | 0.8 ± 0.1 | 2.0 ± 0.2 | 11.6 ± 0.5 | 1.9 ± 0.2 | 11.4 ± 0.6 | ** |
| phenethyl acetate | 1269 | 1256 | 222 ± 16.8 | 140.1 ± 6.0 | 16.7 ± 0.9 | 113 ± 4.2 | 15.5 ± 0.4 | ** |
| methyl decanoate | 1324 | 1324 | 387 ± 81 | 368 ± 81 | 56.8 ± 13 | 363 ± 70 | 51.3 ± 11 | ** |
| 2-methylpropyl octanoate | 1348 | 1345 | 6.0 ± 1.7 | 6.3 ± 0.8 | 1.1 ± 0.2 | 6.4 ± 1.1 | 1.0 ± 0.2 | ** |
| ethyl decanoate | 1395 | 1392 | 2177 ± 445 | 1832 ± 160 | 279 ± 41 | 1 941 ± 245 | 230 ± 40 | ** |
| 3-methylbutyl octanoate | 1448 | 1450 | 104 ± 30 | 88.7 ± 10.8 | 9.2 ± 1.6 | 94.8 ± 18.1 | 7.2 ± 1.2 | ** |
| methyl dodecanoate | 1525 | 1526 | 276 ± 16.6 | 291 ± 41 | 61.8 ± 7.0 | 285 ± 26.2 | 52.8 ± 5.2 | ** |
| hexyl octanoate | 1584 | 1571 | 3.3 ± 1.2 | 8.5 ± 1.0 | 0.9 ± 0.2 | 9.0 ± 2.2 | 0.8 ± 0.2 | ** |
| ethyl dodecanoate | 1594 | 1597 | 547 ± 194 | 395 ± 61 | 53.3 ± 12.2 | 533 ± 134 | 43.4 ± 10.4 | ** |
| 3-methylbutyl decanoate | 1647 | 1649 | 108.9 ± 31.2 | 96.0 ± 10.5 | 6.4 ± 1.5 | 109 ± 18.4 | 5.1 ± 1.1 | ** |
| 2-methylbutyl decanoate | 1651 | 1647 | 16.4 ± 5.4 | 17.0 ± 2.1 | 2.5 ± 0.2 | 21.5 ± 4.5 | 2.3 ± 0.2 | ** |
| 2-phenylethyl hexanoate | 1664 | 1650 | 5.4 ± 0.7 | 4.5 ± 0.2 | 0.6 ± 0.1 | 3.8 ± 0.2 | 0.6 ± 0.1 | ** |
| ethyl tetradecanoate | 1794 | 1794 | 26.9 ± 8.8 | 17.7 ± 2.6 | 3.6 ± 1.6 | 26.1 ± 5.8 | 5.7 ± 0.8 | ** |
| 3-methylbutyl dodecanoate | 1848 | 1844 | 6.8 ± 1.6 | 4.8 ± 0.9 | 0.9 ± 0.2 | 7.2 ± 0.7 | 0.8 ± 0.1 | ** |
| methyl hexadecanoate | 1927 | 1933 | 132 ± 32 | 135 ± 38 | 86 ± 12 | 147 ± 41 | 89 ± 19 | no |
| ethyl-9-hexadecenoate | 1979 | 1977 | 7.2 ± 1.2 | 16.6 ± 1.8 | 4.2 ± 0.7 | 13.3 ± 0.9 | 2.9 ± 0.2 | ** |
| ethyl hexadecanoate | 1994 | 1978 | 53.1 ± 3.1 | 35.7 ± 4.3 | 25.8 ± 2.2 | 53.0 ± 5.3 | 24.2 ± 1.3 | ** |
| linalool oxide (furanoid) | 1083 | 1073 | 5.9 ± 0.1 | 9.0 ± 0.5 | 9.2 ± 0.5 | 7.4 ± 0.0 | 8.1 ± 0.2 | ** |
| linalool | 1103 | 1101 | 3.6 ± 0.5 | 5.5 ± 0.1 | 4.8 ± 0.1 | 5.1 ± 0.1 | 4.4 ± 0.2 | ** |
| α-farnesene | 1517 | 1508 | 2.0 ± 0.3 | 7.3 ± 0.5 | 9.2 ± 1.7 | 6.3 ± 0.8 | 8.1 ± 1.0 | ** |
| MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |

Share and Cite
Ruppert, V.; Innerhofer, G.; Voit, J.; Hiden, P.; Siegmund, B. The Impact of the Fermentation Strategy on the Flavour Formation of Ilzer Rose ( Malus domestica Borkh.) Apple Wine. Foods 2021 , 10 , 2348. https://doi.org/10.3390/foods10102348
Ruppert V, Innerhofer G, Voit J, Hiden P, Siegmund B. The Impact of the Fermentation Strategy on the Flavour Formation of Ilzer Rose ( Malus domestica Borkh.) Apple Wine. Foods . 2021; 10(10):2348. https://doi.org/10.3390/foods10102348
Ruppert, Valerie, Georg Innerhofer, Jörg Voit, Peter Hiden, and Barbara Siegmund. 2021. "The Impact of the Fermentation Strategy on the Flavour Formation of Ilzer Rose ( Malus domestica Borkh.) Apple Wine" Foods 10, no. 10: 2348. https://doi.org/10.3390/foods10102348
Article Metrics
Article access statistics, further information, mdpi initiatives, follow mdpi.

Subscribe to receive issue release notifications and newsletters from MDPI journals
- DOI: 10.1007/s13197-019-03771-0
- Corpus ID: 145854007
Development of apple wine from Golden Delicious cultivar using a local yeast isolate
- Sukhvir Sukhvir , G. S. Kocher
- Published in Journal of food science and… 11 April 2019
- Agricultural and Food Sciences
10 Citations
Production and characterization of wine from golden delicious apples using saccharomyces cerevisiae isolated from selected nigerian indigenous alcoholic beverages.
- Highly Influenced
Standardization of seed and peel infused Syzygium cumini -wine fermentation using response surface methodology
An overview of the factors influencing apple cider sensory and microbial quality from raw materials to emerging processing technologies, the use of γ-aminobutyric acid-producing saccharomyces cerevisiae sc125 for functional fermented beverage production from apple juice, a review on the production of wine as a post- harvest processing alternative for mango, banana, and purple sweet potato, effect of inorganic and organic nitrogen supplementation on volatile components and aroma profile of cider., volatilomics of fruit wines, mango wine making process optimization based on artificial intelligence deep learning technology, fermentative processing of unexploited fruit, karonda (carissa carandus l.) into alcoholic beverages, the influence of two yeast strains on fermentation and flavour composition of cider, 30 references, the influence of process optimization on the fermentation profile of mango wine prepared from the apple mango variety, influence of different sugar sources, nitrogen sources and inocula on the quality characteristics of apple tea wine, apple wine processing with different nitrogen contents, fermentative production of guava wine from pectinase treated and untreated juice of ‘punjab pink’ cultivar of psidium guajava l., fermentation of guava pulp with grape-grown yeast (saccharomyces cerevisiae var. ellipsoideus) for wine production, assessment of yeasts for apple juice fermentation and production of cider volatile compounds, panorma of research and development of wines in india, development of alcoholic and malolactic fermentations in highly acidic and phenolic apple musts., malic acid in wine : origin, function and metabolism during vinification, polyphenol profiles of apple juices., related papers.
Showing 1 through 3 of 0 Related Papers
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Microorganisms

Microorganisms in Fermented Apple Beverages: Current Knowledge and Future Directions
Production of fermented apple beverages is spread all around the world with specificities in each country. ‘French ciders’ refer to fermented apple juice mainly produced in the northwest of France and often associated with short periods of consumption. Research articles on this kind of product are scarce compared to wine, especially on phenomena associated with microbial activities. The wine fermentation microbiome and its dynamics, organoleptic improvement for healthy and pleasant products and development of starters are now widely studied. Even if both beverages seem close in terms of microbiome and process (with both alcoholic and malolactic fermentations), the inherent properties of the raw materials and different production and environmental parameters make research on the specificities of apple fermentation beverages worthwhile. This review summarizes current knowledge on the cider microbial ecosystem, associated activities and the influence of process parameters. In addition, available data on cider quality and safety is reviewed. Finally, we focus on the future role of lactic acid bacteria and yeasts in the development of even better or new beverages made from apples.
1. Introduction
Although styles of cider are extremely diverse and not easy to categorize, depending on the type of apple juices used and the degrees of sweetness, from extra dry to sweet, and alcohol content, ranging from 1.2–8% ( v / v ), cider can be defined as a fermented alcoholic beverage made from apple juice [ 1 ]. Cider production is encountered in more than 25 countries around the world in temperate regions where apple trees can flourish. The highest production is in Europe where the term cider refers strictly to fermented products [ 2 , 3 ]. Within Europe, the main cider-producing countries are England, Spain, France, Germany and Ireland, while smaller productions are found in Finland, Poland, Austria and Switzerland. The consumption of cider remains mainly European, accounting in 2016 for about 60% of world consumption compared with only 12% in North America [ 4 ]. There are several cider types, and traditional cider countries like Great Britain and France have their own specialties [ 5 ]. French cider tends to be sweeter than the sharper, drier cider of Great Britain, which has an alcohol content up to 8.5% ( v / v ). The fruity characteristics and aromas of French cider often are the result of ‘defecation’, in which pectins and other substances are separated from the juice. Then, the clear juice is raked off and fermented slowly and not to complete dryness [ 6 ]. In North America and Australia, the word ‘cider’ refers to the raw pressed unfermented apple juice, while ‘hard cider’ denotes a fermented product [ 7 ].
Cider is one of the oldest known beverages with a long and fascinating history. Historians broadly agree that apple trees existed along the Nile River Delta as early as 1300 BC [ 8 ], and a number of written documents citing alcoholic beverages made from apple and pear date back to ancient times, notably from Pliny, St. Augustin and Palladius [ 9 ]. By the beginning of the ninth century, cider drinking was well established in Europe, and a reference made by Charlemagne clearly confirms its popularity [ 10 ]. After the Norman Conquest of 1066, cider consumption became widespread in England, and orchards were established specifically to produce cider apples. In the first half of the twentieth century, cider was the second most consumed drink in France, behind wine, but ahead of beer [ 11 ]. Unfortunately, the damage caused to the Norman orchards during World War II together with the lack of public support resulted in a drastically reduced production, marking the decline of cider consumption in France. The current methods of cider production (quality of equipment, control assemblies and processes, stability, hygiene, neutralization of microorganisms, bottling, etc.) limit defects in the final product and make it possible to meet consumer requirements. Current cider producers use high quality standards, and ciders are elaborated under controlled conditions throughout the process.
In brief, the cider-making process ( Figure 1 ) typically involves three main stages: apple crushing and pressing out the juice, followed by the most important stages of elaboration, fermentation. This includes classical alcoholic fermentation of sugars into ethanol performed by yeast strains and malolactic fermentation (MLF) processed by lactic acid bacteria (LAB) that can occur during the maturation. Although external sources of microorganisms may be added to the must in French traditional cider-making, alcoholic and malolactic fermentations are mainly performed by indigenous flora present on apples, on production equipment and in the cellar. Spontaneous fermentation begins within a few hours if the temperature of the must rises above 10 °C. This process is usually slow requiring at least 2–3 weeks for the main fermentation and several months for the maturation. Maturation takes place in wooden, polyester or stainless-steel casks at a controlled temperature of 3 °C–12 °C. The entire process can take from 1–6 months depending on the country. First, during alcoholic fermentation, sugars are converted mainly into ethanol and carbon dioxide by yeasts (mainly Saccharomyces sp.). The varietal choice and maturity of the fruits influence the sugar content of the starting must and, thus, the final ethanol level. Then, the malolactic fermentation involves the conversion of malic acid into lactic acid and carbon dioxide. Finally, the cider is bottled when its density is between 1009 and 1029 depending on the degree of sweetness desired (in France, typically extra dry, dry, half-dry and sweet). Active dry yeast (ADY) may be added in cider before bottling to obtain a naturally-carbonated beverage. The amount of residual sugar in the cider is essentially a consequence of the technological choice of the main alcoholic fermentation stoppage density and of the cider density at bottling. As shown in Figure 1 , sulfites may be added at different stages of the process. Before fermentation, sulfites are added to control the natural microflora and to minimize oxidation of apple juice constituents. At bottling, sulfites are used to prevent oxidative changes and to inhibit secondary contamination [ 12 ]. After pressing, malic acid may also be added; this practice is a simple and effective way to change the acidity of the must.

Cider-making process in France. Legend: + , optional addition; alcohol degree ; density ; ADY: active dry yeast.
Cider is a fermented beverage for which the recognition of ‘territoriality’ is important for its appreciation. The sensory profile of cider is significantly associated with microbial activities, and indigenous microorganisms may actively contribute to the expression of cider typicity. The microbial ecology of ciders is complex and includes several genera, species and strains of yeasts and bacteria [ 13 , 14 ]. During must production, fermentation and in the post-fermentative stage, apple juice or cider is susceptible to alteration by oxygen, enzymes, heat and/or microorganisms that can lead to a loss of nutritional and organoleptic qualities. With the increasing demand of consumers for nutritious, healthy and fresh-looking products with high organoleptic qualities, measures have been developed to prevent such alterations and to control the organoleptic characteristics of the product.
This review aims at describing the role of microbial flora in the fermentation of apple juices, highlighting the links between ecological factors, yeasts and LAB diversities and the organoleptic properties of ciders. To date, even if ciders are safe products, research has focused mainly on the quality and safety of ciders through studies on the limitation of the development of spoilage and pathogenic microorganisms. This review will focus more on microbial quality referring to the overall effects of microbial activity, including growth, enzymatic activity and metabolic byproducts. Finally, a review of microbial diversity and the microbial contribution to the quality and safety of ciders will give us the opportunity to propose new perspectives for research on apple fermented beverages, especially through LAB activities.
2. Microbial Diversity: From Apple to Cider
Regarding the microbial ecosystem, next generation sequencing strategies bring many new whole ecosystem pictures, especially regarding non-cultivable bacteria [ 15 , 16 ]. Such studies are very rare in the cider research area, with only one paper revealing the microbiota of wine and organic apple cider submerged vinegar production [ 17 ]. Another study described the yeast biodiversity in must and during alcoholic fermentation [ 18 ]. There is a major lack of data on cider microbiota and its dynamics during the process. Current microbial research has moved into the genomic era with increasing amounts of data available, along with decreasing costs for sequencing, especially for LAB [ 19 ] and more specifically lactobacilli [ 20 ]. Better knowledge could be easily obtained by specific (meta-)genomic analysis of cider microbiomes.
2.1. Yeast and Mold Diversity
Fungi (yeasts and molds) are naturally present on apples and can be found at each step of cider production. The presence of yeasts at the early stage of flower blossom has been described in various plants [ 21 , 22 ]. In flower nectar, yeast levels can reach densities up to 4 × 10 8 cells/mL, and their frequency and abundance are directly correlated with the proportion of floral visits by bumble-bees, which thus appear as potential transmission vectors of yeasts from one flower to another in an orchard [ 21 ]. However, it seems that for the majority of plant nectars, the diversity of yeast communities is rather low [ 22 ]. On apple blossoms, yeasts have been isolated from both stigma and hypanthium surfaces, at frequencies similar to or greater than bacteria, particularly in hypanthia [ 23 ].
The apple surface is also a natural reservoir of fungi. In freshly-cut apples, fungi levels can range from 3.6–7.1 log CFU/g [ 24 ]. The dominant species identified in these cut apples were Candida sake and Pichia fermentans . Some of the fungi species on apples can be phytopathogenic species mainly included in the class Dothideomycetes, with about 95% of these in the order Capnodiales that causes damaging blemishes on apples [ 25 , 26 ]. A PCR-DGGE based-study of the microbiota of five varieties of Asturian apples used for the production of PDO (Protected Designation of Origin) ciders in Spain also identified Exobasidium sp., responsible for galls and leaf malformations, and Mycosphaerellaceae and Dissoconiaceae families, which produce sooty blotch and flyspeck on apples [ 27 ]. In this work, little variation in microbial diversity was found amongst the five apple varieties studied, without identifying the usual species associated with spontaneous fermentation. The authors conclude that the surface microbiota of the apples does not seem to be a determinant in the subsequent fermentation process. In contrast, another study showed that apples themselves can be the source of yeasts of technological interest [ 28 ]. This was the case with Saccharomyces cerevisiae yeasts, which could be found in high numbers on apples used for traditional Irish cider fermentations. In the same way, Hanseniaspora and Brettanomyces/Dekkera in ciders could be tracked back to the fruits.
The main yeasts found in cider are Saccharomyces yeasts. A study of unpasteurized ciders and cider musts obtained from different cider houses from northwestern regions of France reported 15 yeast species among 208 picked isolates [ 29 ]. The main species in this study was Saccharomyces bayanus accounting for 34.5% of the isolates, followed by Saccharomyces cerevisiae , Lachancea cidri , Dekkera anomala and Hanseniaspora valbyensis representing 16%, 15%, 10.5% and 6.5% of the isolates, respectively. The proportions of each of the 10 other species, i.e., Candida oleophila , C. sake , C. stellate , C. tropicalis , H. uvarum , Kluyveromyces marxianus , Metschnikowia pulcherrima , Pichia delftensis , P. misumaiensis and P. nakasei , never exceeded 3.5% of the total isolates. Yeast diversity was higher in cider musts than bottled ciders. Regarding the dominance of S. bayanus , the same observation was made in natural cider from Asturias (Spain) [ 30 ]. Saccharomyces bayanus was the predominant species from the beginning to the middle steps of the fermentation process, accounting for up to 41% of the picked isolates, whereas S. cerevisiae took over the process in the final stages of fermentation. H. valbyensis was always present at the end of fermentations regardless of the fermentation process used. The variations in the proportions of the different identified yeasts are connected to the occurrence of a sequential succession of yeast species throughout the cider-making process. Morrissey et al. thus identified three phases in the cider process based on the dominant yeast species present [ 28 ]. The first phase, which they called ‘the fruit yeast’ phase, is dominated by Hanseniaspora uvarum / Kloeckera apiculata yeasts, along with a few S. cerevisiae yeasts [ 14 , 28 ]. The second phase, or ‘fermentation phase’ where the alcoholic fermentation occurs, is characterized by the replacement of oxidative or slightly fermentative non- Saccharomyces yeasts by the strong fermenting Saccharomyces yeasts, such as S. bayanus and S. cerevisiae . The last ‘maturation phase’ is dominated by Brettanomyces/Dekkera yeasts. The yeast population fluctuates from one year of production to the next [ 31 ]. This is visible in the variations in the proportions of the main yeast species constituting a resident mycoflora throughout cider cellars and by the intermittent apparition of some species constituting a ‘transitory mycoflora’.
2.2. Bacterial Diversity
Bacteria are present from the apple flowers to the final product ( Table 1 ). In 2013, Shade et al. studied the apple flower microbiome by pyrosequencing and described the presence of diversified bacterial communities evolving differently from the bud to the fruit [ 32 ]. This study highlighted that apple flowers carry bacteria that will be involved in the process of cider or vinegar making (mainly Lactobacillaceae and Acetobacteraceae families, respectively). Surprisingly, bacteria from Deinococcus-Thermus phylum were found in abundance. This phylum was not known to be related to fruit crop. Enterobacteriaceae , commonly isolated on apple fruits, were present at every stage of the flower maturation.
Bacterial diversity found in apple juice-related products.
| Family | Origin | Genus/Species | References |
|---|---|---|---|
| Apple flower Fresh-cut apple Cider | [ , , , , ] | ||
| Cider | [ , ] | ||
| Apple flower Apple cider vinegar | sp. | [ , ] | |
| sp. | |||
| sp. | |||
| Cider | sp. | [ ] | |
| Cider | [ , ] | ||
| Apple surface Apple flower | Coliforms | [ , , ] |
a Genus unspecified.
In 2015, Graça et al. detected principally mesophilic and psychrotrophic microorganisms on fresh cut apple while coliforms and LAB were isolated on apple flowers [ 24 ]. Focusing on cider apples, Alonso et al. used PCR-DGGE to study the native microbiota of five apple varieties commonly used in the Asturian cider-making process. Predictably, Enterobacteriaceae were present due to the ubiquity in nature of this genus, but bacterial species usually associated with spontaneous fermentation were not [ 27 ]. The apple surface microbiota may not be a determinant in the fermentation process. The microbiota of apple cider is strongly influenced by other factors such as harvest techniques, quality sorting and storage. In 2004, Keller et al. brought to light the influence of picking techniques on the microbiota [ 42 ]. Cider apples picked from the ground after their fall bring more bacterial diversity than those tree harvested. After grinding, no difference between bacteria counts were found, whether they were stored or not. However, significant differences in bacterial counts between apple varieties were identified.
Bacterial starters do not exist yet in cider; thus, Sanchez et al. investigated LAB prevalence during the malolactic fermentation in Asturian cider cellars in order to find the most efficient fermentative strains [ 33 ]. They mostly isolated strains of Lactobacillus brevis and Oenococcus oeni. This last species is already known to be very tolerant to low pH and to the presence of alcohol [ 38 ]. According to a fermentation capacity evaluation of the selected strains, O. oeni strains were the most efficient. Salih et al. also highlighted the importance of O. oeni during the malolactic fermentation, and the presence of Lactobacillus brevis in some of the ciders tested [ 37 ]. Different behaviors of the LAB flora depend on the kind of apples used for cider-making (sweet cider apples, sweet dessert apples, bitter cider apples). The influence of the geographical origins of the indigenous cider LAB was determined by Sanchez et al. using the RAPD (Random Amplification of polymorphic DNA) technique on O. oeni strains. Five distinct groups, specific to only one producing area, were identified and had an identical RAPD profile. This significant result brought to light the link between O. oeni strains and their geographical origin [ 33 ]. A recent study focusing on the biogeography of O. oeni confirmed the importance of genetic adaptation of this species in cider and also highlighted that O. oeni from wine or from cider were genetically different [ 38 ]. The first genome of O. oeni has been sequenced and annotated in 2005 [ 43 ]. Many studies have investigated the genome of this bacterium and have shown that O. oeni strains from wine or cider present a different genomic content [ 44 , 45 , 46 ]. A recent study of Sternes et al. analyzed the pan-genome of O. oeni with 191 strains, of which only four have been isolated from cider [ 46 ]. They showed again that three out of four of the cider isolates cluster closely together. The presence of neighboring wine-derived strains suggests that information from additional strains isolated from cider is required before any conclusion regarding the possibility of a cider-specific subset of O. oeni can be reached. The other source of genomic data from LAB isolated from cider is related to their technological or probiotic potential [ 47 , 48 ].
Lactobacillus sp. and Oenococcus sp. are the most common LAB identified in apple juice byproducts. In apple cider vinegar, which is the result of acetic fermentation, both of them were detected, even if acetic acid bacteria, such as Acetobacter sp., Komagataeibacter sp. or Gluconobacter sp., were the most abundant [ 17 ]. In 2010, Sanchez et al. studied the LAB diversity during malolactic fermentation in an industrial cider [ 13 ]. Using molecular tools, such as 16S rRNA gene sequencing, Lactobacillus collinoides , O. oeni , Pediococcus parvulus and, with minor content, bacteria like L. casei or P. ethanolidurans were identified. Acetic acid bacteria are necessary for vinegar production, but can ruin cider production. In contrast, LAB are essential in malolactic conversion during cider production, but some can damage the product by producing spoilage compounds.
2.3. Factors Influencing Microbial Diversity
Variations in the microbial ecosystem of ciders are associated with several factors, from the orchards to the final product. First, microbial diversity is determined by the growing conditions of the fruits such as the apple varieties, the climate and the production process. The cultivation practices have an impact on the fruit microbial composition in terms of abundance and diversity. Organic and conventional apple bacterial communities were shown to be significantly different [ 49 , 50 ]. The organic apple phyllosphere displayed higher numbers of bacteria than the conventional apple phyllosphere. A comparison of integrated and organic growing systems for Golden Delicious apple production also revealed significantly higher frequencies of filamentous fungi, greater abundance of total fungi and of taxon diversity in organic apples than in integrated apples [ 51 ]. The crop management methods thus influence the microbial communities associated with the surface of apple fruits used for cider production. The apple variety also has an influence on the microbial composition of the fruits. Keller et al. showed that significant differences exist in total aerobic bacterial and fungal populations among apple varieties in relation to their pH, Brix and titratable acidity [ 42 ]. The apple varieties with the lowest titratable acidity, highest pH and highest Brix have the highest microbial concentrations (≥2.5 log CFU/g). The method of harvesting also plays a role in microbial diversity. Microbial populations on apples, in pomace and in cider are higher when apples are harvested off the ground rather than tree-picked. In the final cider, the average aerobic plate counts for all pooled varieties tested in the ground-harvested group was 4.89 log CFU/g compared with 2.88 log CFU/g for the fresh tree-picked group [ 42 ].
After fruit harvesting, the cider process modulates the microbial composition of ciders. The culling of apples result in ciders with higher microbial numbers than those made from unculled apples [ 42 ]. A strong link exists between the temperature profile of the cider fermentations and the yeast population dynamics of the predominant yeast species, present within the fermentations [ 28 ]. Another piece of research also showed that the musts obtained by pneumatic pressing were dominated by non- Saccharomyces yeasts ( Hanseniaspora genus and Metschnikowia pulcherrima ), whereas in the apple juices obtained by traditional pressing, Saccharomyces together with non- Saccharomyces were always present [ 30 ].
Cider processing facilities and cellars walls, floors and surfaces also constitute reservoirs of bacteria and fungi throughout the cider process. For example, one source of S. cerevisiae yeasts appears to be the process utensils, the press house and the vat-house, in which this resident flora can be found even six months after the last pressing [ 28 ].
Even if microbial reservoirs are broad, the microbial diversity and microflora successions also greatly depend on the aptitudes of the bacterial and fungal strains to resist or adapt to the process conditions such as depletion in oxygen levels, sulfites presence, CO 2 and alcohol productions and essential nutrients’ availability. It also depends on the differences in their specific growth rates, in their sugar uptake capabilities, on inter-specific competition, cell death, flocculation and/or natural sedimentation characteristics [ 52 ].
3. Microbial Contribution to Cider Organoleptic Quality
3.1. yeast contribution.
During alcoholic fermentation, many byproducts such as esters, higher alcohols and phenolic compounds are produced as secondary metabolites. Esters provide mainly fruity and floral notes; higher alcohols provide ‘background flavors’; whereas the phenolic compounds can generate interesting or unpleasant aromatic notes. Esters are the main volatile compounds in cider behind ethanol [ 53 ]. They are characterized by a high presence of ethyl acetate, which alone can represent up to 90% of the total esters [ 54 , 55 ]. The amount of acetates produced by yeasts seems to be strongly related to the nature of the strains leading to alcoholic fermentation: Saccharomyces sp. produce fewer acetate amounts than non- Saccharomyces yeasts. Comparing the potential of H. valbyensis and S. cerevisiae to produce volatile compounds, Xu et al. [ 55 ] showed that H. valbyensis yielded higher concentrations of ethyl acetate and 2-phenylethyl acetate, while S. cerevisiae kept more free (non-esterified) isoamyl alcohol and isobutanol. A small variation in the ester concentration of ciders may have significant consequences on their final sensory quality [ 56 ]. Most of the esters are responsible for the fruity characteristics of ciders. However, an excessive amount of ethyl acetate may lead to an unpleasant smell of solvent.
Higher alcohols are directly derived from the metabolism of yeasts. They are synthesized during fermentation from oxo-acids originating in amino acids and sugar metabolism [ 57 ]. In ciders, they are mostly represented by isopentanols (2- and 3-methylbutanol) followed by isobutanol, propanol, butanol or hexanol [ 58 ]. Although they constitute a relatively low amount of the total substances, higher alcohols may greatly influence sensory characteristics. Rapp and Mandery [ 59 ] found the total higher alcohols in wine to be in the range 80 ± 540 mg/mL ; concentrations up to 300 mg/L contribute to pleasant flavor, but concentrations above 400 mg/mL provoke unpleasant flavor and harsh taste. Some higher alcohols, particularly iso-amyl alcohol, contribute to unpleasant flavor [ 60 ], although a positive correlation has been reported between n-butanol and the aroma quality of apple juice [ 61 ].
The third class of secondary products, i.e., the phenolic compounds, also have important effects on the sensory properties of apple ciders by either their content or their profile. These compounds derived from raw material have an impact mainly on color, bitterness, and astringency [ 62 ]. High molecular weight procyanidins in ciders are known to contribute to astringency, whereas the smaller compounds contribute to bitter taste [ 63 , 64 , 65 ]. Simultaneously, they influence the sweetness and sourness, thus further highlighting their importance in overall flavor development [ 64 ]. In addition to the non-volatile phenolic compounds, the volatile phenolics mainly formed by enzymatic decarboxylation during fermentation contribute to aroma [ 66 ].
It has been reported that during the early stages of fermentation, excess growth of the apiculated yeast Kloeckera can generate high levels of esters and volatile acids [ 67 ]. In wine, the aromatic profile is negatively influenced by the yeast Brettanomyces / Dekkera and is characterized by mousy, medicinal, wet wool, burnt plastic or horse sweat smells [ 68 ]. Buron et al. have shown that Brettanomyces / Dekkera cider strains were able to produce 4-ethylcatechol, 4-ethylphenol and 4-ethylguaiacol from caffeic, p -coumaric and ferulic acids, respectively [ 69 ]. These volatile phenols are associated with organoleptic defects. In contrast, in some beers, this yeast is considered essential and beneficial [ 70 ]. In wine- and cider-making on an industrial scale, the control of Brettanomyces / Dekkera is usually achieved through the addition of sulfur dioxide (SO 2 ) to the fermentation medium [ 71 ]. In cider-making, the concentration of SO 2 is in the range of 50–150 mg/mL at pH 3.0–3.8, not exceeding 200 mg/mL in total [ 72 ]. However, some strains of Brettanomyces / Dekkera are naturally resistant to SO 2 , and elimination of this yeast by physical treatments (filtration) has a limited efficiency (due to the cell size of this yeast) and does not prevent subsequent recontamination.
3.2. Bacterial Contribution
Transformation of malic acid, lowering total acidity, is the major organoleptic change induced by LAB. During MLF, the strong green taste of malic acid is replaced by the less aggressive taste of lactic acid [ 73 ]. However, LAB are also responsible for other changes in aromas increasing flavor complexity, involving changes of fruity, flowery and nutty flavors, as well as the reduction of vegetative/herbaceous aromas by reduction of acetaldehyde metabolism [ 74 , 75 , 76 ].
Lactobacillus , Leuconostoc , Oenococcus and Pediococcus are genera of special interest as they are able to survive cider environments (low pH, high ethanol content and low nutrients). Research focuses on the contribution of O. oeni , but other genera, particularly Lactobacillus species, should not be underestimated [ 77 ]. In wine, it is well known that some varietal aromas revealed during alcoholic fermentation by yeast disappear or change after malolactic fermentation [ 73 ]. For example, the concentration of some esters can be either increased or decreased by MLF, according to the type of bacterial strain used [ 78 ]. Apart from esters, aroma compounds such as higher alcohols, fatty acids, lactones and sulfur and nitrogen compounds can be produced by LAB [ 77 ].
LAB contribution to aromatic profiles of ciders has been explored less than it has been in wine. A few studies are available, principally linked on the use of O. oeni strains as starters rather than studying LAB metabolism in the cider environment. In wine, LAB contribution is focused on citric acid metabolism that induces the production of compounds linked to buttery descriptors: diacetyl, 2,3-butanediol and acetoin [ 79 ]. Together with acetonic compounds, citric acid degradation involves the production of acetic acid that can significantly modify the aromatic profile. Citric acid metabolism with the production of diacetyl cannot be responsible for the whole panel of flavor modifications, and the mechanisms should be further studied.
Along with the favorable sensory changes that can occur during cider elaboration, LAB can be also responsible for undesirable reactions. The frequent cider alteration known as ‘piqûre acroléique’ is mainly caused by a heterofermentative LAB commonly encountered in cider, Lactobacillus collinoides [ 80 , 81 ]. In apple-derived products, this alteration results from glycerol degradation to 3-hydroxypropionaldehyde (3-HPA) under the action of L. collinoides via the diol-dehydratase enzyme. In addition to L. collinoides , some other cider species, like L. hilgardii [ 34 ] or L. diolivorans [ 82 ], are able to produce 3-HPA. Glycerol is one of the major products of yeasts metabolism during cider alcoholic fermentation and is important for the sensorial quality of fermented beverages. Due to its high instability, during the distillation process, the 3-HPA is transformed by dehydration [ 83 ] in acrolein, a lachrymatory chemical generating a peppery flavor, which can spoil the product, giving a bitter taste [ 84 , 85 ].
One major spoilage microorganism is the Gram-negative, facultative anaerobic bacterium Zymomonas mobilis isolated from various alcoholic beverages, including ciders, beers and perries. Z. mobilis is a remarkable bacterium and a very promising microorganism for industrial ethanol production because its catabolism follows the Entner–Doudoroff pathway, thus giving a near-theoretical yield of ethanol from glucose, fructose and sucrose, the only carbon and energy sources that support its growth [ 40 ]. As a cider spoilage microorganism, growth of Z. mobilis is correlated with the production of large quantities of acetaldehyde along with an almost explosive production of gas and a marked turbidity of the product, an alteration known as ‘framboisé’ in French ciders or ‘cider-sickness’ in English ciders [ 41 , 86 ]. Associated with these symptoms is a marked change in the flavor of the beverage, the original fruity character being lost or hidden by a strong and characteristic taste, reminiscent of raspberry. Malolactic fermentation (MLF) is considered to enhance the risk of ‘framboisé’, and Bauduin et al. [ 41 ] have shown that the relationship between MLF and ‘framboisé’ is mainly associated with the increase of pH correlated with the conversion of malic acid to lactic acid rather than with nutritional factors produced by LAB. In fact, the amount of residual nitrogen in cider appears to be the main factor controlling the growth of Z. mobilis , and thus, a solution for the prevention of this alteration consists of reducing the amount of residual nitrogen as soon as possible [ 41 ].
Therefore, a greater knowledge of cider LAB flora and their metabolisms in a cider environment could provide laboratory and practical cellar tools for a better control of cider quality.
4. Safety Assessment of Fermented Apple Beverages
Fermented foods and beverages are known to be safer than unfermented counterparts. The improved food safety arising from fermentation is largely due to LAB, a predominant group of organisms in most fermented foods and beverages. Occasionally, bacterial pathogens such as Salmonella spp., Escherichia coli and Staphylococcus aureus , originating from orchard soil, farm and processing equipment or human sources, may occur in apple juice. However, both apple juice and fermented cider contain organic acids, mainly malic acid (≅5 g/L) in apple juice and lactic acid (3–4 g/L) in fermented cider, generating acidity (pH level ranging from 3.0–3.5 and 3.3–4.0, respectively) that usually prevents the growth of these pathogens, which can survive for only a few hours. The growth and metabolism of LAB usually inhibit the growth of normal spoilage flora of the matrix and of any bacterial pathogens that it may contain. Therefore, apple cider is traditionally not regarded as a potentially hazardous food [ 87 ]. However, the monitoring of food-borne hazards in cider such as the pathogenic bacteria E. coli , protozoan Cryptosporidium , biogenic amines or mycotoxins still requires vigilance on the part of cider producers.
4.1. Biogenic Amines
Biogenic amines (BA) are low molecular weight organic bases with an aliphatic, aromatic or heterocyclic structure frequently occurring in foods and beverages involving fermentation or the ripening process. The formation of these molecules is achieved through the removal of the alpha carboxyl group from amino acids [ 88 ]. The most abundant BA found in foods are histamine, tyramine, putrescine, cadaverine and phenyl ethylamine. In fermented beverages, such as beer, wine and cider, production is influenced by microorganisms present [ 88 , 89 ], environmental factors such as pH, ethanol [ 90 , 91 ], sulfur anhydride level [ 92 ], raw material quality and fermentation, as well as technological conditions [ 90 , 93 ]. Consumption of food containing high level of BAs can induce adverse reactions such as headache, hyper- or hypo-tension and rashes. Such disorders may become serious especially for consumers whose detoxification system is impaired either by genetic disorders or medical treatments [ 89 ]. Histamine and tyramine are considered as most toxic and particularly relevant for food safety, while putrescine and cadaverine are known to potentiate these effects [ 94 ].
In cider, as microbiological stabilization is not performed after MLF, indigenous heterofermentative LAB constitute the predominant flora capable of promoting the production of BAs [ 36 , 39 , 95 ]. As shown in Table 2 , among LAB, Oenococcus and Lactobacillus were found to be the most representative genera of BA producers in cider.
Potential bacterial species producers of biogenic amines in Spanish and French ciders.
| Biogenic Amine | Producer | References |
|---|---|---|
| Histamine | [ , , ] | |
| Putrescine | [ , ] | |
| Tyramine | sp. | [ , , ] |
Studies conducted on commercial cider from Spain and France revealed the presence of BA in almost 90% of the analyzed products with a higher prevalence of tyramine, histamine, putrescine and cadaverine among other amines [ 89 , 95 ]. Nevertheless, BA content of cider seems to be lower than that detected in other fermented foods and beverages [ 88 ]. Some differences in amount and composition were also found between French and Spanish samples. More precisely, cadaverine and putrescine were detected at a maximal concentration of 34 mg/L in 20% and 57% of Spanish cider samples, respectively, while only in trace amounts in only a third of French cider samples (1 mg/L). Tyramine was the most frequently detected BA in French samples (present in 70% of samples in concentrations below 14 mg/L). Histamine was detected at relatively low levels in both French and Spanish samples (26% of total samples, below 16 mg/L) [ 89 , 95 , 96 ]. As mentioned by Ladero et al., the characteristics of the apple variety used and/or the different elaboration processes, as well as possible microbiota differences could explain the differences [ 89 ]. The global amount and profile of BA produced do not appear to be driven by cider-making steps and types of press [ 95 ]. Therefore, some strategies have been proposed to decrease the formation of BA, such as (a) reducing amino acid precursor levels (generally decreasing with fruit ripening), (b) limiting the growth of spoilage bacteria, (c) inoculating starter cultures without amino acid decarboxylase and (d) inoculating biogenic amine-degrading microorganisms [ 94 , 97 ].
4.2. Mycotoxins
Mycotoxins are secondary metabolites of filamentous fungi mainly triggered by Aspergillus , Fusarium and Penicillium genera [ 98 ]. In apple and apple-derived products, patulin represents the most relevant mycotoxin. The toxin is an unsaturated heterocyclic lactone toxin produced by a wide range of mold species [ 99 ]. Among these species, P. expansum, as the main pre-harvest and post-harvest contaminant in pomaceous fruits (apples and pears), is considered as the major source of patulin in these fruits [ 100 ]. The level in food and beverages is regulated in Europe by the European Commission and in the United States by the U.S. Food and Drug Administration (FDA) to a maximum acceptable concentration of 50 µg/L for fruit juices and derived products (including cider) [ 101 ]. Indeed, Michigan apple cider mills were analyzed for patulin concentration. The mycotoxin was detected in almost 20% of cider mill samples with 2% of samples having concentration higher than 50 µg/L [ 102 ]. Temperature, activity of water (a w ) and pH were found to influence P. expansum growth, as well as patulin production. The fungi is able to produce the toxin around 16 °C. P. expansum can produce the mycotoxin only at an a w of 0.99, which is the approximate a w of fresh fruits. Finally, patulin production was found to be optimum at pH 4 [ 103 ]. Apple contains natural acids (citric and malic acids) that lead to the reaching of these optimal conditions (pH of fruit varies from <2.5–5) [ 104 ]. It is commonly admitted that the toxin is generally unstable during fermentation, so that products such as cider are usually free of patulin. In a recent study, the level of patulin in contaminated musts was shown to have decreased six-fold after two days of fermentation [ 105 ]. Reports of patulin in cider are likely due to the adjunction of apple juice to produce ‘sweet cider’ or low-fermented cider [ 102 ].
4.3. Pathogens
As previously mentioned, unpasteurized apple cider is historically considered to be a safe product, free of microbial pathogens due to its acidic level and to the fermentation process. However, some bacterial and parasitic pathogens can survive and may remain infectious [ 106 ]. To date, enterohemorrhagic E. coli (EHEC) serotype O157:H7, as well as the protozoan Cryptosporidium parvum have been linked to several outbreaks since the 1980s due to apple cider consumption [ 106 ]. Nevertheless, it is important to notice that such outbreaks only occurred in North America and mainly in unfermented apple ciders [ 106 ]. European apple cider has never been implicated in any outbreaks of this kind due to alcoholic fermentation process byproduct, ethanol, which is toxic for most potential pathogens in cider [ 107 ]. EHEC O157:H7 is known to have a fecal origin and may contaminate apples, juice and cider directly from animal/human feces or by indirect contact (equipment, contaminated water, etc.) [ 108 ]. Cryptosporidium spp. is an intracellular parasite with an infectious stage known as oocysts. Oral transmission of the parasite is facilitated by the ability of oocysts to survive for weeks to months in the environment. The study of contamination sources in unpasteurized apple cider revealed that the parasite is found in washed apples, water, fresh and finished cider [ 108 ]. Kniel et al. studied the potential of malic acid, as well as hydrogen peroxide to reduce the infectivity of C. parvum in apple cider. Interestingly, infectivity was completely inhibited by incubation of oocysts in apple cider plus 0.025% H 2 O 2 and inhibited (up to 88%) by the addition of 5% malic acid [ 109 ].
5. Functional Improvement of Apple Fermented Beverages
5.1. control of the microbial ecosystem to improve or modulate cider quality, 5.1.1. rational design of starter cultures.
The selection of appropriate starter strains is key in the control of the cider fermentation process and characteristics of the final beverage. Microbial starters, especially O. oeni , are less used in cider production than in wine production, but their role might be crucial for the quality of the final product [ 110 ]. This leads to many studies focusing on the selection of microbial starters [ 33 , 111 ], their improvement [ 112 , 113 ] and the use of other LAB than O. oeni [ 114 ]. The use of isolated strains of S. cerevisiae is an interesting strategy for maintaining the quality and reproducibility of fermented beverages. This is especially true for the Champenoise method, typical of Asturian PDO ciders, and based on a secondary fermentation in bottle. The screening and the selection of local yeast strains is believed to be more effective than using commercial starters, as these endemic strains are potentially better acclimated to the environmental conditions than industrial starters [ 115 ]. These authors thus proposed a methodology for the rapid screening and selection of autochthonous yeast strains based on their oenological and technological properties. The ciders obtained with the selected yeast strains were scored as good after sensory analysis. The choice in species driving the fermentation is important for technological purposes and also for the aroma profile development in cider. For example, the presence of Hanseniaspora sp. yeast strains during apple fermentation results in the production of considerable amounts of esters and alcohols, contributing to fruity sensory notes, compared with apple musts fermented only with Saccharomyces sp. yeasts, which provide rather neutral sensory notes [ 116 ]. The fermentation performance has also been improved by the use of a hybrid strain between S. eubayanus and S. cerevisiae [ 111 ]. Recently, in order to control the proliferation of Brettanomyces / Dekkera in wine, Ngwekazi et al. [ 71 ] have identified and characterized killer toxins secreted by non- Saccharomyces yeasts related to wine. Their results, although preliminary, show that killer toxins have a high potential to control the population of large numbers of Brettanomyces / Dekkera strains. These results are especially encouraging, as none of the killer toxins characterized inhibit the fermentative yeast Saccharomyces .
Another characteristic to be considered for the bacterial strains used in the cider fermentation process is their aptitude in resisting bacteriophages (phages). The presence of lytic or lysogenic phages of Oenococcus has previously been described in wine [ 117 ]. Recently, Constanti et al. characterized O. oeni bacteriophages and the related implications for malolactic fermentation in wine [ 118 ]. They reported that pH and ethanol affect the lytic activity of Oenococcus phages, especially when wine alcohol content is low. The presence of phages in cider has not yet been investigated. It is thus a point of interest that should be examined, as anti- Oenococcus phages could be at the origin of cider fermentation problems by disturbing the malolactic fermentation driven by phage-sensitive Oenococcus strains. Still, phages could be used as antimicrobial agents against spoilage and pathogenic bacteria in ciders and therefore help in controlling the safety and quality of these fermented beverages. Phage therapy in the food industry has been extensively studied [ 119 ]. However, phage applications to apple fermented beverages are scarce. One main obstacle to their efficient antimicrobial action would be their potential sensitivity to acidity found in apple products [ 120 ].
5.1.2. Control of the Fermentation Process Parameters
Spoilage and pathogenic flora in apple juice and by extension in apple fermented beverage can be reduced by physical methods, which have already been reviewed [ 121 ] and will not be further discussed. The control of the fermentation process parameters such as the time of inoculation of mixed cultures, the temperature of fermentation steps in the process or prefermentative steps is a key element in the modulation of the cider ecosystems throughout processing and thus of the quality of the final product. Aroma production during cider fermentation is greatly dependent on the yeast species in the presence and their sequential succession throughout the process. A study of the co-culture of Wickerhamomyces anomalus and S. cerevisiae showed that the association could help improve the quality and add complexity to the cider [ 122 ]. Controlling the strain association parameters during the fermentation process, i.e., the inoculation time and the sequential or simultaneous mixed cultures, is crucial for the optimization of the desired kind of cider. In the same way, the fermentation of a vegetable juice using a mixed culture of S. cerevisiae and L. plantarum resulting in an enhancement of the nutritional content of the final beverage [ 123 ] emphasizes the feasibility of the chosen co-fermentation by the selection of the right microbial associations for designing new functional apple fermented beverages.
Yeast metabolism is greatly dependent on the temperatures applied during the fermentation process. Peng et al. [ 124 ] have shown that variations of the fermentation temperature have a direct influence on the aromatic profile of the final cider. In their study, the ciders fermented at 20 °C seemed to result in the best acceptance by the consumer and displayed the highest aromatic characteristics. This is probably due to modifications in the microbial metabolism that result in variations in the production of esters, volatile compounds and alcohols according to the fermentation temperatures. Variations in the fermentation temperature are nonetheless to be considered with care, as the increase of temperature can also lead to the formation of undesired compounds by the expression of unsuitable microbial metabolic pathways [ 124 , 125 ]. In the same way, fruit processing, pectinolytic enzyme application, cell yeast immobilization on alginate and the type of fermentation have all a significant influence on the antioxidant capacity, polyphenol profile and volatile composition of ciders [ 126 ]. Prefermentative treatments such as pulp fermentation induced the formation of higher amounts of ethanol, procyanidins B2 and C1, epicatechin and catechin and resulted in a higher antioxidant activity than in non-pulp fermented ciders. Cell immobilization positively affected the ethanol content, but decreased the antioxidant activity of ciders. Ciders obtained with spontaneous fermentation contained more esters and methanol compared to inoculated ciders [ 126 ]. The MLF is also a bottle-neck in the cider production process, and one area of research is looking for strategies to control/improve this natural phenomenon [ 127 ]. Such process parameters can thus be used as levers to modulate the quality of the final product.
5.1.3. Control of Cider Quality by LAB
Microbial quality is obviously microorganism dependent and is highly affected by chemical, physical and biological factors pertaining to the environment. Maintaining microbiological quality and the maximum sensory and nutritional quality of fermented beverages requires a combination of antimicrobial hurdles in order to limit the growth of undesired microorganisms. By producing organic acids as a fermentation metabolite, antimicrobial peptides and hydrogen peroxide, LAB strains may contribute to improving the quality of apple ciders. Bacteriocins are generated from bacteria and, usually, are inhibitory towards phylogenetically-related species. There are only a few reports about the inhibitory activity of bacteriocins against yeasts [ 128 , 129 , 130 ]. To our knowledge, the effectiveness of bacteriocins from LAB for controlling the growth of undesirable yeasts in cider or wine has never been studied, although bacteriocins produced by LAB have received considerable attention over the years for their possible use as biopreservatives in food, to reduce the use of chemical preservatives. It could therefore be interesting to screen new bacteriocins from LAB isolated from fermented beverages.
Bacteriocins could also be effective against spoilage bacteria. L. collinoides exhibits natural resistance to conditions encountered during the fermentative process [ 131 ]. In order to avoid this alteration, the bacteriocin enterocin AS-48, a broad-spectrum antimicrobial peptide produced by Enterococcus faecalis [ 132 ], was tested against two 3-HPA-producing L. collinoides strains causing apple cider spoilage. The two L. collinoides strains tested were rapidly inactivated by low concentrations of enterocin AS-48 in fresh apple juice (2.5 µg/mL) and also in Basque cider (2.5–5 µg/mL) [ 133 ]. Another classical disorder, which does not affect flavor, is known as ‘ropiness’. This microbiological problem arises when certain bacteria synthesize exopolysaccharides (EPS), thus increasing the viscosity of the cider [ 134 ]. The EPS show a large variation in composition, molecular mass and structure and, once secreted into the medium, play an important role in the rheology and texture of fermented beverages, enhancing naturally the texture and viscosity [ 135 ]. As a consequence of the increase of viscosity, the cider flows like oil; hence the term ‘ropiness’. In addition to being a biothickener, prebiotic effects of several EPS have been demonstrated [ 136 ]; however, despite these interesting properties, a high level of EPS production in cider is unwanted, as it is prejudicial to the organoleptic quality of the product. Although ropiness is mainly caused by some strains of LAB [ 137 , 138 , 139 ], it has been shown that one strain belonging to the Bacillus genus can be responsible for this alteration [ 140 ]. Among the alternative methods suggested to avoid this alteration in beverages, Grande et al. [ 141 ] have tested the efficacy of the E. faecalis enterocin AS-48 against a slime-producing B. licheniformis strain in apple cider. Their results show that enterocin AS-48 is also active against the EPS-producing strain either in culture medium or in apple cider, suggesting a possible use of this enterocin to prevent ropiness. These results are of great interest for the development of tools allowing for the control of undesired bacteria in fermented apple cider.
Thanks to amino oxidase enzymatic activity, some species of LAB appear to be of great interest for the potential control of BA-related health risk. Hitherto, many studies have been conducted with the purpose to identify LAB isolated from fermented foods with BA degrading capability, but only a few concern fermented beverages. To our knowledge, no study has been conducted on LAB isolated from cider. A collection of 85 LAB isolated from wines, must and lees was screened for their ability to degrade histamine, tyramine and/or putrescine. Twenty-five percent of the LAB were able to degrade histamine, 18% tyramine and 18% putrescine. The strains with highest activity belonged to Lactobacillus and Pediococcus groups, and most of them were able to degrade simultaneously at least two BAs [ 108 ]. In the future, it might be of interest to screen the potential of cider-associated LAB to reduce potential BA level in this beverage.
Large numbers of studies attribute antifungal activity to LAB strains thanks to the production of various organic acids (such as lactic, acetic, caproic, formic, propionic, phenyl lactic and butyric), fatty acids and peptides [ 142 , 143 ]. LAB may represent interesting biological control agents in apple fermented beverages by means other than bacteriocin. The detoxification of patulin through binding to bacterial surface proteins is an example [ 144 , 145 ]. Recently, Zoghi et al. identified two probiotic strains of L. acidophilus and L. plantarum able to catch the toxin through their surface layer proteins (fructooligosaccharide content). In the best conditions and after six weeks of refrigerated storage, more than 90% of initial patulin were removed from apple juice with no significant difference in organoleptic properties [ 144 ].
5.2. Health Benefits of Apple Fermented Beverages
Fermented beverages and especially non-dairy probiotic beverages are believed to be the next functional foods for probiotic delivery. Likely candidates are chilled fruit juices or fermented vegetable juices [ 146 ]. For the consumer, they present the advantages of lacking dairy allergens such as lactose, containing low cholesterol and having a vegan-friendly status [ 147 ]. The health benefits of fermented beverages have been described. The improvement of gastrointestinal health associated with the microbial content of fermented beverage is thought to be responsible for perceived health outcomes. Evidence of the direct or indirect action of the beverage microbiota on gastrointestinal health have been given over the years, even if the mechanisms involved are still unclear for the most part [ 148 ]. The health benefits of apple beverages have been the subject of much scrutiny, for many years. For example, apple beverages, including cider, have been shown to have anti-viral properties [ 149 ]. Some apple juices are already used as vectors of probiotic lactobacilli strains [ 150 , 151 ]. Several traditional cereal and vegetal fermented beverages are the source of probiotic bacteria [ 152 ]. Apple fermented beverages can therefore be sources and vectors of probiotics. Spent cider yeast, a by-product of the fermentation process, was used as a dietary supplementation in a piglet model. This supplementation proved to enhance gut functions and to reduce Salmonella and Escherichia carriage in porcine gut [ 153 ]. Some probiotic potential has also been demonstrated for lactobacilli [ 48 ] or pediococci [ 47 , 154 ]. A probiotic beverage from apple fermented with L. casei has recently been developed for human consumption [ 151 ].
A recent review detailed the role of LAB as an efficient cell factory for the production of functional biomolecules and food ingredients to enhance the quality of cereal-based beverages [ 155 ]. These LAB assets could be transposed to apple fermented beverages. They encompass the LAB-mediated inhibition of spoilage or pathogenic microorganisms through antibacterial compound production, the reduction of potential antinutritive factors, the amelioration of the apple fermented beverage nutritional value, the LAB aroma and flavor compound production, the production of EPS related to texture development, organoleptic changes and the prebiotic nature of those beverages, the production of nutraceutical compounds and anti-allergenic biomolecules. Some LAB, isolated from wine or cider, also showed potential intrinsic (without grape/apple matrix) health benefits [ 156 ]. For example, O. oeni can harbor anti-inflammatory potential [ 157 ] or produce EPS [ 158 ]. This EPS production could even help with the industrial production of food products containing lyophilized O. oeni strains [ 158 ]. The EPS production by LAB could also be related to industrial perspectives such as viscosity and mouth feel enhancement properties [ 159 ].
The development of functional apple fermented beverages is promising. For example, strategies combining apple juice and a novel whey-based beverage fermented by kefir grains have already been designed [ 160 ]. The combination of apple juice and kefir grains resulted in a beverage with high total phenolic content and antioxidant activity.
6. Conclusions
This review emphasized the microbial ecosystem of musts and showed how mastering the quality and the safety of cider production is reliant on a better understanding of the mechanisms of LAB and yeast metabolism involved in the transformation of precursors into potent flavor components. The present review further paved the way for the optimization of the industrial scale-up for artisanal cider production using the integrated metabolomics and molecular phylogeny approaches to identify and select strains of LAB, particularly O. oeni , to improve the flavor/aroma profiles of ciders. Indeed, although considerable efforts have been made in recent decades to optimize and improve the production of cider, cider remains a product with a great variability related in particular to the notion of ‘terroir’ that can be defined as a homogeneous territory from a soil and climate point of view. Therefore, pedoclimate factors together with indigenous microorganisms may significantly influence the quality and typicity of the cider produced in a specific location. The apple benefits from a good and healthy image that could be combined with new microbial characteristics with a special focus on LAB. Specific research on microbiomes using ‘omics’ tools will give rapid insights into the potential of strains associated with these products. For these reasons, studies on apple fermentation beverages comprise a promising field of research with great potential for available new, healthy and pleasant products on the market.
Conflicts of Interest
The authors declare no conflict of interest.
Academia.edu no longer supports Internet Explorer.
To browse Academia.edu and the wider internet faster and more securely, please take a few seconds to upgrade your browser .
Enter the email address you signed up with and we'll email you a reset link.
- We're Hiring!
- Help Center

Wine Production from Mixed Fruits (Pineapple and Watermelon) Using High Alcohol Tolerant Yeast Isolated from Palm Wine

Related Papers
IOSR Journals
This Study Was Aimed At Investigating The Suitability Of Two Fruits (Pineapple And Soursop) As
Acta Scientifci Nutritional Health
Folasade Adeboyejo
Constance C Ezemba
This research was carried out to produce wine from banana (Musa sapientum) pulp using yeast (Saccharomyces cerevisiae) isolated from grape (Vitis vinifera).The fermentation of the banana wine lasted for 21 days. Physico-chemical parameters were determined during fermentation using standard procedures. Liquor of the fermenting must was removed for every 48 hours from the fermentor for analysis of pH, titratable acidity, specific gravity and reducing sugar using standard procedures. The results from the experiment showed that specific gravity of the wine was observed to reduce drastically as the fermentation progressed. The pH of the banana wine during fermentation increased from 4.16-4.22 at day to the last day while the titrable acidity (% w/v tartaric acid) of the banana wine produced increased from 1.05-1.77. The alcohol content of the wine increased with time. The specific gravity values were observed to range from 1.266 to 1.184 kg/m 3 which gradually decreased throughout the period of fermentation. At the end of the fermentation,
SpringerPlus
Chukwuma Stephen Ezeonu
PRODUCTION OF WINE FROM THE FERMENTATION OF ORANGE JUICE BY Saccharomyces cerevisiae
Oladimeji ishaq Hassan
The fermentation of orange juice by Saccharomyces cerevisiae isolated from palm wine was carried out. The fermentation was done in two phases; the aerobic phase which lasted for 5 days and the anaerobic phase which lasted for 9 days. Some physicochemical parameters were monitored during the aerobic and anaerobic phase of fermentation. These were pH, titratable acidity, specific gravity, sugar content and alcohol concentration. The result of the physicochemical analysis showed that during aerobic fermentation there was a decline in the pH from 3.6 to 2.4; an increase in titratbable acidity from 9.10 to 16.8g/l. During anaerobic phase the pH increased from 3.0 to 4.3 while the titratable acidity decreased from 16.4 to 9.7g/l. The yeast counts increased from 5.5 ×106 to 6.9×106 cells/ml; alcohol content increased from 0 to 5.4% during aerobic fermentation, while during anaerobic there was a drop in yeast counts from 7.1×106cells/ml to 6.3×106 cells/ml; and the alcohol content increased from 5.8 to 9.6%. The sugar content and specific gravity in the wine dropped throughout during aerobic and anaerobic fermentation with sugar content dropping from initial value of 50.80 mg/ml to 2.87 mg/ml, while the Specific gravity dropped from 1.040 to 0.980osp.gr. On the 9th day of the anaerobic phase the fermentation was terminated by opening the fermentation tank, the wine was then clarified, racked and bottled.
The study was aimed at the production of apple (Malus pumila) fruit wine with the use of yeast Saccharomyces cerevisiae isolated from palm wine. Both primary and secondary fermentation of the apple lasted 28 days. Aliquot samples were removed and used daily from the fermentation tank for analysis of alcohol content, specific gravity, pH, titratable acidity, and reducing sugar using standard procedures. During fermentation, pH of the fruit must range from 5.0 to 3.2. There was an increase in alcohol content, which was observed with time. Finally at the end of the 28 th day's fermentation, the alcohol concentration in the fruit wine was observed to be 3.2%. Also titratable acidity concentration of the wine shows steady increase with time throughout the fermentation period. This study has revealed that much acceptable wine with quality could be produced from apple with Saccharomyces cerevisiae isolated from palm wine. Sensory evaluation results showed there were no significant differences (p > 0.05) in flavor, taste, clarity and overall acceptability between apple wine and a reference wine. The apple wine was generally accepted.
Journal of Food Science and Engineering 9 (2019) 199-209
Caroline Ebere
The aim of this study was to produce and evaluate table wine from two different varieties of pawpaw (rose red and yellow pawpaw). The must was evaluated for physicochemical and microbiological changes during fermentation while the wine was analyzed for physicochemical characteristics, microbiological quality and sensory properties and compared with commercial grape wine. Specific gravity of the “must” during fermentation decreased from 1.059-0.995 for rose red pawpaw and 1.005-0.990 for yellow pawpaw. The sugar content decreased from 13-3% on the 14th day of fermentation for rose red pawpaw while yellow pawpaw “must” decreased from 12.5-3%. pH drop for the yellow pawpaw “must” was 4.7-3.4 on the 14th day and 4.0-3.4 for rose red pawpaw “must”. Titratable acidity of the pawpaw “must” increased from 0.16-0.32% for rose red pawpaw “must” and 0.20-0.52% for yellow pawpaw “must”. Microbial analysis of the “must” during fermentation showed that yeast count increased from no growth to 3.0 × 106 cfu/mL for yellow pawpaw must and 4.0 × 106 cfu/mL for rose red pawpaw, respectively while total bacterial count decreased from 5.4 × 107-l.5 × 107 cfu/mL for yellow pawpaw must and 5.2 × 107-1.2 × l07 cfu/mL for rose red pawpaw “must”. Coliform recorded no growth throughout the period of fermentation. Physicochemical analysis of the wine showed that the yellow pawpaw wine has a specific gravity of 0.999, alcohol content 8.00%, titratable acidity of 0.59%, pH of 3.5 and sugar content of 3%. The rose red pawpaw wine had sugar content of 3%, titratable acidity of 0.38%, alcohol content 7.69%, specific gravity 0.997 and pH of 3.5. Microbial analysis of the wine showed no growth of coliform and yeast while bacterial count was 1.0 × 106 cfu/mL for both wines. Sensory results for the pawpaw wine showed no significant (p > 0.05) difference in the clarity and overall acceptability from the commercial wine.
Integrative Food, Nutrition and Metabolism
Pratap Chandran
Kehinde P Akinrotoye
The effect of fermented palm wine tapped from Raphia palm tree (Raphia hookeri) on the growth of some common diarrhoeagenic bacteria such as Staphylococcus aureus, Escherichia coli, Salmonella typhi, Shigella dysentariae and Esherichia coli 0157:H7 was carried out using agar diffusion method. The palm wine used had growth inhibitory effect on all the test organisms with diameter of zones of inhibition ranging from 2.20mm to 28.20mm. Palm wine subjected to fermentation for 168hours (7 days) exerted the highest growth inhibitory effect on all the test bacteria. The inhibition mediated by this palm wine was superior to that of some conventional antibiotics used such as tetracycline, ampiclox and ampicillin. It is conceivable therefore that palm wine subjected to natural fermentation or freshly tapped could be used to treat diarrhoea caused by these organisms.
Chigozie E Ofoedu
Physicochemical and sensory acceptability of wine made from soursop (Annonamuricata) were evaluated. A “must” (soursop juice before or during fermentation) sample of the soursop pulp was prepared and replicated. Its replica is referred to as sample B in this work. The “must” samples were treated with 0.543/litre of sodium metabisulphite, inoculated with 5grams reconstituted active baker’s yeast and allowed to ferment. The fermentation lasted for 11days. The wine sample produced had a final alcohol content of 12.99%, pH of 3.42, and total acidity of 0.82%. The green wine (young) was aged for 12weeks to reduce the acidity and to develop a characteristic bouquet. It was packaged and presented for sensory evaluation using Don Morris white wine as a standard. The sample wine compared favorably with the standard with no significant difference (p>0.05) in odor and taste but with a significant difference (p<0.05)in color acceptability. The green wine was also compared with the replica and no significant difference obtained. The wine at 9months old was tested again and 3.35, 0.9826, 13.95% and 2.15% for pH, specific gravity, alcohol content and titratable acidity respectively was obtained. Sensory evaluation at 9months old was also carried out and result obtained was used in evaluating analysis of variance. No significant difference existed in color, odor, taste and acceptability.
Loading Preview
Sorry, preview is currently unavailable. You can download the paper by clicking the button above.
RELATED PAPERS
Chemical Science Review and Letters
EZENWA MARTINS
Enya Emmanuel
Pr. Roger DJOULDE Darman
International Journal of Current Microbiology and Applied Sciences
Pooja Nikhanj
Victor S I N G H Ayam
International Journal of Microbiological Research
Dr. P. Saranraj
ighedi israel
priya sekar
Journal of Food Processing & Technology
harmeet chauhan
Indian Journal of …
Newitchaya Wutthinithisanand
Oranusi Solomon
Runu Chakraborty
Journal of Applied …
Karan Bagde , pranali waghdhare
konfo Christian
J M Noel MUNYANEZA
Oluwatoyin Olugbemi
Journal of Applied Biology & Biotechnology
vandana ranganathan
UMYU Journal of Microbiology Research
Adebare J. Adeleke
Paul Osei-fosu , Henry Mensah-Brown
La Granja: Revista de Ciencias de la Vida
Plant Foods for Human Nutrition (Formerly Qualitas …
MOHAMMAD AIZAT JAMALUDIN , Mohd Anuar Ramli
Fred O.J. Oboh
Iyamenye Nibeho Clet
Antonella Pasqualone
Nausheen Saba
Piyush Kashyap
- We're Hiring!
- Help Center
- Find new research papers in:
- Health Sciences
- Earth Sciences
- Cognitive Science
- Mathematics
- Computer Science
- Academia ©2024

IMAGES
COMMENTS
Abstract. The study was aimed at the production of apple (Malus pumila) fruit wine with the use of yeast Saccharomyces cerevisiae isolated from palm wine. Both primary and secondary fermentation ...
Fruit Wine Production: A Review. Shrikant Baslingappa Swami*, N.J. Thakor. and A.D. Divate. Department of Agricultural Process Engineering, College of Agricultural Engineering and Technology, Dr ...
This study has revealed that much acceptable wine with quality could be produced from apple with Saccharomyces cerevisiae isolated from palm wine. Sensory evaluation results showed there were no significant differences (p > 0.05) in flavor, taste, clarity and overall acceptability between apple wine and a reference wine.
This paper provides a production technology to process Golden Delicious apple cultivar to apple wine using a local yeast isolate from apple. Materials and methods Apple juice extracted from apples (cultivar Golden Delicious) having brix of 9.6 ± 1.5 °B and pH of 3.5 ± 0.2 was procured from local market during 2015 and 2016.
The present study was conducted to optimize fermentation parameters for apple wine production using Golden Delicious apples. Physicochemical analysis of the cultivar revealed a °Brix-acid ratio of 24.61 with ample amount of total and reducing sugars (9.6 and 6.03% w/v); making it a suitable substrate to produce ethanol. Microbiological analysis lead to isolation of a yeast strain (namely A2 ...
Current Journal of Applied Science and Technology 41(3): 1-6, 2022; Article no.CJAST.57683 ISSN: 2457-1024 (Past name: British Journal of Applied Science & Technology, Past ISSN: 2231-0843, NLM ID: 101664541) Wine Production from Apple (Malus pumila) Using Yeast Isolated from Palmwine C. C. Ezemba a*, V. N. Anakwenze b and A. S. Ezemba b a ...
Jagtap and Bapat (2014), evaluated the potential of custard apple in the production of a beverage fermented using S. cerevisiae (NCIM 3282) yeast and assessed the antioxidant capacity, total phenolic content and DNA damage-protecting activity of custard apple fruit wine. Custard apple wine showed free radicle scavenging activity towards DPPH ...
production of wines from available fruits could reduce the level of post harvest losses and also increase variety of wines [12,9,8]. Amerine and Kunkee, [13] observed that the type of wine to be produced dictates and strain of yeast to be involved and also the fruit to be used. Some of the preservatives used in wine making
The study was aimed at the production of apple (Malus pumila) fruit wine with the use of yeast Saccharomyces cerevisiae isolated from palm wine. Both primary and secondary fermentation of the apple lasted 28 days. Aliquot samples were removed and used daily from the fermentation tank for analysis of alcohol content, specific gravity, pH, titratable acidity, and reducing sugar using standard ...
The flavour and the volatilome of apple wines made from the Austrian heritage variety Ilzer Rose was in the scope of this study. The apple wines were produced by adopting oenological practises that are not commonly used in fruit wine production. Different fermentation strategies including the addition of enzymes with β-glucosidase activity, addition of a fining agent, maceration of the mash ...
of different nitrogen concen trations in apple wine fermentation. The a verage total nitrogen content in 51 d ifferent. apples juices was 155.81 mg/L, with 86 .28 % of the values above 100 mg/L ...
The study revealed a process for apple wine preparation using an indigenous yeast and also optimized and compared malolactic and non-malolactic fermented ciders. The present study was conducted to optimize fermentation parameters for apple wine production using Golden Delicious apples. Physicochemical analysis of the cultivar revealed a °Brix-acid ratio of 24.61 with ample amount of total and ...
1. Introduction. Apple wine and cider are traditional alcoholic beverages with an alcohol content lower than 8.25%. The European Cider and Fruit Wine Association (AICV) states that within the European Union, cider and fruits wines have some of the fastest growth rates of all alcoholic beverages [].This increase in popularity is also reflected by the number of scientific papers that were ...
A biocatalyst was prepared by immobilization of Saccharomyces cerevisiae strain AXAZ-1 on apple pieces. It was examined by electron microscope and studied during the fermentation of grape must for batch wine-making. The immobilized yeast showed an important operational stability without any decrease of its activity even at low temperatures (1−12 °C).
The engineered wine yeast strains described in this paper show the potential of novel yeast strain development to improve wine quality. But molecular biologists face a major obstacle to this progress: near world-wide refusal to permit the use of GMOs in the production of foods and beverages, at least in 'developed' countries ( Gross, 2009 ...
The selection of apple fruit for making wine depends upon several factors such as market demand, availability of raw material, production facilities, and sound economic reasons. ... Influence of juice contents on quality of apple wine prepared from apple juice concentrate. Research and Industry 39 (4), 250. 4.3.6. Inoculation With Yeast Culture.
Introduction. The world area under vines and the volume of wine production in 2017 have not changed significantly over the last 15 years since 2002, however, the value of wine exports in US$ has more than doubled (Anderson and Pinilla 2017).Wine production faces new challenges such as global warming (van Leeuwen and Darriet 2016) and increasing competition from the emergence of new markets and ...
The nutritive value of wine is increased due to the release of amino acids and other nutrients from yeast during fermentation. Fruit wines contain 8-11% alcohol and 2-3% sugar with energy value ranging between 70 and 90 kcal per 100 ml. The present explained about the fermentation of wine and its quality analysis.
2. Microbial Diversity: From Apple to Cider. Regarding the microbial ecosystem, next generation sequencing strategies bring many new whole ecosystem pictures, especially regarding non-cultivable bacteria [15,16].Such studies are very rare in the cider research area, with only one paper revealing the microbiota of wine and organic apple cider submerged vinegar production [].
This work has shown that the pH ranges of the pineapple, watermelon, and mixed fruit juice used for the production of the wine for this research were 4.22 ± 0.01, 5.07 ± 0.01 and 4.47 ± 0.01 respectively, while there was no significant difference in the values of the reducing sugars amongst the three samples.
2.2 Preparation of yeast. Prepared a specialized yeast medium 1 L and autoclaved for 15 min at 121 °C. After cooling to room. temperature, 0.05 % active dry yeast was added and, activated f or 24 ...
Production of Wine and Vinegar from Cashew (Anacardium occidentale ) "Apple" Samuel Lowor 1*, Daniel Yabani 1, Kumi Winifred 1 and C. K. Agyente-Badu 1 1Cocoa Research Institute of Ghana, P.O.Box 8, Akim-Tafo, Ghana. Authors' contributions The present work was result of the efforts of all authors. The lead author SL designed the
This study was aimed at comparative evaluation of red wine produced from Hibiscus sabdariffa L. and Citrus sinensis juice using Saccharomyces cerevisiae isolated from palm wine Original Research ...