
Permission Letter To Conduct Research: How To Draft It Right!
In this article, I’ll share my insights and provide you with a step-by-step guide, including customizable templates , to craft your own effective permission letter for research.
Permission Letter Generator
Disclaimer: This is a generated template and should be tailored to your specific needs. Ensure all legal or organizational requirements are met before sending.
Key Takeaways Understand the purpose and importance of a permission letter for research. Learn the essential components to include in your letter. Get a step-by-step guide to writing a compelling permission letter. Benefit from a customizable template to streamline your writing process. Discover practical tips from my personal experience to enhance your letter.
Understanding the Importance of a Permission Letter for Research
A permission letter for research is a crucial document that formally requests authorization to conduct a study in specific locations or collect data from a particular group.
It serves as a formal agreement between the researcher and the authority or individuals involved, ensuring that the research is conducted ethically and legally.
Step-by-Step Guide to Writing Your Permission Letter
Step 1: start with contact information and date.
Always begin your letter by stating your contact information at the top, followed by the date. This should include your name, address, phone number, and email address.
Step 2: Address the Recipient Properly
Address the recipient by their proper title and name. If you’re unsure, a general “To Whom It May Concern” can suffice, but personalized greetings are always more impactful.
Step 3: Introduce Yourself and Your Affiliation
Introduce yourself, your position, and your affiliation. This sets the context and establishes your credibility.
Step 4: Clearly State the Purpose of Your Letter
Be clear and concise about your intent to seek permission for research. Mention the research topic and why the specific site or group is essential for your study.
Step 5: Provide Details of Your Research
Explain the scope of your research, the methodology you’ll use, and the expected duration. Transparency is key to gaining trust and approval.
Step 6: Assure Ethical Compliance
Highlight your commitment to ethical standards, including how you’ll ensure participant confidentiality and data protection.
Step 7: Request for Approval
Politely request permission to proceed with your research, expressing your willingness to comply with any required protocols or guidelines.
Step 8: Include Contact Information for Follow-up
Offer your contact information again, encouraging the recipient to reach out with any questions or requests for further details.
Step 9: Close with a Professional Salutation
End your letter with a professional closing, such as “Sincerely,” followed by your name and signature.
Template for a Permission Letter To Conduct Research
[Your Name] [Your Address] [City, State, Zip Code] [Phone Number] [Email Address] [Date]
[Recipient’s Name or Title] [Organization’s Name] [Address] [City, State, Zip Code]
Dear [Recipient’s Name or Title],
I am writing to request permission to conduct research at [location/site/group], as part of my [research project/study] on [topic]. My name is [Your Name], and I am a [Your Position] at [Your Institution or Organization].
The purpose of my research is to [briefly state the objective]. I believe that [location/site/group] is essential for my study because [reason]. The research will involve [describe the methodology], and I anticipate it will take approximately [duration] to complete.
I assure you that all research activities will adhere to the highest ethical standards. Participant confidentiality and data protection will be strictly maintained throughout the research process.
Your approval to conduct this research would be greatly appreciated. I am more than willing to adhere to any specific protocols or requirements you may have. Please feel free to contact me at [Your Phone Number] or [Your Email Address] if you have any questions or need further information.
Thank you for considering my request. I look forward to your positive response.
[Your Name] [Your Signature, if sending a hard copy]
Personal Tips from My Experience
- Personalize Your Letter: Tailoring the letter to the recipient shows respect and attention to detail.
- Be Concise but Thorough: Provide enough detail to inform but not so much that it overwhelms the reader.
- Follow-Up: Don’t hesitate to follow up if you haven’t received a response within a reasonable time frame.
- Show Appreciation: Always express gratitude for the recipient’s time and consideration.
I hope this guide helps you craft an effective permission letter for your research. I’d love to hear about your experiences or any additional tips you might have. Please share your thoughts and questions in the comments below!
Related Posts
- Free Templates for Research Permission Letters
- 3 Must-Have Templates for Requesting Permission Easily
- Sample Letter To Request To Attend A Conference: Free & Effective
Frequently Asked Questions (FAQs)

Q: What is a permission letter to conduct research?
Answer : A permission letter to conduct research is a formal request to obtain permission from an organization or individual to conduct research on a particular topic. This type of letter is commonly used by students, researchers, and scholars who require permission to carry out their research.
Q: Why is a permission letter to conduct research important?
Answer : A permission letter to conduct research is important because it shows that the researcher has obtained the necessary permissions to conduct their research. It also provides a clear understanding of the scope and nature of the research and how it will be conducted, which can help to prevent misunderstandings or legal issues.
Q: Who should I address my permission letter to?
Answer : You should address your permission letter to the individual or organization that has the authority to grant permission for your research. This could be the head of the organization, a department manager, or an individual who is responsible for the area that you wish to conduct research in.
Q: What should I include in my permission letter to conduct research?
Answer : Your permission letter to conduct research should include an introduction that outlines your research topic and objectives, an explanation of why you need permission, an overview of your research methodology, details on the timeline and logistics of your research, and a formal closing that thanks the recipient for their time and consideration.
Q: How do I ensure that my permission letter to conduct research is effective?
Answer : To ensure that your permission letter to conduct research is effective, make sure that it is clear, concise, and polite. Provide detailed information about your research and the nature of your request, and address any potential concerns or objections that the recipient may have. Finally, proofread your letter carefully to ensure that it is free from errors and typos.
Related Articles
Sample request letter for air conditioner replacement: free & effective, goodbye email to coworkers after resignation: the simple way, unbelievable absence excuse letter for work template, salary negotiation counter offer letter sample: free & effective, formal complaint letter sample against a person: free & effective, medical reimbursement letter to employer sample: free & effective, leave a comment cancel reply.
Your email address will not be published. Required fields are marked *
Sample Letter Hub

Letter Of Permission To Conduct Research
By Mubashir
July 3, 2024
A Letter of Permission to Conduct Research is a formal request to an individual or organization seeking permission to conduct research. It outlines the purpose, methodology, and ethical considerations of the research project.
In this article, we will provide you with templates, examples, and samples of Letters of Permission to Conduct Research. These samples will guide you in drafting a well-written letter that effectively communicates your research intentions and secures the necessary approvals.
Letter of Permission to Conduct Research
Dear [Recipient Name],
I am writing to request permission to conduct research at your organization. I am a researcher at [Your Institution] and I am currently working on a project that investigates [Research Topic].
I am interested in collecting data from [Data Source] at your organization. I believe that this data will be valuable in helping me to understand [Research Question].
I understand that you may have concerns about the confidentiality of your data. I assure you that I will take all necessary precautions to protect the privacy of your participants. I will only collect data that is necessary for my research and I will not share it with any third parties.
I would be grateful if you would consider my request. I am available to meet with you at your convenience to discuss my research project in more detail.
Thank you for your time and consideration.
Sincerely, [Your Name]

How to Write a Letter of Permission to Conduct Research
When you are conducting research, it is important to obtain permission from the individuals or organizations that you will be studying. This is especially important if your research involves collecting data from human subjects or if you will be using copyrighted materials.
1. Identify the appropriate person or organization to contact
The first step is to identify the appropriate person or organization to contact for permission. This may be the individual who is the subject of your research, the head of an organization, or a copyright holder.
2. Write a clear and concise letter
Your letter should be clear and concise, and it should state the purpose of your research and the specific data or materials that you would like to use. You should also include a brief description of your research methods and how you will protect the privacy of your subjects.
3. Be respectful and professional
It is important to be respectful and professional in your letter. You should address the person or organization by their proper title and use formal language. You should also be clear about your intentions and avoid making any promises that you cannot keep.
4. Include a self-addressed stamped envelope
If you are requesting a written response, be sure to include a self-addressed stamped envelope. This will make it easier for the person or organization to respond to your request.
5. Follow up
If you do not receive a response within a few weeks, you may want to follow up with a phone call or email. Be polite and persistent, but do not be pushy.
6. Be prepared to negotiate
In some cases, the person or organization that you are contacting may be willing to grant you permission, but they may have certain conditions. Be prepared to negotiate and compromise in order to reach an agreement that is acceptable to both parties.
7. Get everything in writing
Once you have reached an agreement, be sure to get everything in writing. This will protect both you and the person or organization that you are studying.
FAQs about Letter Of Permission To Conduct Research
What is a letter of permission to conduct research.
A letter of permission to conduct research is a formal document that grants permission to a researcher to conduct research on a specific topic or within a specific setting. It typically outlines the scope of the research, the methods to be used, and the expected duration of the study.
Who needs a letter of permission to conduct research?
Researchers who plan to conduct research involving human participants, sensitive data, or access to restricted areas or populations may need to obtain a letter of permission. This is especially important when the research involves vulnerable populations or involves the collection of personal information.
What are the key elements of a letter of permission to conduct research?
A letter of permission to conduct research should typically include the following elements:
- The name and affiliation of the researcher
- The purpose and objectives of the research
- The methods to be used
- The expected duration of the study
- A statement of confidentiality and ethical considerations
- The signature of the authorized individual granting permission
How do I obtain a letter of permission to conduct research?
The process for obtaining a letter of permission to conduct research can vary depending on the institution or organization involved. In general, researchers should contact the appropriate authority (e.g., an institutional review board, research ethics committee, or gatekeeper) to inquire about the requirements and procedures for obtaining permission.
What are the consequences of not obtaining a letter of permission to conduct research?
Failing to obtain a letter of permission to conduct research can have several consequences, including:
- Denial of access to research participants or data
- Delay or suspension of the research project
- Ethical concerns and potential harm to research participants
Reach out to us for a consultation.
SLH is your favorite destination for all types of letter samples and templates.
+923498230044
© 2024, SampleLetterHub
WTO / Letters and Emails / Permission / Permission Letter to Use Laboratory (Sample Letters)
Permission Letter to Use Laboratory (Sample Letters)
A written permission letter is a vital document that grants access to laboratory facilities, and it is frequently required of students and researchers for their projects, experiments, and research studies. This article aims to simplify the concept of permission letters, shedding light on their importance, the typical addressees , and the process of crafting an effective one.
A permission letter is a written request from a student or researcher seeking approval to utilize laboratory resources for academic or research purposes. It is generally addressed to the relevant authority, such as a lab supervisor, department head, or school administrator. This document serves as a formal request, outlining the writer’s intent, the specific activities they plan to undertake, and any necessary assurances regarding safety and responsibility.
Understanding how to compose an effective permission letter is paramount for students and researchers aiming to conduct experiments or research in a laboratory setting. It not only demonstrates respect for established protocols but also ensures a clear and documented understanding between the student and the authority granting access.
Samples in Word Format

Free Template for Laboratory Use Permission Letter
[Your Full Name]
[Your Position/Title – if applicable]
[Your Institution or Organization]
[Your Address]
[City, State, Zip Code]
[Your Email Address]
[Your Phone Number]
[Laboratory Supervisor’s or Facility Manager’s Name]
[Their Position/Title]
[Name of the Laboratory or Facility]
[Institution or Organization]
[Laboratory Address]
[City, State, Zip Code]
Dear [Laboratory Supervisor’s or Facility Manager’s Name],
Subject: Request for Permission to Use [Name of Laboratory or Facility]
I am writing to request permission to use the [specific name of the laboratory or facility] at [Institution or Organization] for [state the purpose, e.g., conducting research, performing experiments, etc.]. My name is [Your Full Name], and I am a [Your Position/Title, e.g., graduate student, research assistant, independent researcher] at [Your Institution or Organization].
The nature of my work involves [briefly describe your research or project, focusing on the aspects that necessitate the use of the laboratory facilities]. Access to the [Name of Laboratory or Facility] is crucial for [mention specific equipment, resources, or environment needed for your work].
I plan to use the laboratory facilities from [start date] to [end date], and my work schedule will be [mention the expected frequency, days, and times you plan to use the facilities]. I assure you that I will adhere to all laboratory safety protocols and guidelines, and I am willing to undergo any necessary training or orientation required to use the facilities safely and effectively.
Additionally, I am supervised by [Supervisor’s or Advisor’s Name], who is a [Their Position/Title] at [Your Institution or Organization]. [He/She] is fully aware of my project and the need to access these facilities.
I am committed to maintaining the integrity of the laboratory and will ensure that all equipment and resources are used responsibly and left in proper condition after each use.
I would be grateful for the opportunity to discuss this request further and provide any additional information or documentation you may require. Your approval of this request would greatly contribute to the success of my [research/project].
Thank you for considering my application to use the [Name of Laboratory or Facility]. I look forward to your positive response.
[Your Position/Title]
[Your Institution or Organization]
Sample Permission Letters to Use Laboratory
Sample 1: from a graduate student for thesis research.
Subject : Request for Laboratory Access for Thesis Research Project
Dear Professor Henderson,
I hope this letter finds you in good health. My name is Emily Turner, and I am a graduate student pursuing a Master’s in Chemistry. I am currently in the thesis track and am writing to formally request permission to utilize the laboratory facilities under your supervision for my thesis research project.
My research focuses on developing environmentally friendly polymers for sustainable packaging materials. The experiments require specialized equipment available in the Advanced Materials Research Lab, and I believe that access to these facilities is crucial for the success of my project. I am seeking permission to use the lab for a duration of approximately six months, starting from March 1, 20XX.
I have carefully reviewed and will strictly adhere to all safety protocols and guidelines established by the university. I am committed to ensuring minimal disruption to the lab schedule and will coordinate with you and other lab members to find suitable times for my work. Additionally, I am open to any suggestions or guidelines you may have to enhance the efficiency and safety of my research.
I appreciate your time and consideration of my request. If approved, I am eager to commence my research promptly and contribute to the academic advancements within our department.
Thank you for your attention to this matter. I look forward to discussing this further with you.
Emily Turner
Enrollment number: 1234
Sample 2: From a Researcher for a Collaborative Project
Subject: Request for Collaborative Laboratory Access for Research Project
Dear Dr. Mitchell,
I trust this letter finds you well. My name is Dr. Michael Reynolds, and I am a researcher at the Center for Biomedical Advancements. I am writing to seek your permission to access and utilize the laboratory facilities at the Biomedical Engineering Lab for a collaborative research project between our institution and the University of Innovation and Technology.
Our project focuses on developing novel biomaterials for neural tissue engineering. The experiments are estimated to take approximately three months, and we are seeking permission to commence our work starting from April 1, 20XX. After reviewing the state-of-the-art facilities available at your laboratory, I am convinced that they would significantly enhance the quality and scope of our experiments. The collaborative nature of this project aligns seamlessly with the interdisciplinary approach fostered at your university.
I assure you that our team will strictly adhere to all established protocols, safety measures, and scheduling requirements. We are open to collaboration and knowledge-sharing with the members of your lab, aiming for a mutually beneficial research endeavor.
I appreciate your consideration of this request and would be grateful for an opportunity to discuss this further at your earliest convenience. Thank you for your time and support.
Dr. Michael Reynolds
About This Article

Was this helpful?
Great! Tell us more about your experience
Not up to par help us fix it, keep reading.

Letters and Emails , Permission
Sample permission letter for photoshoot (use location).

Sample Permission Letters to Use Library (Examples)

Sample Letters of Permission for Construction – Templates

Letters and Emails
Travel permission letter: sample letters & writing tips.

Education , Letters and Emails , Permission , Request
Sample permission letter to visit a museum (email examples).

Sample Permission Letter to Principal for Conducting Seminar

Asking Permission to Leave School Early (Letter & Application)

Sample Permission Letters to Come Late to Work

Business , Debt , Letters and Emails
Debt collection letter templates (late payment).

Letters and Emails , Recommendation
Recommendation letter for student from teacher (samples).

Employment , Letters and Emails , Verification
42 employment verification letter samples & templates.

Letters and Emails , Verification
Proof of funds letter (requirements + template), thank you for your feedback.
Your Voice, Our Progress. Your feedback matters a lot to us.
- Skip to main content
- Skip to primary sidebar
The Measured Mom
Education resources for parents and teachers
PS PK K 1 2 3 13 Comments
Science experiments from A-Z
This post contains affiliate links. As an Amazon Associate I earn from qualifying purchases.
Sharing is caring!
- Pinterest 22100
Looking for science experiments for preschoolers that are are sure to keep your child’s interest? We’ve found the best of the best – from A to Z!

Preschoolers have a natural fascination for the world. It’s a fabulous time to soak up science experiments! Here some of the best from the blogosphere.

A – Apple rotting experiment – What will happen to an apple when it’s left to sit in water, vinegar, or oil? Check out this rotting apple experiment from Gift of Curiosity.
B- Blow up a balloon with a bottle – We repeated this baking soda and vinegar experiment a number of times at my kids’ request. We followed this tutorial at Frogs and Snails and Puppy Dog Tails.
C- Cloud in a jar – You’ll need some boiling water, ice cubes, aerosol spray, and a mason jar with a lid to make a cloud in a jar with No Time for Flashcards.
D – Density experiment – Fill a clear bowl half-full of water. Then gather small items for this simple (but high interest!) density experiment from Inspiration Laboratories.

E – Easter egg sink or float – I love how this sink or float experiment from Happy Toddler Playtime involves a lot of testing and predicting. What a fun way to develop problem solving!
F – Dyed flowers – My kids loved the classic dyed flower experiment. Learn more at The Imagination Tree.
G – Grass in a cup – Teach your child about plants any time of year with this seed planting activity from Creative Connections for Kids.

H – Humpty Dumpty egg drop – This is the most brilliant twist on the classic egg drop experiment that I’ve seen! I Heart Crafty Things has a free printable for you as you find different ways to cushion an egg.
I – Ice Experiment – Teach your child how to lift ice with a string with this experiment from Mess for Less.
J – Jar Rainbow – Learn about density with this beautiful rainbow in a jar from Primary Playground.

K – Kitchen Counter Science – My five-year-old was busy for more than half an hour doing kitchen counter science with just a few basic ingredients . Check it out at No Time for Flashcards.
L – Light experiment – Help your preschooler see that seeds need light to grow with this experiment from Gift of Curiosity.
M – Magnetic objects – Which objects are magnetic and which aren’t? Grab our free recording sheet as your child has fun exploring with magnets.

N – Nature sink or float – Collect objects on a nature walk and do a sink or float activity.
O – Ocean in a bottle – Help your child see that oil and water don’t mix with this beautiful ocean in a bottle from Happy Hooligans.
P – Make a pulley – You just need a few basic materials to make your own pulley like they did at Kids Activities Blog.

Q – Quicksand science – Learn about the properties of quicksand by making your own! Check it out at Preschool Powol Packets.
R – Rain in a jar – This experiment is super easy to set up, but it fascinates preschoolers! Learn Play Imagine shows you how to make rain in a jar.
S – Smelling bottles – Explore the sense of smell with this collection of smelling bottle ideas from Gift of Curiosity.
T – Temperature experiment – You need just a thermometer and three glasses of water – one cold, one lukewarm, and one warm/hot. Talk about how thermometers rise and fall with temperatures. Have your child feel the outside of each cup and predict whether the thermometer will rise or fall when he puts it in the cup. Test your predictions!
U – Up, up, and away – Try this helium balloon experiment from Mess for Less. How many balloons will it take to make the bag go up?

V – Volcano – We had so much fun doing the classic volcano experiment in our backyard one year. Try this paint and erupt volcano from Fun at Home with Kids… it combines art and science for a super cool effect!
W – Walking water – Coffee Cups and Crayons has a striking walking water experiment that requires just a few materials you’ve already got at home!
X – Explore X-rays – You can make your own light box and print X-rays onto transparencies for observation. Learn more here.
Y – Yeast experiment – Help your preschooler understand how yeast makes bread rise with this yeast experiment from Steam Powered Family.
Z – FiZZy science – Just put a layer of baking soda in a rimmed baking sheet and give your child some food-colored vinegar in a spray or squirt bottle. Let the fizzing begin!
Free Alphabet Printables
Join our email list and get this free sample of alphabet activities from our membership site! Students will practice identifying and forming letters, matching upper to lowercase, and identifying beginning sounds.
You May Also Enjoy These Episodes:

Reader Interactions
13 comments.
June 1, 2018 at 2:17 am
June 2, 2018 at 3:43 pm
You’re welcome, Joanne!
May 17, 2016 at 9:13 am
Such wonderful ideas! Thanks so much for sharing! I’m going to do many of these with my kids as well as share on my blog page, Emily’s Puzzle. Thanks again!
Anna Geiger
May 21, 2016 at 7:50 pm
Thanks for reading, Emily!
Nancy Penfield
July 9, 2015 at 8:48 pm
I really enjoy your website! I look around for activities that might help me in my work and I also send Facebook comments to my step-daughter and daughter-in-law as they both homeschool their children. Thank you so much for the wonderful things that you share!
July 10, 2015 at 4:44 pm
You’re very welcome, Nancy, and thanks so much for passing along my resources! 🙂
Kari Molise @ Pre-K Complete
June 30, 2015 at 11:17 am
This is such a great list of science experiments! I love bringing learning to life for preschoolers and using hands-on activities. Thank you for putting this great list together!
~ Kari http://www.PreKComplete.com
July 3, 2015 at 5:43 pm
You’re very welcome, Kari!
June 25, 2015 at 5:57 am
I am really grateful to you for your resources. The more you give the more you receive!!! You have been doing a great job. Keep it up.
Thanks and regards,
July 3, 2015 at 5:42 pm
Thank you so much for reading, Priya! I’m glad these resources can help you. 🙂
[…] The Measured Mom […]
[…] was Science. I found the best preschool science experiments from A-Z so we did an experiment with an apple to go with the letter of the week. I cut an apple […]
[…] http://www.themeasuredmom.com/the-best-science-experiments-for-preschoolers […]
Leave a Comment Cancel reply
Your email address will not be published. Required fields are marked *
This site uses Akismet to reduce spam. Learn how your comment data is processed .
Science of Reading Resources
Popular freebies.
Get instant access to science of reading workshops and over 2000 printable resources!
Become a Member
- Application Letter For Permission To Conduct Research In Lab
These four templates serve as formal requests for permission to conduct research in a laboratory setting, addressing various scenarios and academic levels. Whether you are an undergraduate or graduate student seeking to explore your research interests, an external researcher hoping to collaborate with a renowned lab, or any other individual in need of lab access, these templates provide structured, respectful, and well-organized introductions. Each template emphasizes the importance of the research project, highlights the alignment with the lab's expertise, expresses commitment to safety and ethical standards, and kindly requests permission to access the lab facilities. These introductions set the tone for a professional and persuasive application for lab access, showcasing a strong desire to contribute to the scientific community while respecting the lab's protocols and expectations.
Template Formal Research Lab Permission Request
[Your Name] [Your Address] [City, State, Zip Code] [Email Address] [Phone Number] [Date]
[Lab Supervisor's Name] [Lab Name] [Institution Name] [Institution Address] [City, State, Zip Code]
Dear [Lab Supervisor's Name],
Subject: Request for Permission to Conduct Research in Your Lab
I hope this letter finds you in good health and high spirits. I am writing to seek your permission to conduct research in your esteemed laboratory for my [Your Research Topic] project. I am a [Your Academic Level] student at [Your University] and have a strong interest in [Relevant Research Area].
My research aims to [Briefly Describe Your Research Objectives] and is an integral part of my academic pursuits. I believe that your lab, with its state-of-the-art equipment and expertise, would provide an ideal environment for me to carry out this research effectively.
I assure you that I will adhere to all lab protocols, safety guidelines, and ethical standards. I am willing to participate in any necessary training and orientation programs to ensure the responsible and safe conduct of my research.
I kindly request your permission to access the lab facilities and materials required for my research from [Proposed Start Date] to [Proposed End Date]. I understand that lab availability may vary, and I am flexible with scheduling to accommodate the needs of the lab and its ongoing research projects.
I am more than willing to discuss this request further at your convenience and provide any additional information or documentation you may require. Your guidance and mentorship would be invaluable to the success of my research project.
Thank you for considering my request. I look forward to the opportunity to contribute to the research conducted in your lab and to learn from the experts in the field.
[Your Name] [Your University ID (if applicable)]
Template Permission Request for Lab Research (Graduate Student)
I hope this message finds you well. I am writing to formally request your permission to carry out my graduate research in your laboratory. As a [Your Academic Level] pursuing a degree in [Your Program] at [Your University], my research topic is centered around [Briefly Describe Your Research Objectives], which aligns closely with the work being done in your lab.
I am impressed by the innovative research being conducted at [Lab Name] and believe that the resources and expertise available there would greatly enhance the quality and impact of my research. I am committed to upholding the highest standards of safety, ethics, and professionalism while working in the lab.
I kindly request permission to access your lab facilities and collaborate with your research team from [Proposed Start Date] to [Proposed End Date]. I am more than willing to accommodate the lab's schedule and contribute to its ongoing projects in any way possible.
I would appreciate the opportunity to meet with you to discuss this request further and address any questions or concerns you may have. Your mentorship and guidance in this research endeavor would be invaluable to me.
Thank you for considering my request. I eagerly await your response and the potential opportunity to conduct research in your esteemed lab.
Template Research Lab Access Request (Undergraduate Student)
I trust this letter finds you in good health. I am writing to request your permission to conduct research in your laboratory as part of my undergraduate academic journey. I am currently enrolled in the [Your Program] program at [Your University] and am passionate about exploring [Relevant Research Area].
My research project focuses on [Briefly Describe Your Research Objectives], and I believe that your lab's facilities and the expertise of your team would significantly contribute to the success of my research. I am committed to following all lab protocols and safety guidelines diligently.
I kindly request permission to access your lab facilities and work under your guidance from [Proposed Start Date] to [Proposed End Date]. I am flexible and willing to accommodate the lab's operational hours and ongoing projects.
I would greatly appreciate the opportunity to meet with you to discuss this request in more detail and address any questions or concerns you may have. Your mentorship and support would be instrumental in my academic and research pursuits.
Thank you for considering my request. I look forward to the possibility of conducting research in your lab and contributing to the scientific advancements of the institution.
Template Lab Research Permission Request (External Researcher)
[Your Name] [Your Affiliation/Institution] [Your Address] [City, State, Zip Code] [Email Address] [Phone Number] [Date]
Subject: Request for Permission to Conduct Research in [Lab Name]
I hope this message finds you well. I am writing to request permission to conduct research in your laboratory as an external researcher. My research interests align closely with the work being carried out at [Lab Name], and I believe that collaborating with your team would be mutually beneficial.
My research project, [Briefly Describe Your Research Objectives], requires access to specialized equipment and expertise that I understand your lab can provide. I am committed to adhering to all lab protocols, safety measures, and ethical standards during my research activities.
I kindly request permission to access your lab facilities and collaborate with your research team from [Proposed Start Date] to [Proposed End Date]. I am open to discussing any scheduling or resource-sharing arrangements that may be necessary.
I would be grateful for the opportunity to meet with you to discuss this request further and explore potential avenues of collaboration. Your guidance and support would be invaluable in advancing the goals of my research project.
Thank you for considering my request. I look forward to the possibility of conducting research in your esteemed lab and contributing to the scientific community.
[Your Name] [Your Affiliation/Institution]
We are delighted to extend our professional proofreading and writing services to cater to all your business and professional requirements, absolutely free of charge at Englishtemplates.com . Should you need any email, letter, or application templates, please do not hesitate to reach out to us at englishtemplates.com. Kindly leave a comment stating your request, and we will ensure to provide the necessary template at the earliest.
Posts in this Series
- Common Reason For Leave Application In School
- Eid Leave Application Samples
- Employee Leave Application Form Sample
- Asking Tuition Fee Certificate From School For Income Tax Rebate
- Application To Traffic Police For Cancellation Of Invalid Ticket
- Application To Union Council For 2nd Marriage In English
- Application To Your Principal To Grant You The Permission To Join The Football Team, And Participate In The Inter School League
- Application To Your Principal To Issue The College Continuing Certificate For Scholarships
- Applications For Jobs, Leaves, Officer, Students, And General Formats
- Apply A Leave Certification For Checking Up, Or Medical Findings Atherosclerotic Aorta
- Apply For A Post Of Trainee Engineer Electrical
- Apply For Been A Choir In Church
- Application To Road Construction Minister For Grant Our Village Road-Path
- Application To Shift Family To My Site Location
- Application To Stop My Medical Facility Provided By The Company
- Application To The Bank Manager To Transfer Account
- Application To The Chairman Of Civil Engineering Department To Permit Me To Get Job-Internship At Any Company During Vacation Days
- Application To The College Principal To Allow, Or Give Me Permission To Open A Food Stall In The College Premises
- Application To The Department Railway Office Transfer My Job To Other Place
- Application To The District Collector To Construct A Godown
- Application To The Principal For The Change Of Name
- Application To The Principal Requesting Him To Start The Classes For Foreign Language
- Application To The Railway Minister For New Railway Division At Our Place
- Application To The Sdm For Correction In Caste Certificate
- Application To The Society For Permission of A Stall
- Application To Purchase Scraps In An Organisation
- Application To Register Fir On Missing of Bank Pass Book
- Application To Remove Bus Service For School
- Application To Request Hod To Release Salaries of All The Employees
- Application To Retake Exam Due To Low Attendance
- Application To Revenue Department For Recovery of Land From Land Grabbers, And Grant Passbook Patta Passbook To The Owner
- Application To Principal It Is Going To Be Late For Admission
- Application To Principal Requesting Her To Grant You Exemption From Class Test As You Would Need To Join The Practice Session For Inter School Dance Event
- Application To Principal To Avail Bus Facility
- Application To Principal To Organize Winter Camp In College
- Application To Principal To Preponement Of Examination Date
- Application To Promote Associate Professor
- Application To Office-Institution For Missing Interview of Admission In University-School-Work
- Application To Open An Account In A Bank For A Union, Or Association
- Application To Organize Mela Event
- Application To Participate In Art Competition
- Application To Participate In Singing
- Application To Police Commissioner To Check Reckless Driving In Their Area
- Application To Police Station Against A Person Who Is Not Paying My Money
- Application To Principal About To Swap The Ip Class To Maths Class On Alternate Days
- Application To Principal For Change of Section
- Application To Principal For Change Of Subject Teacher
- Application To Principal For Cleanliness Program By Students
- Application To Principal For Increasing Cupboards In Library
- Application To Principal For Taking Tc, And Character Certificate
- Application To Consulate General For Passport Reissue For Study Permit Extension
- Application To Convert My Commercial Meter To Domestic Meter
- Application To Correction Of Dob In My Policy To The Insurance Company
- Application To Deduct Hostel Fees From College Fees
- Application To Food, And Supply For Surrender Ration Card
- Application To Higher Authority For Transfer To Other Places Due To Family Person Illness
- Application To Labour Commissioner For Rehabilitation
- Application To Ministry of Interior For Issuing Noc
- Application To Move Into Higher School Branch, How To Write An Email To Principal And Hr?
- Application To Change Marital Status
- Application To Change Name In Marksheet
- Application To Change Subject In College Math To Psychology
- Application To Close The Bank Account Sample
- Application To College Library For Additional Books
- Application To Bank Manager For Opening Account
- Application To Bank Manager To Open An Account of My Sister On My Behalf
- Application To Bank Manager To Open Bank Account of Daughter
- Application To Board Of School Education For Xerox Copies Of Answer Sheets
- Application To Cancel Hostel Booking
- Application Of Transfer From Security Department To A Different Department
- Application On Issuance of Discharge Certificate
- Application Regarding Husband'S Transfer Near To Wife'S Service Area
- Application Regarding Submission Of Affidavit To Sit In Exams
- Application Request For Staff Recruitment Or To Appoint New Staff
- Application Requesting For A Job
- Application Requesting For Ac Repair-Installment
- Application Requesting For Issuance Of Suspension Letter Of A Junior Due To Certain Issues
- Application Requesting House Rent Allowance From Company
- Application Requesting Posting On Another Place For My Mother Treatment
- Application Seeking Confirming My Job
- Application To Add Wife Name In Office Records
- Application To Adviser For Semester Result
- Application To Apply For Cancellation Of Admission
- Application To Apply For Home In Govt Housing Scheme
- Application To Arrange Summer Camp For Class 4
- Application To Authority Filling A Complaint Against Faculty- Change Of Faculty In An Institution
- Application To Authority Requesting For Change In Age
- Application To Bank For Name Change Of Account Holder
- Application To Bank In Order To Change The Signatures
- Application To Bank Manager For Father'S Death
- Application Letter To Get Police Verification Certificate
- Application Letter To Join An Association
- Application Letter To Mayor For Promotion Of Tourism Spot
- Application Letter To Principal For Require Fee Structure
- Application Of Degree From College
- Application of Different Kinds of Visa
- Application Of Lost School Id Card
- Application of Noc Certificate In Bank
- Application Of The Post Of A Salesman In A Frame Dealing In Educational Equipments
- Application Letter For Road Construction
- Application Letter For Shop Manager
- Application Letter For Spouse Visa
- Application Letter For Stall Booking
- Application Letter For Tender Submission
- Application Letter For The Post Of House Master
- Application Letter For The Post of Pay Master
- Application Letter For Transfer Of Electricity Meter
- Application Letter For Water Supply Connection Sample
- Application Letter of Railway Medical Card Replacement
- Application Letter Requesting A Referral From School
- Application Letter Requesting For Construction Of Rain Water Harvesting Tanks
- Application Letter Saleswomen To Work At Cars Showroom
- Application Letter To Change College
- Application Letter For Pharmacist In Hospital
- Application Letter For Pilot Training
- Application Letter For Release Of Fund
- Application Letter For Renovation Contract Works
- Application Letter For Repair Of Pipe Leakage In The Street
- Application Letter For A Residential Plot
- Application Letter For Allotment of Staff Quarter Due To Some Personal Issues
- Application Letter For Cement Dealership
- Application Letter For Change of Toilet Seat
- Application Letter For Converting Joint Account To Single Account
- Application Letter For Correction Of Mark Sheet
- Application Letter For Cricket Club
- Application Letter For Environmental Officer
- Application Letter For Football Match
- Application Letter For Government Promotion
- Application Letter For Health Care Assistant Job
- Application Letter For High Level Inquiry of Officers
- Application Letter For Hotel Job
- Application Letter For Hotel Receptionist Job
- Application Letter For Industrial Training Sample
- Application Letter For Job In Ngo
- Application Letter For Paid Leave
- Application Format For Adding Wife Name In Service Book
- Application Format For Advance Housing
- Application Format For Advance Housing Allowance
- Application Format For Electricity Meter Change
- Application Format For Surname Correction In Mutual Funds Office
- Application Format For The Post of Legal Advisor
- Application Letter Asking For Cash From Other Branch
- Application Letter Asking Government To Replace Government Vehicle Which Is In Bad Condition
- Application Letter For A Position of A Non Academic Staff In A University
- Application For Transport Contract
- Application For Tube Light Replacement
- Application For Use Of School Buildings Facilities, And Grounds
- Application For Volunteer Work In Organisations Or Companies
- Application For Wrong Marks In Marksheet
- Application For The Temporary Appointment In The School
- Application For Tlb Operator Job
- Application For Train Tickets Reservation
- Application For Transfer From One School To Another
- Application For Transfer of Headmasters
- Application For Student Visa In Australia
- Application For Student Visa In Canada
- Application For Study Tour To Industries, Factories Or Other Places
- Application For Subject Change By Teacher To Principal
- Application For The Post of Physics Teacher
- Application For The Sales Manager Position
- Application For Shift Change In Office
- Application For Single Or Separate Room In Hostel
- Application For Student Visa To Embassy
- Application For Study About Aeronautical Engineering
- Application For The Position of Fuel Retail Clerk
- Application For The Post of Cash Transfer Assistant
- Application For Refund Amount of Booking Flat , Due To Home Loan Has Been Cancelled
- Application For Refund of Fees Paid In School
- Application For Refund of Security Deposit
- Application For Registration Of Vendor
- Application For Reimbursement Of Medical Expenses-Charges
- Application For Security Guard License
- Application For Seeking Asylum In Foreign Country
- Application For Student Visa In Spain
- Application For Student Visa In Usa
- Application For Testimonial Certificate From University
- Application For The Noc In Concern of Change of Department
- Application For Readmission In The Same Class
- Application For Realizing Bill Of Govt Development Work
- Application For Receiving My Documents For Company
- Application For Recheck My Physical Education Paper, And Accountancy Paper
- Application For Recovering The Courier Goods & Power To Receive-Handover The Said Courier Goods To 3rd Persons
- Application For School Leaving Certificate Complaining Regarding Dissatisfaction
- Application For School Room For Competitive Exam
- Application For Student Visa In Malaysia
- Application For Student Visa In Norway
- Application For Teaching Of Law Subject
- Application For Testimonial Certificate From School
- Application For Purchase Of Sports Equipment For College
- Application For Railway Pass
- Application For Re Evaluation Of Marks, And Result
- Application For Re-Engagement As A Volunteer Teacher
- Application For Reactivation Of Salary
- Application For Salary Slip For Loan Purpose
- Application For School Bus Route Change
- Application For Student Visa In Finland
- Application For Student Visa In Germany
- Application For Taking Makeup Exam
- Application For Taking Permission Organizing A Self Defence Training In Institute
- Application For Promotion Under Career Advancement Scheme
- Application For Providing Water Tank
- Application For Providing Water Tanker
- Application For Provisional Admission In College
- Application For Purchase of Computer
- Application For Release Of Provident Fund
- Application For Release of Surety
- Application For Replacement Of Medical Card
- Application For Replacement of Security Guard
- Application For Safety Of Playground
- Application For Salary Increase By The School Administrator
- Application For Promotion To Assistant Professor
- Application For Promotion To Associate Professor
- Application For Promotion To Full Professor
- Application For Promotion To Senior Lecturer
- Application For Promotion To Supervisor
- Application For Release Of Passport
- Application For Release of Pension
- Application For Repair Of Water Tank
- Application For Repair Water Taps In Hostel
- Application For Room Allotment In Guest House
- Application For Room Allotment In Hostel
- Application For Permission To Sit In Class Without Books
- Application For Permission To Sit In Exam
- Application For Prayer Road Repairing
- Application For Promotion In Designation
- Application For Rejoining The Job By Employees
- Application For Release of Impounded Vehicle
- Application For Renewal Of Security Agreement
- Application For Reopening Case In Honorable Court By The Plaintiff
- Application For Residential Society Approval
- Application For Retake Exam Because Of The Bad Result
- Application For Permanent Residence Certificate
- Application For Permission To Conduct A Lucky Draw?
- Application For Permission To Join The Football Team Of School
- Application For Permission To Play A Friendly Football Match
- Application For Permission To Purchase A Plot (Write To My Office Authority)
- Application For Rejoining Coaching Class
- Application For Rejoining College, Or University As Professor-Lecturer
- Application For Relief In Cost For Society'S Road Work To Corporation
- Application For Renewal Of Employment Exchange Registration Certificate
- Application For Requesting A New Cricket Ground
- Application For Requesting Deduction of Provident Fund
- Application For Mortgage Modification
- Application For My Travel Arrangements (I Am Going On Project Work So Please Make The Arrangements For Travel)
- Application For Name Change After Marriage
- Application For New Bus Service
- Application For New Electricity Meter Connection
- Application For Not Wearing School Uniform
- Application For Nursing Job Samples
- Application For Opening A Lab In School
- Application For Out Leaving Sanctioned In Army Person
- Application For Participation In Extracurricular Activities, And Sports
- Application For Rejoining Batch In Mahendra (State Level Examinations)
- Application For Rejoining Classes Of Particular Subjects
- Application For Release of Vehicle From Court
- Application For Reporting Of Teacher Absence In Class
- Application For Requesting A Driver In Company
- Application For Marriage Allowance
- Application For Medical Allowance Issuance Sample
- Application For Medical Promotion Officer
- Application For Mortgage Broker License
- Application For Mortgage Insurance
- Application For Lost New Zealand Passport
- Application For Lost Of School Bag
- Application For Lost University Id Card
- Application For Lunch At Home From School
- Application For Major Change In University
- Application For Loan To Bank Manager To Open A New Business
- Application For Looking A Job When You Are Waiting For Result
- Application For Lost Atm Card For Bank
- Application For Lost Degree To The Authorities
- Application For Lost Library Book
- Application For Library Fine Forgiveness
- Application For Loan For Renovation Of House
- Application For Loan From Company
- Application For Loan From School By Teacher Or Other Staff Member
- Application For Loan On The Security Of Postal Insurance Policy
- Death Leave Application Samples For Office-Schools
- Demand Application To Hire More Peons Staff For The School
- Application For Leave Of Absence
- Application for Leaving Government Quarter
- Application For Leaving Hostel, And Permission-Allow Me To Take My Luggage
- Application For Library Card Issue
- Application For Library Fine Concession
- Application For Job In A Statistics Entity
- Application For Job In Place of Father
- Application For Job Leaving Certificate
- Application For Job Vacancy As An It Technician
- Application For Joining New Batch
- Application For Land Allotment To Deputy Commissioner
- Application For Late Fee Submission Due To Financial Problems
- Application For Late Joining Of Internship Program
- Application For Learning English Language
- Application For Leave From School
- Application For Irish Passport
- Application For Irish Visa
- Application For Issuance of Bank Statement
- Application For Issuance of Birth Certificate
- Application For Issuance of Health Certificate
- Application For Issuance Of Provisional Certificate
- Application For Issuance Of School Bus Service
- Application For Issue Of Dependent Identity Card
- Application For Issued Qualifying Certificates To The Company
- Application For Hostel Allotment Sample
- Application For Hostel Room
- Application For Inclusion of Name In Seniority List
- Application For Increase Rent For Generator Supply In Bank
- Application For Increasing Rates of Cylinder
- Application For Installments of Advance Loan From Salary
- Application For Installments of Tax Payment From Salary
- Application For Internship In Library
- Application For Irish Birth Certificate
- Application For Irish Citizenship
- Application For Food Allowance
- Application For Football Academy
- Application For Fuel Allowance Increment
- Application For Grade Promotion
- Application For Grant For Development-Repair-Maintenance
- Application For Grant For Non Profit Organization
- Application For Grant For Organizing Conference-Seminar-Symposium
- Application For Grant of Educational Stipend
- Application For Grant Of Quarter Allowance
- Application For Half Class
- Application For Extension Of The Time Submission For The Original Certificate
- Application For Extra Plumber In Hospital
- Application For Fee Concession Due To Father'S Death By Mother
- Application For Fee Instalments In College (As My Father Is A Small Shopkeeper)
- Application For Fee Waiver After Father'S Death
- Application For Finance, And Accounts Job
- Application For Financial Assistance For Organizing Cultural Show
- Application For Financial Assistance Under Capacity Building Scheme For The Unemployed Youths
- Application For Financial Secretary
- Application For English Teacher Job Position
- Application For Equivalence Of Foreign Degree
- Application For Extending Teaching Job
- Application For Extension of Govt Quarter
- Application For Extension of Service
- Application For Electrification To Supplier
- Application For Employment As A Journalist
- Application For Employment As A Laborer
- Application For Employment As Sales Boy
- Application For Employment As Secretary
- Application For Duplicate Social Security Card
- Application For Duplicate Title
- Application For Electricity Meter Change
- Application For Electricity Meter Not Working
- Application For Duplicate Higher Secondary Certificate
- Application For Duplicate Library Card
- Application For Duplicate Passport
- Application For Duplicate Diploma Certificate
- Application For Duplicate Driving Licence
- Application For Duplicate Fee Receipt
- Application For Duplicate Green Card
- Application For Disconnection of Water Supply
- Application For Duplicate Bank Passbook
- Application For Duplicate Birth Certificate Letter
- Application For Demolishing The Unused Building, And Construction Of New One Sanctioned.
- Application For Department Change In Company
- Application For Departmental Promotion
- Application For Dependent Certificate of My Family Members
- Application For Death Certificate
- Application For Degree Issuance Before Convocation
- Application For Delay Intimation Of Death Claim
- Application For Delay of Intimation of Death Claim(Lici Insurance Policy)
- Application For Company Residence
- Application For Compensation Of Expenses
- Application For Complaint That Electric Transformer Is Overloaded
- Application For Consideration For Promotion
- Application For Considering My Fee By Bank Draft
- Application For Construction of Computer Lab In School
- Application For Continuation In Job As A Teacher
- Application For Continuing The Service
- Application For Correction In Seniority List
- Application For Correction Of My Name In Service Records
- Application For Correction Of The Name Of My Mother In My Mark Sheet
- Application For Cricket Pitch In Our Society Ground
- Application For Dealership Cancellation
- Application For Dealership Letter Sample
- Application For Change of Workplace In Company, Factory Or Office
- Application For Changing Coaching Batch
- Application For Changing Hobby Class
- Application For Character Certificate By School-College Student
- Application For Civil Sub Engineer
- Application For Clearance Of Hostel Dues, And Security
- Application For College Fees Refund
- Application For Company Registration
- Application For Company Registration With Roc
- Application For Change In Class Schedule
- Application For Change In Pick & Drop Stop, And Route
- Application For Change My Gas Connection Name
- Application For Change My Id Card Photo
- Application For Change Of Address In School
- Application For Change of Bank Security
- Application For Change Of Class Time In School, College, Or University
- Application For Change Of Department In A Government Job
- Application For Change Of Department On Govt Job
- Application For Change Of Stream In School
- Application For Change of Subjects In University, College, Or School
- Application For Change of Transport In School
- Application For Cancellation of Transfer Orders
- Application For Canteen Contract
- Application For Cantonment Board Job
- Application For Car Parking In The College, Or School
- Application For Certificate of Title
- Application For Being An Agent of Pan Card Company
- Application For Bill Instalments
- Application For Booking A Banquet For Reception Party
- Application For Bus Pass For Students
- Application For Business Loan To Bank Manager
- Application For Buying More Books For School Library
- Application For Backup Class
- Application For Batch Change In Institute
- Application For Batch Transfer In Coaching
- Application About Electric Meter Checking, And Access Meter Bill
- Application About The Partiality Done By Teacher In Exam Paper Checking
- Application Applying For The Job In Food Authority Agency
- Application Asking For Construction Of Retaining Wall I-C.C Drainage
- Application Asking For Job As A Banker
- Application For 2nd Installment Of Education Loan
- Application For A Bank Loan
- Application For A Post Office Box
- Application For A Reduction In Tax Deductions At The Source
- Application For A Security Guard Sample
- Application For Adding Spouse Name In Service Book
- Application For Admission Cancellation Due To Poverty
- Application For Admission Fee Installments
- Application For Admission For Kindergarten
- Application For Advance Salary Due To Eid
- Application For Advance Salary For Christmas
- Application For Advance Salary For Festival Diwali, Deepawali
- Application For Advance Salary For House Maintenance
- Application For Advance Salary For Sacrifice of Animal On Eid Ul Adha
- Application For Allotment Of A Room Multipurpose Letters
- Application For Allotment of Govt Married Accommodation
- Application For Allotment of Railway Quarter
- Application For Allotment Of Room For Guests
- Application For Allow Me To Come Late
- Application For Applying As A Teacher In Engineering College
- Application For Applying Job At A Call Centre
- Application For Appointment of Legal Advisor
- Application For Approval For Bill Payment
- Application For Asking Regularization Of An Adhoc Basis
- Application For Asking Some Sports Instruments To The Director Elementary Education For School Students
- Application For Attestation Letter
Home » Letters » School » Request Letter to Principal for Permission to Use Laboratory – Sample Letter Requesting Access to Laboratory
Request Letter to Principal for Permission to Use Laboratory – Sample Letter Requesting Access to Laboratory
When writing a request letter for permission to use the laboratory, it is important to be clear and polite. Introduce yourself and provide detailed information about the purpose of your request. Clearly state why you need access to the laboratory and how it will benefit your studies or projects. Always thank the recipient for considering your request. Avoid unclear language and ensure all necessary information is included to support your request.
Sample Letter: Request Letter to Principal for Permission to Use Laboratory
To, The Principal, ___________ (Name of the school), ___________ (Address of the school),
Date: __/__/____ (Date)
Subject: Request for Permission to Use Laboratory
Respected Sir/Madam,
With due respect, my name is _______________ (name) and I am a student of __________ (mention class) having roll number _____________ (mention roll number).
I am writing this letter to request permission to use the laboratory for ___________ (mention purpose, e.g., conducting experiments, project work, research/other). I believe that access to the laboratory will greatly enhance my ability to ___________ (explain how access to the laboratory will benefit your studies or projects).
Specifically, I need access to ___________ (mention specific equipment or resources needed) to complete my ___________ (mention specific project, research, or academic requirement). I assure you that I will adhere to the laboratory rules and regulations and maintain the decorum expected in the laboratory.
I kindly request your permission to access the laboratory during ___________ (mention preferred times, e.g., after school hours, weekends, specific periods). My contact information is __________ (contact details), and my parents can be reached at __________ (parent’s contact details) for any further discussion.
Thank you for your kind consideration and time. I shall be highly obliged.
Yours faithfully, ___________ (Name) ___________ (Roll number) ___________ (Class) ___________ (Parent Contact Number)
By lettersdadmin
Related post, request letter to principal for organizing science fair – sample letter requesting for organization of science fair, request letter for participation in cultural event – sample letter requesting for participation in cultural event, request letter for changing optional subject – sample letter to school principal requesting for change of optional subject, request letter for permission to start a new club – sample letter requesting to start a new club in school, privacy overview.

Permission Letter To Conduct Research
[Your Name]
[Your Address]
[City, State, Zip Code]
[Email Address]
[Phone Number]
[Recipient's Name]
[Title/Position]
[Institution/Organization]
Subject: Request for Permission to Conduct Research
Dear [Recipient's Name],
I hope this letter finds you in good health and high spirits. My name is [Your Name], and I am a [your academic/professional background or affiliation] with a keen interest in [briefly describe your area of interest or expertise]. I am writing to request your kind permission to conduct research at [Institution/Organization name] as a part of my [mention degree program, if applicable] research project.
The purpose of my research is to [briefly explain the main objectives of your research and its potential benefits or contributions to the field]. The study will involve [describe the research methodology, such as surveys, interviews, observations, experiments, etc.] and is expected to be conducted from [start date] to [end date], though the duration may vary depending on the scope of the research.
I have chosen [Institution/Organization name] as the ideal setting for my research due to [explain why the chosen location is suitable for your research, such as access to resources, expertise, or relevant data]. I assure you that the research will be conducted with the utmost professionalism, adhering to all ethical guidelines and ensuring the privacy and confidentiality of all participants and collected data.
The data collected during the research will be used solely for academic purposes and may be included in my thesis/dissertation or other academic publications. I am committed to sharing the findings with the institution and am open to providing a summary of the results upon completion of the research.
Before proceeding with the research, I kindly request your formal permission to conduct this study at [Institution/Organization name]. Additionally, if there are any specific guidelines, procedures, or forms required by the institution for granting research permissions, please do let me know, and I will be glad to comply with all necessary requirements.
Thank you for considering my request. I am eager to receive your approval to commence my research at [Institution/Organization name]. If you have any questions or require further information, please feel free to contact me via email at [Your Email Address] or by phone at [Your Phone Number].
I look forward to your favorable response, and I sincerely appreciate your time and consideration.
Yours sincerely,
[Your Signature, if submitting a printed copy]


- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
- Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
IRB Consent Form Templates
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
General Consent Form Templates
Social and Behavioral Research Projects (last updated 03/16/2023)
Biomedical Research Projects (last updated 07/18/2022)
Consent Form Templates for Specific Biomedical Procedures
MRI and fMRI
Blood Collection by Finger Stick
Blood Collection by Venipuncture
Oral Consent Template
Guidance for Protocols Involving Oral Consent
Debriefing Template
Guidance and Template for Debriefing Participants
Studies Involving Children (Assent/Permission Forms)
Parent-Guardian Permission for Studies Involving Children
Sample Parental Notification Form
Sample Child Assent Form
Performance Release for Minors
Performance Releases
Performance Release for Adults
Sample consent and permission forms
General consent form to participate in research (DOC)
Two stage project consent form (DOC)
Parent permission form for research with child (DOC)
Child assent form (DOC)
Multiple consent form including audio-recording and quotations (DOC)
Photo and video consent form (DOC)
Video-recording consent form (DOC)
Re-contact agreement form (DOC)
Post-debriefing consent form (DOC)
- Privacy Policy

Home » Informed Consent in Research – Types, Templates and Examples
Informed Consent in Research – Types, Templates and Examples
Table of Contents

Informed Consent in Research
Informed consent is a process of communication between a researcher and a potential participant in which the researcher provides adequate information about the study, its risks and benefits, and the participant voluntarily agrees to participate. It is a cornerstone of ethical research involving human subjects and is intended to protect the rights and welfare of participants.
Types of Informed Consent in Research
There are different types of informed consent in research , which may vary depending on the nature of the study, the type of participants, and the context. Some of the common types of informed consent in research include:
Written Consent
This is the most common type of informed consent, where participants are provided with a written document that explains the study and its requirements. The document typically includes information about the purpose of the study, procedures involved, risks and benefits, confidentiality, and participant rights. Participants are asked to sign the document as an indication of their willingness to participate.
Oral Consent
In some cases, oral consent may be used when a written document is not practical or feasible. Oral consent involves explaining the study and its requirements to participants verbally and obtaining their consent. This method may be used for studies with illiterate or visually impaired participants or when conducting research remotely.
Implied Consent
Implied consent is used in studies where participants’ actions are taken as an indication of their willingness to participate. For example, a participant may be considered to have given implied consent if they show up for a scheduled appointment for the study.
Opt-out Consent
This method is used when participants are given the opportunity to decline participation in a study. Participants are provided with information about the study and are given the option to opt-out if they do not wish to participate. This method is commonly used in population-based studies or surveys.
Assent is used in studies involving minors or participants who are unable to provide informed consent due to cognitive impairment or disability. Assent involves obtaining the agreement of the participant to participate in the study, along with the consent of a legally authorized representative.
Informed Consent Format in Research
Here’s a basic format for informed consent that can be customized for specific research studies:
- Introduction : Begin by introducing yourself and the purpose of the study. Clearly state that participation is voluntary and that participants can withdraw at any time without penalty.
- Study Overview : Provide a brief overview of the study, including its purpose, methods, and expected outcomes.
- Procedures : Describe the procedures involved in the study in clear, concise language. Include information about the types of data that will be collected, how they will be collected, and how long the study will take.
- Risks and Benefits : Outline the potential risks and benefits of participating in the study. Be honest and upfront about any discomfort, inconvenience, or potential harm that may be involved, as well as any potential benefits.
- Confidentiality and Privacy : Explain how participant data will be collected, stored, and used, and what measures will be taken to ensure confidentiality and privacy.
- Voluntary Participation: Emphasize that participation is voluntary and that participants can withdraw at any time without penalty. Explain how to withdraw from the study and who to contact if participants have questions or concerns.
- Compensation and Incentives: If applicable, explain any compensation or incentives that will be offered to participants for their participation.
- Contact Information: Provide contact information for the researcher or a representative from the research team who can answer questions and address concerns.
- Signature : Ask participants to sign and date the consent form to indicate their voluntary agreement to participate in the study.
Informed Consent Templates in Research
Here is an example of an informed consent template that can be used in research studies:
Introduction
You are being invited to participate in a research study. Before you decide whether or not to participate, it is important for you to understand why the research is being done, what your participation will involve, and what risks and benefits may be associated with your participation.
Purpose of the Study
The purpose of this study is [insert purpose of study].
If you agree to participate, you will be asked to [insert procedures involved in the study].
Risks and Benefits
There are several potential risks and benefits associated with participation in this study. Some of the risks include [insert potential risks of participation]. Some of the benefits include [insert potential benefits of participation].
Confidentiality
Your participation in this study will be kept confidential to the extent allowed by law. All data collected during the study will be stored in a secure location and only accessed by authorized personnel. Your name and other identifying information will not be included in any reports or publications resulting from this study.
Voluntary Participation
Your participation in this study is completely voluntary. You have the right to withdraw from the study at any time without penalty. If you choose not to participate or if you withdraw from the study, there will be no negative consequences.
Contact Information
If you have any questions or concerns about the study, you can contact the investigator(s) at [insert contact information]. If you have questions about your rights as a research participant, you may contact [insert name of institutional review board and contact information].
Statement of Consent
By signing below, you acknowledge that you have read and understood the information provided in this consent form and that you freely and voluntarily consent to participate in this study.
Participant Signature: _____________________________________ Date: _____________
Investigator Signature: ____________________________________ Date: _____________
Examples of Informed Consent in Research
Here’s an example of informed consent in research:
Title : The Effects of Yoga on Stress and anxiety levels in college students
Introduction :
We are conducting a research study to investigate the effects of yoga on stress and anxiety levels in college students. We are inviting you to participate in this study.
If you agree to participate, you will be asked to attend four yoga classes per week for six weeks. Before and after the six-week period, you will be asked to complete surveys about your stress and anxiety levels. Additionally, we will measure your heart rate variability at the beginning and end of the six-week period.
Risks and Benefits:
There are no known risks associated with participating in this study. However, the benefits of practicing yoga may include decreased stress and anxiety levels, increased flexibility and strength, and improved overall well-being.
Confidentiality:
All information collected during this study will be kept strictly confidential. Your name will not be used in any reports or publications resulting from this study.
Voluntary Participation:
Participation in this study is completely voluntary. You are free to withdraw from the study at any time without penalty.
Contact Information:
If you have any questions or concerns about this study, you may contact the principal investigator at (phone number/email address).
By signing this form, I acknowledge that I have read and understood the above information and agree to participate in this study.
Participant Signature: ___________________________
Date: ___________________________
Researcher Signature: ___________________________
Importance of Informed Consent in Research
Here are some reasons why informed consent is important in research:
- Protection of participants’ rights : Informed consent ensures that participants understand the nature and purpose of the research, the risks and benefits of participating, and their rights as participants. It empowers them to make an informed decision about whether to participate or not.
- Ethical responsibility : Researchers have an ethical responsibility to respect the autonomy of participants and to protect them from harm. Informed consent is a crucial way to uphold these principles.
- Legality : Informed consent is a legal requirement in most countries. It is necessary to protect researchers from legal liability and to ensure that research is conducted in accordance with ethical standards.
- Trust : Informed consent helps build trust between researchers and participants. When participants understand the research process and their role in it, they are more likely to trust the researchers and the study.
- Quality of research : Informed consent ensures that participants are fully informed about the research and its purpose, which can lead to more accurate and reliable data. This, in turn, can improve the quality of research outcomes.

Purpose of Informed Consent in Research
Informed consent is a critical component of research ethics, and it serves several important purposes, including:
- Respect for autonomy: Informed consent respects an individual’s right to make decisions about their own health and well-being. It recognizes that individuals have the right to choose whether or not to participate in research, based on their own values, beliefs, and preferences.
- Protection of participants : Informed consent helps protect research participants from potential harm or risks that may arise from their involvement in a study. By providing participants with information about the study, its risks and benefits, and their rights, they are able to make an informed decision about whether to participate.
- Transparency: Informed consent promotes transparency in the research process. It ensures that participants are fully informed about the research, including its purpose, methods, and potential outcomes, which helps to build trust between researchers and participants.
- Legal and ethical requirements: Informed consent is a legal and ethical requirement in most research studies. It ensures that researchers obtain voluntary and informed agreement from participants to participate in the study, which helps to protect the rights and welfare of research participants.
Advantages of Informed Consent in Research
The advantages of informed consent in research are numerous, and some of the most significant benefits include:
- Protecting participants’ autonomy: Informed consent allows participants to exercise their right to self-determination and make decisions about whether to participate in a study or not. It also ensures that participants are fully informed about the risks, benefits, and implications of participating in the study.
- Promoting transparency and trust: Informed consent helps build trust between researchers and participants by providing clear and accurate information about the study’s purpose, procedures, and potential outcomes. This transparency promotes open communication and a positive research experience for all parties involved.
- Reducing the risk of harm: Informed consent ensures that participants are fully aware of any potential risks or side effects associated with the study. This knowledge enables them to make informed decisions about their participation and reduces the likelihood of harm or negative consequences.
- Ensuring ethical standards are met : Informed consent is a fundamental ethical requirement for conducting research involving human participants. By obtaining informed consent, researchers demonstrate their commitment to upholding ethical principles and standards in their research practices.
- Facilitating future research : Informed consent enables researchers to collect high-quality data that can be used for future research purposes. It also allows participants to make an informed decision about whether they are willing to participate in future studies.
About the author
Muhammad Hassan
Researcher, Academic Writer, Web developer
You may also like

Implications in Research – Types, Examples and...

Research Paper Outline – Types, Example, Template

Research Paper Title – Writing Guide and Example

Research Techniques – Methods, Types and Examples

References in Research – Types, Examples and...

Background of The Study – Examples and Writing...
- Academic Advising & Accessibility
- Academic and Cultural Enrichment
- Alumni & Friends
- Anthropology Department
- Arts Administration
- Biological Sciences Department
- Business, Nicolais School
- Business Office
- Campus Life
- Campus Safety
- Campus Services
- Career Planning & Development
- Center for Intercultural Advancement
- Center for Leadership & Community Engagement
- Center for Spirituality
- Children & Teens Programs
- Commencement
- Communications & Marketing
- Conference Services
- Continuing Education
- Dance Program
- Dining Services
- Diversity, Equity and Inclusion
- Early Childhood Center
- Education Department
- English Department
- Expanding Your Horizons
- Film & Media
- Film & Photo Shoots
- Financial Aid
- Gender Studies
- Government & Politics Department
- Give to Wagner
- HawkTalk Blog
- Health & Wellness
- History Department
- Holocaust Center
- Honors Program
- Human Resources
- Information Technology
- Institutional Advancement
- Lifelong Learning Department
- Math & Computer Science
- Modern Languages, Literatures & Cultures
- Music Department
- New Students Hub
- Nursing, Evelyn L. Spiro School
- Occupational Therapy
- Performing Arts
- Philosophy & Religious Studies Department
- Physical Sciences Department
- Physician Assistant
- Planetarium
- Pre-Health Science Program
- Pre-Law Program
- President’s Office
- Provost’s Office
- Psychology Department
- Residential Education
- Sociology Department
- Student Engagement and Activities
- Student Government Association
- Study Abroad
- Theatre Season
- Veteran’s Resources
- Visual Arts Department
- Wagner Fund
- Wagner Magazine
- WCBG Student Radio
- Writing Center
- Follett Discover Access
- Events Calendar
- Job Opportunities
- Registrar’s Office
- For Employees
- For Faculty
- For Current Students
- For New Students
- For Community
- For Parents
Sample Informed Consent Form (HERB)
Informed Consent Form
The Department of Psychology at Wagner College supports the practice of protection of human participants in research. The following will provide you with information about the experiment that will help you in deciding whether or not you wish to participate. If you agree to participate, please be aware that you are free to withdraw at any point throughout the duration of the experiment without any penalty [Note: the penalty statement is only appropriate for students] .
In this study we will ask you to __________________ * . If you have any [ insert reason why they should not participate if applicable ], please inform the experimenter and the study will end now. All information you provide will remain confidential and will not be associated with your name. If for any reason during this study you do not feel comfortable, you may leave the laboratory and receive credit for the time you participated and your information will be discarded. Your participation in this study will require approximately _____ minutes. When this study is complete you will be provided with the results of the experiment if you request them, and you will be free to ask any questions. If you have any further questions concerning this study please feel free to contact us through phone or email: RESEARCHER NAME at NAME@wagner.edu (718-) or SUPERVISOR NAME at NAME@wagner.edu (718-). Please indicate with your signature on the space below that you understand your rights and agree to participate in the experiment.
Your participation is solicited, yet strictly voluntary. All information will be kept confidential and your name will not be associated with any research findings. ______________________________ _______________________________ Signature of Participant NAME, Investigator
______________________________ Print Name ------------------------------------- PLEASE NOTE: If your participants cannot legally give consent (those under 18, for example), the form must be addressed to the parent or guardian.
*If you are asking the participant to read something, view something, reveal personal information, eat something, taste/smell something, you must inform them. You must warn participants if it is possible something you ask them to read or view may be offensive or explicit. Please describe how long (approximately) the procedure will take. Potential participants must be able to make an informed consent to participate! **If your consent form is more than one page long, be sure to number the pages in the manner shown below with a space for the participant to initial each page (so they it can be confirmed that they read each page). example: "page 1 of 4 _____" for the first page of a four-page form.
As the nation’s largest public research university, the Office of the Vice President for Research (OVPR) aims to catalyze, support and safeguard U-M research and scholarship activity.
The Office of the Vice President for Research oversees a variety of interdisciplinary units that collaborate with faculty, staff, students and external partners to catalyze, support and safeguard research and scholarship activity.
ORSP manages pre-award and some post-award research activity for U-M. We review contracts for sponsored projects applying regulatory, statutory and organizational knowledge to balance the university's mission, the sponsor's objectives, and the investigator's intellectual pursuits.
Ethics and compliance in research covers a broad range of activity from general guidelines about conducting research responsibly to specific regulations governing a type of research (e.g., human subjects research, export controls, conflict of interest).
eResearch is U-M's site for electronic research administration. Access: Regulatory Management (for IRB or IBC rDNA applications); Proposal Management (eRPM) for the e-routing, approval, and submission of proposals (PAFs) and Unfunded Agreements (UFAs) to external entities); and Animal Management (for IACUC protocols and ULAM).
Sponsored Programs manages the post-award financial activities of U-M's research enterprise and other sponsored activities to ensure compliance with applicable federal, state, and local laws as well as sponsor regulations. The Office of Contract Administration (OCA) is also part of the Office of Finance - Sponsored Programs.
Ethics & Compliance
- eResearch IRB NextGen Project
- Class Assignments & IRB Approval
- Operations Manual (OM)
- Authorization Agreement Process
- ORCR Policies and Procedures
- Self-Assessment Tools
- Resources and Web Links
- Single IRB-of-Record (sIRB) Process
- Certificate of Confidentiality Process
- HRPP Education Resources
- How to Register a Clinical Trial
- Maintaining and Updating ClinicalTrial.gov Records
- How to Report Clinical Trial Results
- Research Study Participation - FAQ
- International Research
- Coordinated Services & Practices (CSP)
- Collaborative Research: IRB-HSBS sIRB Process
- Data Security Guidelines
- Research Incentive Guidelines
- Routine fMRI Study Guidelines
- IRB-HSBS Website Directory and Guidance
- Waivers of Informed Consent Guidelines
- IRB Review Process
- IRB Amendment Process
- Continuing Review Process
- Incident Reporting (AE/ORIO)
- IRB Repository Application
- IRB-HSBS Education
- Newsletter Archive
You are here
- Human Subjects
- IRB Health Sciences and Behavioral Sciences (HSBS)
Informed Consent Guidelines & Templates
U-m hrpp informed consent information.
See the HRPP Operations Manual, Part 3, Section III, 6 e .
The human subjects in your project must participate willingly , having been adequately informed about the research.
- If the human subjects are part of a vulnerable population (e.g., prisoners, cognitively impaired individuals, or children), special protections are required.
- If the human subjects are children , in most cases you must first obtain the permission of parents in addition to the consent of the children.
Contact the IRB Office for more information .
See the Waiver Guidelines for information about, and policies regarding, waivers for informed consent or informed consent documentation.

See the updated Basic Informed Consent Elements document for a list of 2018 Common Rule basic and additional elements.
Informed Consent Process
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent document containing the required information (i.e., elements of informed consent) and the presentation of that information to prospective participants.
In most cases, investigators are expected to obtain a signature from the participant on a written informed consent document (i.e., to document the consent to participate) unless the IRB has waived the consent requirement or documentation (signature) requirement .
- Projects which collect biospecimens for genetic analysis must obtain documented (signed) informed consent.
- It is an ethical best practice to include an informed consent process for most exempt research . IRB-HSBS reviews, as applicable, the IRB application for exempt research, but not the informed consent document itself. A suggested consent template for exempt research can be found below under the References and Resources section. A companion protocol template for exempt research may be found in the feature box, Related Information (top right).
Informed consent documents
An informed consent document is typically used to provide subjects with the information they need to make a decision to volunteer for a research study. Federal regulations ( 45 CFR 46.116 ) provide the framework for the type of information (i.e., the "elements") that must be included as part of the consent process. New with the revised 2018 Common Rule is the requirement that the consent document begin with a "concise and focused" presentation of key information that will help potential participants understand why they might or might not want to be a part of a research study.
Key Information Elements
The image below displays the five elements identified in the preamble to the revised Final Rule as suggested key information.
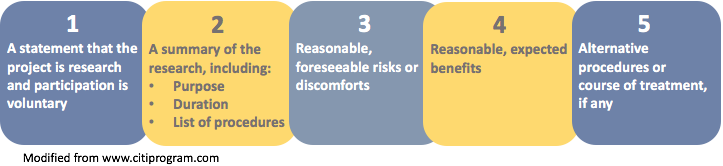
Note: Element number 5 (alternative procedures) applies primarily to clinical research.
General Information & Tips for Preparing a Consent Document
Reading level.
Informed consent documents should be written in plain language at a level appropriate to the subject population, generally at an 8th grade reading level . A best practice is to have a colleague or friend read the informed consent document for comprehension before submission with the IRB application. Always:
For guidance on using plain language, examples, and more, visit: http://www.plainlanguage.gov/
- Tailor the document to the subject population.
- Avoid technical jargon or overly complex terms.
- Use straightforward language that is understandable.
Writing tips
The informed consent document should succinctly describe the research as it has been presented in the IRB application.
- Use the second (you) or third person (he/she) to present the study details. Avoid use of the first person (I).
- Include a statement of agreement at the conclusion of the informed consent document.
- The consent doucment must be consistent with what is described in the IRB application.
Document Formating for Uploading into eResearch
- Remove "track changes" or inserted comments from the consent documentation prior to uploading the document into the IRB application (Section 10-1) for review.
- Use a consistent, clearly identified file naming convention for multiple consent/assent documents.
Informed Consent Templates
IRB-HSBS strongly recommends that investigators use one of the informed consent templates developed to include the required consent elements (per 45 CFR 46.116 ), as well as other required regulatory and institutional language. The templates listed below include the new consent elements outlined in the 2018 Common Rule.
References and Resources
PDF. Lists the basic and additional elements required for inclusion or to be included, as appropriate to the research, in the informed consent documentation, along with the citiation number [e.g., _0116(b)(1)] within the revised Common Rule. New elements associated with the 2018 Common Rule are indicated in bold text.
Strongly recommended for studies that involve the collection of biospecimens and/or genetic or genomic analysis, particularly federally sponsored clinical trials that are required to post a consent document on a public website. Last updated: 04/10/2024.
Informed Consent documents are not reviewed by the IRB for Exempt projects. However, researchers are ethically bound to conduct a consent process with subjects. This template is suggested for use with Exempt projects. Last updated 4/17/24
(Word) Blank template with 2018 revised Common Rule key information and other required informed consent elements represented as section headers; includes instructions and recommended language. It is strongly advised that you modify this template to draft a project-specific informed consent document for your study for IRB review and approval. Last updated: 04/10/2024
(Word) General outline to create and post a flyer seeking participation in a human subjects study. Includes instructions.
(Word) Two sample letters for site approval cooperation between U-M and other institutions, organizations, etc. Letters of cooperation must be on U-M letterhead and signed by an appropriate official. These letters are uploaded into the Performance Site section of the eResearch IRB application.
For use by U-M Dearborn faculty, staff, and students conducting non-exempt human subjects research using subject pools. Last updated 4/10/24
For use by U-M Dearborn faculty, staff, and students conducting exempt human subjects research using subject pools
Researchers who will conduct data collection that is subject to the General Data Protection Regulation (GDPR) must use this template in tandem with a general consent for participation template/document.
- Child assent ages 3-6
- Child assent 7-11
- Parent permission
- Brief protocol for exempt research including data management and security questionnaire
- Child assent 12-14
- Introductory psychology subject pool general consent template
- Introductory psychology subject pool exempt consent template
IRB-Health Sciences and Behavioral Sciences (IRB-HSBS)
Phone: (734) 936-0933 Fax: (734) 936-1852 [email protected]
- Human Subjects Protections

Sample Consent Forms
Consent form templates.
These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find instructions here .
Summary of Changes to the Regulations for Informed Consent: Revised Common Rule Changes to Informed Consent and Waiver Requirements
Summary of Changes to Consent Documents:
- Informed Consent Documents – Version 2.0 Summary of Changes
- Informed Consent Documents – Version 2.1 Summary of Changes
- Informed Consent Documents – 10/26/2020 Summary of Changes
- Informed Consent Documents – 4/10/2023 Summary of Changes
| 2023-07-14 | |
| 2020-01-17 | |
| 2020-01-17 | |
| 2020-01-17 | |
| 2023-04-10 | |
| 2023-06-27 | |
| 2023-04-10 | |
| The following documents are samples. IRBIS does NOT generate these documents with application-specific information. | |
| 2017-10-30 | |
| 2024-08-09 | |
| 2017-04-17 | |
| 2018-04-19 | |
Concise Summary examples can be found here .
Guidance on the use of plain language in consent forms:
- Clinical Research Glossary
- Webinar: The Promise of Plain Language: Launching a Glossary to Support Participant Understanding of Clinical Research – Recording & Slides
There are a few additional forms that are not provided online and may be accessed below. As needed, these should be completed and uploaded to your IRB application.
Foreign Language Consent Forms
COVID-19 Related Forms:
- Spanish-IRB-COVID Information Sheet
- Spanish COVID Consent Letter v2
- Spanish COVID Informational Sheet Translation Certificate
Informed Consent Short Form (for a single subject who may be illiterate, or otherwise unable to read the consent form — used when full consent form has to be read or translated for subject).
- Informed Consent Short Form Guidance
- Simplified Chinese
HIPAA Templates
- Sample HIPAA Authorization Template
- Sample HIPAA Authorization Template in Spanish ( Certification )
Have a language expert improve your writing
Run a free plagiarism check in 10 minutes, generate accurate citations for free.
- Knowledge Base
- Academic writing
- How to write a lab report
How To Write A Lab Report | Step-by-Step Guide & Examples
Published on May 20, 2021 by Pritha Bhandari . Revised on July 23, 2023.
A lab report conveys the aim, methods, results, and conclusions of a scientific experiment. The main purpose of a lab report is to demonstrate your understanding of the scientific method by performing and evaluating a hands-on lab experiment. This type of assignment is usually shorter than a research paper .
Lab reports are commonly used in science, technology, engineering, and mathematics (STEM) fields. This article focuses on how to structure and write a lab report.
Instantly correct all language mistakes in your text
Upload your document to correct all your mistakes in minutes

Table of contents
Structuring a lab report, introduction, other interesting articles, frequently asked questions about lab reports.
The sections of a lab report can vary between scientific fields and course requirements, but they usually contain the purpose, methods, and findings of a lab experiment .
Each section of a lab report has its own purpose.
- Title: expresses the topic of your study
- Abstract : summarizes your research aims, methods, results, and conclusions
- Introduction: establishes the context needed to understand the topic
- Method: describes the materials and procedures used in the experiment
- Results: reports all descriptive and inferential statistical analyses
- Discussion: interprets and evaluates results and identifies limitations
- Conclusion: sums up the main findings of your experiment
- References: list of all sources cited using a specific style (e.g. APA )
- Appendices : contains lengthy materials, procedures, tables or figures
Although most lab reports contain these sections, some sections can be omitted or combined with others. For example, some lab reports contain a brief section on research aims instead of an introduction, and a separate conclusion is not always required.
If you’re not sure, it’s best to check your lab report requirements with your instructor.
Receive feedback on language, structure, and formatting
Professional editors proofread and edit your paper by focusing on:
- Academic style
- Vague sentences
- Style consistency
See an example

Your title provides the first impression of your lab report – effective titles communicate the topic and/or the findings of your study in specific terms.
Create a title that directly conveys the main focus or purpose of your study. It doesn’t need to be creative or thought-provoking, but it should be informative.
- The effects of varying nitrogen levels on tomato plant height.
- Testing the universality of the McGurk effect.
- Comparing the viscosity of common liquids found in kitchens.
An abstract condenses a lab report into a brief overview of about 150–300 words. It should provide readers with a compact version of the research aims, the methods and materials used, the main results, and the final conclusion.
Think of it as a way of giving readers a preview of your full lab report. Write the abstract last, in the past tense, after you’ve drafted all the other sections of your report, so you’ll be able to succinctly summarize each section.
To write a lab report abstract, use these guiding questions:
- What is the wider context of your study?
- What research question were you trying to answer?
- How did you perform the experiment?
- What did your results show?
- How did you interpret your results?
- What is the importance of your findings?
Nitrogen is a necessary nutrient for high quality plants. Tomatoes, one of the most consumed fruits worldwide, rely on nitrogen for healthy leaves and stems to grow fruit. This experiment tested whether nitrogen levels affected tomato plant height in a controlled setting. It was expected that higher levels of nitrogen fertilizer would yield taller tomato plants.
Levels of nitrogen fertilizer were varied between three groups of tomato plants. The control group did not receive any nitrogen fertilizer, while one experimental group received low levels of nitrogen fertilizer, and a second experimental group received high levels of nitrogen fertilizer. All plants were grown from seeds, and heights were measured 50 days into the experiment.
The effects of nitrogen levels on plant height were tested between groups using an ANOVA. The plants with the highest level of nitrogen fertilizer were the tallest, while the plants with low levels of nitrogen exceeded the control group plants in height. In line with expectations and previous findings, the effects of nitrogen levels on plant height were statistically significant. This study strengthens the importance of nitrogen for tomato plants.
Your lab report introduction should set the scene for your experiment. One way to write your introduction is with a funnel (an inverted triangle) structure:
- Start with the broad, general research topic
- Narrow your topic down your specific study focus
- End with a clear research question
Begin by providing background information on your research topic and explaining why it’s important in a broad real-world or theoretical context. Describe relevant previous research on your topic and note how your study may confirm it or expand it, or fill a gap in the research field.
This lab experiment builds on previous research from Haque, Paul, and Sarker (2011), who demonstrated that tomato plant yield increased at higher levels of nitrogen. However, the present research focuses on plant height as a growth indicator and uses a lab-controlled setting instead.
Next, go into detail on the theoretical basis for your study and describe any directly relevant laws or equations that you’ll be using. State your main research aims and expectations by outlining your hypotheses .
Based on the importance of nitrogen for tomato plants, the primary hypothesis was that the plants with the high levels of nitrogen would grow the tallest. The secondary hypothesis was that plants with low levels of nitrogen would grow taller than plants with no nitrogen.
Your introduction doesn’t need to be long, but you may need to organize it into a few paragraphs or with subheadings such as “Research Context” or “Research Aims.”
A lab report Method section details the steps you took to gather and analyze data. Give enough detail so that others can follow or evaluate your procedures. Write this section in the past tense. If you need to include any long lists of procedural steps or materials, place them in the Appendices section but refer to them in the text here.
You should describe your experimental design, your subjects, materials, and specific procedures used for data collection and analysis.
Experimental design
Briefly note whether your experiment is a within-subjects or between-subjects design, and describe how your sample units were assigned to conditions if relevant.
A between-subjects design with three groups of tomato plants was used. The control group did not receive any nitrogen fertilizer. The first experimental group received a low level of nitrogen fertilizer, while the second experimental group received a high level of nitrogen fertilizer.
Describe human subjects in terms of demographic characteristics, and animal or plant subjects in terms of genetic background. Note the total number of subjects as well as the number of subjects per condition or per group. You should also state how you recruited subjects for your study.
List the equipment or materials you used to gather data and state the model names for any specialized equipment.
List of materials
35 Tomato seeds
15 plant pots (15 cm tall)
Light lamps (50,000 lux)
Nitrogen fertilizer
Measuring tape
Describe your experimental settings and conditions in detail. You can provide labelled diagrams or images of the exact set-up necessary for experimental equipment. State how extraneous variables were controlled through restriction or by fixing them at a certain level (e.g., keeping the lab at room temperature).
Light levels were fixed throughout the experiment, and the plants were exposed to 12 hours of light a day. Temperature was restricted to between 23 and 25℃. The pH and carbon levels of the soil were also held constant throughout the experiment as these variables could influence plant height. The plants were grown in rooms free of insects or other pests, and they were spaced out adequately.
Your experimental procedure should describe the exact steps you took to gather data in chronological order. You’ll need to provide enough information so that someone else can replicate your procedure, but you should also be concise. Place detailed information in the appendices where appropriate.
In a lab experiment, you’ll often closely follow a lab manual to gather data. Some instructors will allow you to simply reference the manual and state whether you changed any steps based on practical considerations. Other instructors may want you to rewrite the lab manual procedures as complete sentences in coherent paragraphs, while noting any changes to the steps that you applied in practice.
If you’re performing extensive data analysis, be sure to state your planned analysis methods as well. This includes the types of tests you’ll perform and any programs or software you’ll use for calculations (if relevant).
First, tomato seeds were sown in wooden flats containing soil about 2 cm below the surface. Each seed was kept 3-5 cm apart. The flats were covered to keep the soil moist until germination. The seedlings were removed and transplanted to pots 8 days later, with a maximum of 2 plants to a pot. Each pot was watered once a day to keep the soil moist.
The nitrogen fertilizer treatment was applied to the plant pots 12 days after transplantation. The control group received no treatment, while the first experimental group received a low concentration, and the second experimental group received a high concentration. There were 5 pots in each group, and each plant pot was labelled to indicate the group the plants belonged to.
50 days after the start of the experiment, plant height was measured for all plants. A measuring tape was used to record the length of the plant from ground level to the top of the tallest leaf.
In your results section, you should report the results of any statistical analysis procedures that you undertook. You should clearly state how the results of statistical tests support or refute your initial hypotheses.
The main results to report include:
- any descriptive statistics
- statistical test results
- the significance of the test results
- estimates of standard error or confidence intervals
The mean heights of the plants in the control group, low nitrogen group, and high nitrogen groups were 20.3, 25.1, and 29.6 cm respectively. A one-way ANOVA was applied to calculate the effect of nitrogen fertilizer level on plant height. The results demonstrated statistically significant ( p = .03) height differences between groups.
Next, post-hoc tests were performed to assess the primary and secondary hypotheses. In support of the primary hypothesis, the high nitrogen group plants were significantly taller than the low nitrogen group and the control group plants. Similarly, the results supported the secondary hypothesis: the low nitrogen plants were taller than the control group plants.
These results can be reported in the text or in tables and figures. Use text for highlighting a few key results, but present large sets of numbers in tables, or show relationships between variables with graphs.
You should also include sample calculations in the Results section for complex experiments. For each sample calculation, provide a brief description of what it does and use clear symbols. Present your raw data in the Appendices section and refer to it to highlight any outliers or trends.
The Discussion section will help demonstrate your understanding of the experimental process and your critical thinking skills.
In this section, you can:
- Interpret your results
- Compare your findings with your expectations
- Identify any sources of experimental error
- Explain any unexpected results
- Suggest possible improvements for further studies
Interpreting your results involves clarifying how your results help you answer your main research question. Report whether your results support your hypotheses.
- Did you measure what you sought out to measure?
- Were your analysis procedures appropriate for this type of data?
Compare your findings with other research and explain any key differences in findings.
- Are your results in line with those from previous studies or your classmates’ results? Why or why not?
An effective Discussion section will also highlight the strengths and limitations of a study.
- Did you have high internal validity or reliability?
- How did you establish these aspects of your study?
When describing limitations, use specific examples. For example, if random error contributed substantially to the measurements in your study, state the particular sources of error (e.g., imprecise apparatus) and explain ways to improve them.
The results support the hypothesis that nitrogen levels affect plant height, with increasing levels producing taller plants. These statistically significant results are taken together with previous research to support the importance of nitrogen as a nutrient for tomato plant growth.
However, unlike previous studies, this study focused on plant height as an indicator of plant growth in the present experiment. Importantly, plant height may not always reflect plant health or fruit yield, so measuring other indicators would have strengthened the study findings.
Another limitation of the study is the plant height measurement technique, as the measuring tape was not suitable for plants with extreme curvature. Future studies may focus on measuring plant height in different ways.
The main strengths of this study were the controls for extraneous variables, such as pH and carbon levels of the soil. All other factors that could affect plant height were tightly controlled to isolate the effects of nitrogen levels, resulting in high internal validity for this study.
Your conclusion should be the final section of your lab report. Here, you’ll summarize the findings of your experiment, with a brief overview of the strengths and limitations, and implications of your study for further research.
Some lab reports may omit a Conclusion section because it overlaps with the Discussion section, but you should check with your instructor before doing so.
If you want to know more about AI for academic writing, AI tools, or fallacies make sure to check out some of our other articles with explanations and examples or go directly to our tools!
- Ad hominem fallacy
- Post hoc fallacy
- Appeal to authority fallacy
- False cause fallacy
- Sunk cost fallacy
- Deep learning
- Generative AI
- Machine learning
- Reinforcement learning
- Supervised vs. unsupervised learning
(AI) Tools
- Grammar Checker
- Paraphrasing Tool
- Text Summarizer
- AI Detector
- Plagiarism Checker
- Citation Generator
A lab report conveys the aim, methods, results, and conclusions of a scientific experiment . Lab reports are commonly assigned in science, technology, engineering, and mathematics (STEM) fields.
The purpose of a lab report is to demonstrate your understanding of the scientific method with a hands-on lab experiment. Course instructors will often provide you with an experimental design and procedure. Your task is to write up how you actually performed the experiment and evaluate the outcome.
In contrast, a research paper requires you to independently develop an original argument. It involves more in-depth research and interpretation of sources and data.
A lab report is usually shorter than a research paper.
The sections of a lab report can vary between scientific fields and course requirements, but it usually contains the following:
- Abstract: summarizes your research aims, methods, results, and conclusions
- References: list of all sources cited using a specific style (e.g. APA)
- Appendices: contains lengthy materials, procedures, tables or figures
The results chapter or section simply and objectively reports what you found, without speculating on why you found these results. The discussion interprets the meaning of the results, puts them in context, and explains why they matter.
In qualitative research , results and discussion are sometimes combined. But in quantitative research , it’s considered important to separate the objective results from your interpretation of them.
Cite this Scribbr article
If you want to cite this source, you can copy and paste the citation or click the “Cite this Scribbr article” button to automatically add the citation to our free Citation Generator.
Bhandari, P. (2023, July 23). How To Write A Lab Report | Step-by-Step Guide & Examples. Scribbr. Retrieved September 16, 2024, from https://www.scribbr.com/academic-writing/lab-report/
Is this article helpful?

Pritha Bhandari
Other students also liked, guide to experimental design | overview, steps, & examples, how to write an apa methods section, how to write an apa results section, get unlimited documents corrected.
✔ Free APA citation check included ✔ Unlimited document corrections ✔ Specialized in correcting academic texts

Letter Writing Best Practices
Example letters.
These examples reflect the wide range of formats, reading/writing levels, topics, and interest topics and levels in science. In sharing these letters, we want to give you a general sense of what you can expect from your pre-scientist. But we also hope you notice how different these letters are, despite all students being in middle school with approximately equal time to write. Use your pre-scientist’s letter to gauge their level and try to match their level in your response.
As you browse the letters, consider how you might push those clearly interested in science to start delving deeper into their career search, and connect with and encourage those without much science content in their letter to explore STEM. Seek connections you could make with these students to help you practice for a pen pal of your own.
Additionally, check out our Awesome Letter blog series featuring real letters from STEM professionals that have been annotated by LPS Teachers.
- – Awesome Letter 1: Formatting & kid-friendly language
- – Awesome Letter 2: Enthusiastic writing
- – Awesome Letter 3: Finding ways to connect to your pre-scientist
- – Awesome Letter 4: Pre-scientists love visuals
- – Awesome Letter 5: Bringing closure to your year-long correspondence
Example Letters from Students

Example Letters From STEM Professionals
Intro Scientist Letter 1
Intro Scientist Letter 2
College Scientist Letter 1
College Scientist Letter 2
College Scientist Letter 3
Overcoming Obstacles Scientist Letter 1
Overcoming Obstacles Scientist Letter 2
Overcoming Obstacles Scientist Letter 3
Final Scientist Letter 1
Final Scientist Letter 2
We think these letters are great because they:
- – Are written in student-friendly language.
- – Are well organized with paragraph breaks to separate different topics.
- – Are written neatly or typed in font size of at least 12.
- – Contain use science words with explanations (which is important for vocabulary building).
- – Include visuals (with captions – to explain science concepts, or show different parts of the scientists life).
- – Ask and answer questions from the pre-scientist.
- – Talk about science and non-science topics.
- – Attempt to make connections with the student based on what they know about them.
- – Are excited to write and learn about their pre-scientist.
By no means do we think these are the only great ways to write a letter to your pen pal! Please use your imagination when crafting your letter – the fact that all letters are unique is a big reason this program is special. It’s important to note that these letters are responses to letters STEM professionals received from their pre-scientists. Please attempt to adjust your content and writing level to match the writing sample you receive from your individual student. It’s best to keep most letters to a three page maximum.
Letter Enhancement Ideas
You are welcome to send additional goodies with your letter, but it is not expected or required . If you want to include anything beyond these small items (e.g., books, puzzles, or activity kits), please consider sending it to the teacher so the whole class (and more!) can enjoy. This helps to create a more equitable letter opening experience for our pre-scientists!
Furthermore, this creates a more equitable experience for STEM pen pals as well. STEM pen pals range from undergraduate students to senior scientists; some pen pals do not have the resources or access to opportunities to give gifts/swag to their pre-scientists and this may leave STEM pen pals feeling guilty. The most significant aspect of our program is the time STEM pen pals dedicate to crafting personalized, thoughtful letters to students and we wish to preserve this as our program’s main focus.

- – Stickers
- – Pictures of you and your family, pets, workplace, experiments, travels, etc.e
- – College brochures/pamphlets
- – Postcards
- – Free swag from meetings and conferences
- – Science coloring page
- – Your own drawings/comic strips/diagrams
- – Directions for home science experiments
- – Links for websites and cool science videos and experiments
- – Current science articles related to your field/student interests
- – Pencils/pens
- – College pennants
- – Field work samples/equipment (rocks, fossils, magnifying glass, microscope slides)
- – Science coloring book
Engaging Students Who Say They Don’t Like Science
At LPS we use a no-opt out model , which means we work with all students in a teacher’s class . We believe many of the students who say they don’t like science, and would opt-out, actually just don’t yet know all that science is. This model presents an exciting but potentially frustrating challenge for the STEM pen pals who may get a letter from a student that is short, unrelated to science, or explicitly negative about science. You can find out how excited your student is about science from the initial shared matching information, which will include the science topics your student picked, and a rating of 1-5 about how excited they are about those topics. If you get matched with a student who selected a 1, we hope you will focus on the opportunity you have to greatly broaden a student’s worldview.
Please know that students do receive your letters . Even if they’re “too cool” to respond to your questions during round one, they did read it, and they do appreciate your time. Being consistent to show your student you’re not giving up on them, and trying new topics using different formats are two great ways to hook a more hesitant student. Here are some other ideas:
- – Suggest activities students could do at home to explore science . All students like cool science demonstrations like a baking soda/vinegar volcano eruption! You could send a video link about this experiment along with directions for a simple project they likely already have the materials at home to create.
Input your search keywords and press Enter.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
The PMC website is updating on October 15, 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Breathe (Sheff)
- v.14(2); 2018 Jun

How to obtain informed consent for research
1 University of Messina, “G. Martino” Hospital, Messina, Italy
Amelia Licari
2 University of Pavia, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy
Current biomedical research on human subjects requires clinical trial, which is defined as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions [ i.e. drugs, cells or other biological products, surgical procedures, devices] to evaluate the effects on health outcomes” [1]. In our modern ethical conception, all research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent, which is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate”, as stated in the International Council for Harmonisation Good Clinical Practice guidelines [2]. Informed consent is documented by means of a written, signed and dated informed consent form. This form is required in the following cases: 1) when the research involves patients, children, incompetent/incapacitated persons, healthy volunteers, immigrants or others ( e.g. prisoners); 2) when the research uses/collects human genetic material, biological samples or personal data [3].
Short abstract
The process of obtaining informed consent for clinical trials is tightly regulated; complications arise in circumstances when consent may be waived, or when needed from vulnerable populations http://ow.ly/rEMe30j5MVq
Current biomedical research on human subjects requires clinical trial, which is defined as “any research study that prospectively assigns human participants or groups of humans to one or more health-related interventions [ i.e. drugs, cells or other biological products, surgical procedures, devices] to evaluate the effects on health outcomes” [ 1 ]. In our modern ethical conception, all research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent, which is a process by which “a subject voluntarily confirms his or her willingness to participate in a particular trial, after having been informed of all aspects of the trial that are relevant to the subject’s decision to participate”, as stated in the International Council for Harmonisation Good Clinical Practice guidelines [ 2 ]. Informed consent is documented by means of a written, signed and dated informed consent form. This form is required in the following cases: 1) when the research involves patients, children, incompetent/incapacitated persons, healthy volunteers, immigrants or others ( e.g. prisoners); 2) when the research uses/collects human genetic material, biological samples or personal data [ 3 ].
The informed consent form must be written in language easily understood by the subjects, it must minimise the possibility of coercion or undue influence, and the subject must be given sufficient time to consider participation. However, informed consent is not merely a form that is signed, but is a process in which the subject has an understanding of the research and its risks, and it is tightly described in ethical codes and regulations for human subject research [ 2 ].
Educational aims
- To provide a comprehensive overview of issues in obtaining informed consent in clinical research.
- To describe the process of obtaining informed consent in clinical trials.
- To highlight the circumstances under which informed consent can be waived.
- To review the setting of obtaining informed consent from “vulnerable populations”.
The informed consent process
The voluntary expression of the consent by a competent subject and the adequate information disclosure about the research are critical and essential elements of the informed consent process [ 4 ]. Competent subjects able to comprehend the research-related information should personally decide and provide the consent on research participation. Conditions posing practical challenges in obtaining informed consent from the real subject may include situations of medical emergency or obtaining consent from “vulnerable” subjects and/or children [ 5 ].
Research-related information must be presented to enable people to voluntarily decide whether or not to participate as a research subject. For an ethically valid consent, information provided to a research subject should include, but not be limited to: information about the health condition for which the research is proposed; details of the nature and purpose of the research; the expected duration of the subject’s participation; a detailed description of study treatment or intervention and of any experimental procedures (including, in the case of randomised clinical trials (RCTs), also blinding and randomisation); a statement that participation in research is voluntary; probable risks and benefits associated with research participation; details of the nature of the illness and possible outcome if the condition is left untreated; availability, risks and benefits of alternative treatments; information about procedures adopted for ensuring data protection/confidentiality/privacy, including duration of storage of personal data; details about the handling of any incidental findings of the research; description of any planned genetic tests; details of insurance coverage in case of injury; reference contacts for any further answers to pertinent questions about the research and the subject’s rights and in case of any research-related injury to the subject; and any other information that seems necessary for an informed decision to be taken by the subject. Of particular importance, a statement offering the subject the opportunity to withdraw at any time from the research without consequences must be provided during the information disclosure [ 2 ]. Specific information should be provided in case of research projects involving children, incapacitated adults not able to give informed consent, illiterate populations, etc. (as will be described later in this article).
The information about the research should be given by a physician or by other individuals ( i.e. researchers) with appropriate scientific training and qualifications [ 6 ]. Furthermore, the location where the informed consent is being discussed, and the subject’s physical, emotional and psychological capability, must be taken into consideration when taking consent from a human subject.
Informed consent: when is it not necessary?
After institutional review board (IRB) or independent ethics committee approval is achieved, obtaining informed consent from each human subject prior to his/her participation in clinical trial is mandatory [ 5 ]. However, when specific circumstances occur, the informed consent can be waived, and “research without consent” is possible, which allows enrolment of patients without their consent, under strict regulation [ 7 ]. In order that research without consent is considered justifiable, the following three conditions have to be met: 1) it is impracticable to obtain consent, 2) the research does not infringe the principle of self-determination, and 3) the research provides significant clinical relevance [ 8 ].
The first condition, of “impracticability”, occurs when obtaining informed consent is burdened by high impact in terms of time and economic resources or could compromise the study’s validity [ 8 ]. The second condition means that, although physicians are requested to ensure that the patient has understood the aim of the research and the risks and/or benefits associated with study participation, the researchers are also advised to respect the patient’s decision-making capacity, not interfering with his/her decisions and acting always in the patient’s best interest [ 9 ]. The third condition leads to justification of waiving consent when the clinical relevance and public health importance are potentially high [ 8 ].
The formal literature identifies different types of RCTs and classifies them into three macro-areas: 1) RCTs based on infeasibility of informed consent; 2) RCTs that omit informed consent only for control groups; and 3) RCTs that omit informed consent entirely.
RCTs based on infeasibility of informed consent
Emergency clinical studies, involving critically ill subjects, represent an exception to the requirement of informed consent. The investigated life-saving therapy and the medical intervention may be required immediately, not permitting the researchers to wait and respect all procedures of obtaining informed consent. Within this context, the researchers will be able to proceed with patient recruitment, also without the subject’s consent to treatment, when, prior to the study, the IRB has ascertained the presence of mandatory conditions ( table 1 ) [ 10 ].
Table 1
Conditions to be met in emergency clinical study
| • Subjects affected by a life-threatening condition |
| • The treatment is experimental |
| • The clinical research allows verification of both the effectiveness and safety of the treatment |
| • It is impracticable to obtain consent |
| • The waiver of informed consent is needed for the clinical trial |
| • The researcher will contact the legally authorised representative |
| • The family members can decline the patient’s participation in the study |
Cluster randomised studies include cluster-cluster and individual-cluster research [ 11 ]. In cluster-cluster designs ( e.g. studies on infectious disease prevention), the intervention involves the entire target community, so that single subjects cannot refuse it [ 12 ]. Conversely, in individual-cluster designs ( e.g. studies on primary care), although the intervention involves all the selected community, the right to refuse treatment is allowed. Under this circumstance, the omission of informed consent is justified only when the treatment refusal undermines the validity of the research study and/or procedures [ 13 ].
RCTs that omit informed consent only for control groups
In Zelen’s single-consent model ( e.g. RCTs in infectious or oncological diseases), randomisation occurs prior to any consent, and informed consent is sought only from individuals assigned to experimental treatment [ 14 ]. In the control group, the physicians do not make substantial changes in routine patient care, so informed consent is not required for patient enrolment [ 8 ].
In order to improve study recruitment, Zelen developed the double-consent design. Specifically, informed consent is requested for subjects to be involved in the study but not for the randomisation, preventing psychological distress [ 14 ].
In follow-up studies, the nested consent model ( e.g. for single cohort studies) or cohort multiple RCTs model ( e.g. for multiple cohort studies) is applied. In these variants, patients give their consent for prospective follow-up; however, they remain blinded to any randomised experimental interventions [ 15 ].
In trials using the model of “consent to postponed information”, the informed consent process is carried out after the study is completed [ 16 ].
All these RCT types aim to avoid unnecessary stress in patients who will not receive the new promising experimental treatment. Moreover, these clinical study designs do not affect the standard therapeutic approach or infringe the rights of the patients in the control group; therefore, the clinical trial can proceed without obtaining informed consent [ 8 ].
RCTs that omit informed consent entirely
Based on the fact that patients are assigned to standard care interventions, no informed consent is sought either in low-risk pragmatic RCTs [ 17 ] or in prompted optional randomisation trials [ 18 , 19 ]. However, in a low-risk pragmatic RCT, patients do not have the possibility to choose one of the two standard treatments, whereas in a prompted optional randomisation trial, both the researchers and the enrolled patients can choose one type of treatment over another, despite the randomisation results [ 6 ].
Special needs: vulnerable patients
A “vulnerable population” is defined as a disadvantaged community subgroup unable to make informed choices, protect themselves from inherent or intended risks, or keep their own interests safeguarded [ 20 ]. In the health domain, “vulnerable populations” refers to physical vulnerability ( e.g. pregnant women, fetuses, children, orphans, students, employees, prisoners, the military, and those who are chronically or terminally ill), psychological vulnerability (cognitively and intellectually impaired individuals) and social vulnerability (those who are homeless, from ethnic minorities, are immigrants or refugees) [ 20 ].
Due to a compromised free will and inability to make conscious decisions, several ethical dilemmas (related to communications, privacy and treatment) often arise when research involves these populations. Guaranteeing protection of rights, safety, data privacy and confidentiality of vulnerable subjects are prerogatives of good clinical practice, and law dispositions are regulated and strictly monitored by the applicable authorities [ 21 ].
Physical vulnerability
For a long time, pregnant women were excluded from clinical research because of their “vulnerability”. Although pregnant women are able to make informed and conscious choices, they have been considered “vulnerable” due to the potential risks to the fetus, who is also considered as a “patient” [ 22 ]. More recently, with the consideration of pregnant women as “scientifically complex” rather than “vulnerable” subjects, it has been permitted to involve this category in research trials [ 23 ]. The “scientific complexity” reflects both ethical and physiological complexity. The ethical aspects are secondary to the need to find a balance between interests of the fetus and the mother. The physiological aspects are strictly related to the pregnancy status [ 24 ].
Research studies involving pregnant women and fetuses have to satisfy specific federal regulations ( table 2 ). The following appropriate precautions should be taken in research studies involving pregnant women: no pregnant woman may be involved as a subject in a human clinical research project unless the purpose of the research is to meet the health needs of the mother and the fetus will be placed at risk only to the minimum extent necessary to meet such needs, or the risk to the fetus is minimal [ 25 ].
Table 2
Conditions to be met in research studies involving pregnant women and fetuses
| • studies have also been conducted on pregnant animals |
| • Clinical studies have been conducted on nonpregnant women |
| • Clinical findings assessing potential harms to pregnant women and fetuses are available |
| • The risk to the fetus is minimal and caused exclusively by the procedure/intervention |
| • The study will achieve crucial knowledge not obtainable by any other means |
| • The researchers will have no part in any decision influencing fetal viability or pregnancy |
| • No incentive will be provided to influence the course of pregnancy |
Researchers can enrol pregnant women only when the mother and/or the father are legally competent. In fact, the consent to participate in research may be either self-directed (only the mother’s consent is required) or made with the guidance of the woman’s partner. However, the father’s consent need not be obtained when: 1) the research activity is directed to the health needs of the mother; 2) the father’s identity is doubtful; 3) the father is absent; or 4) a pregnancy from rape has occurred [ 26 ]. The consent signature requirements from the mother and father are summarised in table 3 . Once the informed consent is obtained, the pregnant women will be included into any phase of the study unless the research project will be compromised or the patient’s health (mother and/or fetus) will be in danger.
Table 3
Consent signature requirements for pregnant women and children
| Direct benefit to mother | Mother |
| Direct benefit to mother and fetus | Mother |
| Direct benefit to fetus | Mother and father |
| Direct benefit to individual subjects | One parent or guardian |
| No direct benefit to individual subjects | Both parents |
| No direct benefit to the subject or societal (indirect) benefit | Both parents |
| Medical care related to pregnancy | Parental consent is not needed |
| Medical care related to mental health treatment, or the diagnosis or treatment of infectious, contagious or communicable diseases | Parental consent is not needed |
| Self-sufficient minors | Parental consent is not needed |
| Aged ≥15 years | |
| Living alone | |
| Managing their own financial affairs | |
| Emancipated minors | Parental consent is not needed |
| Married or divorced | |
| On active duty in the US armed forces | |
| By a court | |
| Having the legal right to consent on their own behalf to medical, dental or mental health treatment |
# : consent requirements are the same whether the risk is “no more than minimal” or “more than minimal”.
Medical students and employees, who take part in numerous aspects of patient care in primary, secondary and tertiary care settings, are often invited to participate in human studies as volunteers. Frequently, the requesting researcher is their supervisor or instructor, who may push them to participate in the study, which can negatively influence their decision and also violate the consent legitimacy. Therefore, in order to protect these subjects against “coercion” or “undue influence”, when an investigator wishes to recruit medical students or employees, they must first obtain IRB approval for inclusion in the study of these vulnerable subgroups [ 27 ].
Prisoners, defined as any individual involuntarily confined or detained in a penal institution, are considered as “vulnerable” because they may be coerced into study participation, and also, due to both cognitive and psychiatric disorders, they can show an impaired ability to provide voluntary informed consent [ 28 ]. To protect this population, the Office for Human Research Protections has stipulated federal regulations according to which the only studies that may involve prisoners are those with independent and valid reasons for involving them ( table 4 ) [ 25 ].
Table 4
Studies that may involve prisoners
| • Studies on the possible causes, processes and effects of incarceration |
| • Studies on prisons as institutional structures or on prisoners as incarcerated persons |
| • Studies on special conditions affecting prisoners |
| • Studies on practices of improving the health or well-being of the prisoners |
| • Epidemiological studies |
Due to the context of war in which they work, as well as the critical care setting in which they are treated, military subjects often receive medical care and/or participate in biomedical research under an “implied consent” condition. Moreover, the superior–subordinate relationship contributes to favour coercion or undue influence, making this population vulnerable [ 29 ]. To curb this phenomenon and to ensure that participation is truly voluntary, the US Dept of Defense agencies have adopted requirements similar to those that govern medical research that applies to the civilian population. Accordingly, the medical research recruitment session happens in the absence of superiors, and the informed consent is obtained prior to participating in a medical research study. The presence of an ombudsman guarantees and verifies that the participation is voluntary and that the information provided during recruitment is complete, accurate and clear. A payment as an incentive is acceptable but it must not be used to legitimise a coercive interference. Additional protection is provided to students at service academies, especially those aged <18 years. However, when emergency research is conducted or the research study advances the development of a medical product needed by the armed forces, informed consent will not be required [ 29 ].
Psychological vulnerability
Mental disability may compromise the self-determination and decision-making capacities [ 30 ]. Researchers interested in enrolling individuals with cognitive disorders are invited to apply different strategies to promote a better understanding of information-gathering processes. Simplifying the questions and content, adopting supportive technologies, using a more simple language, and spending more time for the information process have been suggested as useful and valid measures. When all these strategies prove to be insufficient, the investigators are required to obtain consent from a legally authorised representative [ 30 ].
Social vulnerability
Similarly to other vulnerable populations, research involving the homeless, ethnic minorities, immigrants and refugees is regulated by laws and specific procedures. Cultural and language differences, “undocumented” migrant status, and the precarious legal positions of these subjects raise several ethical issues, such as whether the participation is truly voluntary, or there are unrealistic expectations, or any benefits for their “status”.
Obtaining informed consent in these groups is extremely complex. A friendly procedure has been identified as the best way to adequately involve these vulnerable groups. A health centre or community building could represent an accessible location. The reimbursement of travel expenses for applicants can be a valid solution to obtain a representative sample for the clinical research. Clear and simple language, emphasising confidentiality, with the help of professional interpreters, can tempt migrants to sign the consent form. Lastly, the possibility of receiving something back in return for their contribution may enable successful enrolment of migrants in research [ 31 ].
Special needs: children
Because of their young age as well as their limited emotional and intellectual abilities, children are considered to be legally incompetent to give valid informed consent; thus, to enrol a child in a research study, the permission by at least one parent or legal representative is mandatory ( table 3 ). For subjects aged <18 years, biological or adoptive parents or legal guardians (persons having both legal capacity and responsibility) can give consent on behalf of their child, exercising free power of choice without any form of coercion. While married mothers and fathers both have parental responsibility, unmarried parents can exert parental responsibility only if they are named individually on the child’s birth certificate. Also, divorced parents maintain parental responsibility, but it is necessary to know to whom the child’s custody has been assigned [ 32 ]. However, on this matter, the European laws and regulations are not harmonised and several discrepancies are present in each country [ 33 ].
Despite potential benefits for the research subjects, the failure of parents to give consent (or their refusal to give consent) is not a rare circumstance [ 34 ]. It can be the case that researchers are dealing with underage parents, so that, although underage parents are responsible for representing their children, as minors themselves they are not considered to be sufficiently mature; therefore, they will be not able to give valid consent. Literacy and socioeconomic levels have been identified as the most common reasons for parental non-response [ 34 ]. Clarity and adequate explanation of research information materials should be part of effective planning to overcome language and social barriers.
In clinical studies in which the adopted methodology constitutes “less than minimal risks” for children, passive parental consent represents a possible way to more easily obtain informed parental consent [ 34 ]. Furthermore, parents can be informed with regard to a possible study involving their children, and, at the time of data collection, only the child’s assent is required. In fact, although the child’s decision-making capacity and understanding of the research project in which he/she will be involved may be limited, the Medical Research Council have shown that, when study details are provided and communicated in a clear and adequate manner, the child can be able to reach a decision and participate consciously in the research [ 35 ]. “Assent” is the term coined to express the child’s willingness to participate in clinical trials despite their young age. The “assent” should include and respect the following key points: 1) helping the child to acquire disease awareness; 2) explaining the potential impact of the experimental treatment; 3) evaluating the child’s ability to understand and adapt to new situations or challenges; and 4) positively influencing the patient’s willingness to participate in clinical trials [ 36 ]. Although the “assent” is not mandatory for research offering a direct benefit for the child, it arises from the need to respect paediatric research subjects [ 37 ]. The evaluation of the capacity to provide the “assent” is based on developmental stage, intellectual abilities and life or disease experience. Usually, the cut-off age of 7 years is used for the beginning of logical thought processes and rational decision making [ 38 ]. However, “assent” for children aged <7 years can be also required once the ability to read and write has been verified [ 32 ]. Figures 1 and and2 2 summarise the parental and assent permission requirements, respectively.

Flow chart of parental permission requirements.

Flow chart of child assent requirements.
When conducting clinical research, the obtaining of informed consent is required. Informed consent is a procedure through which a competent subject, after having received and understood all the research-related information, can voluntarily provide his or her willingness to participate in a clinical trial. However, when it is impracticable to obtain consent, and the research does not infringe the principle of self-determination and also provides significant clinical relevance, the researcher is legally authorised to proceed without informed consent. Furthermore, in order to preserve the self-determination and decision-making rights, specific law dispositions are applied when vulnerable populations are enrolled in clinical trials.
Self-evaluation questions
- a) Diagnosis
- b) Risks and benefits of treatment
- c) Alternatives to treatment
- d) Family’s wishes
- a) When a minor is considered as emancipated
- b) When a patient is found to be incompetent
- c) When immediate treatment is necessary to prevent death or permanent impairment
- d) When the subject is aged >18 years
- a) Minor is married or divorced
- b) Minor on active duty in the US armed forces
- c) Minor is considered self-sufficient by a court
- d) Minor having a son
Suggested answers
- All research conducted on humans must be pre-emptively accepted by the subjects themselves through the procedure known as informed consent.
- Voluntary expression of consent and adequate information disclosure about the research are critical and essential elements of the informed consent process.
- When specific circumstances occur, informed consent can be waived: if it is impracticable to obtain consent, if the research does not infringe the principle of self-determination, and if the research provides significant clinical relevance.
- Participation of vulnerable patients in clinical trials is regulated by specific law dispositions.
Conflict of interest: None declared.
How to Write a Lab Report
Lab Reports Describe Your Experiment
- Chemical Laws
- Periodic Table
- Projects & Experiments
- Scientific Method
- Biochemistry
- Physical Chemistry
- Medical Chemistry
- Chemistry In Everyday Life
- Famous Chemists
- Activities for Kids
- Abbreviations & Acronyms
- Weather & Climate
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Lab reports are an essential part of all laboratory courses and usually a significant part of your grade. If your instructor gives you an outline for how to write a lab report, use that. Some instructors require a lab report to be included in a lab notebook , while others will request a separate report. Here's how to write a lab report you can use if you aren't sure what to write or need an explanation of what to include in the different parts of the report.
A lab report is how you explain what you did in your experiment, what you learned, and what the results meant.
Lab Report Essentials
Not all lab reports have title pages, but if your instructor wants one, it would be a single page that states:
- The title of the experiment.
- Your name and the names of any lab partners.
- Your instructor's name.
- The date the experiment was performed or the date the report was submitted.
The title says what you did. It should be brief (aim for ten words or less) and describe the main point of the experiment or investigation. An example of a title would be: "Effects of Ultraviolet Light on Borax Crystal Growth Rate". If you can, begin your title using a keyword rather than an article like "The" or "A".
Introduction or Purpose
Usually, the introduction is one paragraph that explains the objectives or purpose of the lab. In one sentence, state the hypothesis. Sometimes an introduction may contain background information, briefly summarize how the experiment was performed, state the findings of the experiment, and list the conclusions of the investigation. Even if you don't write a whole introduction, you need to state the purpose of the experiment, or why you did it. This would be where you state your hypothesis .
List everything needed to complete your experiment.
Describe the steps you completed during your investigation. This is your procedure. Be sufficiently detailed so that anyone can read this section and duplicate your experiment. Write it as if you were giving directions for someone else to do the lab. It may be helpful to provide a figure to diagram your experimental setup.
Numerical data obtained from your procedure usually presented as a table. Data encompasses what you recorded when you conducted the experiment. It's just the facts, not any interpretation of what they mean.
Describe in words what the data means. Sometimes the Results section is combined with the Discussion.
Discussion or Analysis
The Data section contains numbers; the Analysis section contains any calculations you made based on those numbers. This is where you interpret the data and determine whether or not a hypothesis was accepted. This is also where you would discuss any mistakes you might have made while conducting the investigation. You may wish to describe ways the study might have been improved.
Conclusions
Most of the time the conclusion is a single paragraph that sums up what happened in the experiment, whether your hypothesis was accepted or rejected, and what this means.
Figures and Graphs
Graphs and figures must both be labeled with a descriptive title. Label the axes on a graph, being sure to include units of measurement. The independent variable is on the X-axis, and the dependent variable (the one you are measuring) is on the Y-axis. Be sure to refer to figures and graphs in the text of your report: the first figure is Figure 1, the second figure is Figure 2, etc.
If your research was based on someone else's work or if you cited facts that require documentation, then you should list these references.
- The Most Dangerous Elements on the Periodic Table
- Is It Safe to Reboil Water?
- What Are the Most Deadly Poisons and Chemicals?
- How to Draw a Lewis Structure
- Chemistry Glassware Types, Names and Uses
- Back Titration Definition
- Molarity Definition in Chemistry
- Understanding Endothermic and Exothermic Reactions
- Actual Yield Definition (Chemistry)
- How to Balance Equations - Printable Worksheets
- Chemical Properties of Matter
- General Chemistry Topics
- Ionic vs. Covalent Bonds: How Are They Different?
- What Is Muriatic Acid? Facts and Uses
- Synthesis Reaction Description Plus Examples
- Table of Electrical Resistivity and Conductivity
- Research Process
- Manuscript Preparation
- Manuscript Review
- Publication Process
- Publication Recognition
Language Editing Services
- Translation Services

Writing a Scientific Research Project Proposal
- 5 minute read
- 115.3K views
Table of Contents
The importance of a well-written research proposal cannot be underestimated. Your research really is only as good as your proposal. A poorly written, or poorly conceived research proposal will doom even an otherwise worthy project. On the other hand, a well-written, high-quality proposal will increase your chances for success.
In this article, we’ll outline the basics of writing an effective scientific research proposal, including the differences between research proposals, grants and cover letters. We’ll also touch on common mistakes made when submitting research proposals, as well as a simple example or template that you can follow.
What is a scientific research proposal?
The main purpose of a scientific research proposal is to convince your audience that your project is worthwhile, and that you have the expertise and wherewithal to complete it. The elements of an effective research proposal mirror those of the research process itself, which we’ll outline below. Essentially, the research proposal should include enough information for the reader to determine if your proposed study is worth pursuing.
It is not an uncommon misunderstanding to think that a research proposal and a cover letter are the same things. However, they are different. The main difference between a research proposal vs cover letter content is distinct. Whereas the research proposal summarizes the proposal for future research, the cover letter connects you to the research, and how you are the right person to complete the proposed research.
There is also sometimes confusion around a research proposal vs grant application. Whereas a research proposal is a statement of intent, related to answering a research question, a grant application is a specific request for funding to complete the research proposed. Of course, there are elements of overlap between the two documents; it’s the purpose of the document that defines one or the other.
Scientific Research Proposal Format
Although there is no one way to write a scientific research proposal, there are specific guidelines. A lot depends on which journal you’re submitting your research proposal to, so you may need to follow their scientific research proposal template.
In general, however, there are fairly universal sections to every scientific research proposal. These include:
- Title: Make sure the title of your proposal is descriptive and concise. Make it catch and informative at the same time, avoiding dry phrases like, “An investigation…” Your title should pique the interest of the reader.
- Abstract: This is a brief (300-500 words) summary that includes the research question, your rationale for the study, and any applicable hypothesis. You should also include a brief description of your methodology, including procedures, samples, instruments, etc.
- Introduction: The opening paragraph of your research proposal is, perhaps, the most important. Here you want to introduce the research problem in a creative way, and demonstrate your understanding of the need for the research. You want the reader to think that your proposed research is current, important and relevant.
- Background: Include a brief history of the topic and link it to a contemporary context to show its relevance for today. Identify key researchers and institutions also looking at the problem
- Literature Review: This is the section that may take the longest amount of time to assemble. Here you want to synthesize prior research, and place your proposed research into the larger picture of what’s been studied in the past. You want to show your reader that your work is original, and adds to the current knowledge.
- Research Design and Methodology: This section should be very clearly and logically written and organized. You are letting your reader know that you know what you are going to do, and how. The reader should feel confident that you have the skills and knowledge needed to get the project done.
- Preliminary Implications: Here you’ll be outlining how you anticipate your research will extend current knowledge in your field. You might also want to discuss how your findings will impact future research needs.
- Conclusion: This section reinforces the significance and importance of your proposed research, and summarizes the entire proposal.
- References/Citations: Of course, you need to include a full and accurate list of any and all sources you used to write your research proposal.
Common Mistakes in Writing a Scientific Research Project Proposal
Remember, the best research proposal can be rejected if it’s not well written or is ill-conceived. The most common mistakes made include:
- Not providing the proper context for your research question or the problem
- Failing to reference landmark/key studies
- Losing focus of the research question or problem
- Not accurately presenting contributions by other researchers and institutions
- Incompletely developing a persuasive argument for the research that is being proposed
- Misplaced attention on minor points and/or not enough detail on major issues
- Sloppy, low-quality writing without effective logic and flow
- Incorrect or lapses in references and citations, and/or references not in proper format
- The proposal is too long – or too short
Scientific Research Proposal Example
There are countless examples that you can find for successful research proposals. In addition, you can also find examples of unsuccessful research proposals. Search for successful research proposals in your field, and even for your target journal, to get a good idea on what specifically your audience may be looking for.
While there’s no one example that will show you everything you need to know, looking at a few will give you a good idea of what you need to include in your own research proposal. Talk, also, to colleagues in your field, especially if you are a student or a new researcher. We can often learn from the mistakes of others. The more prepared and knowledgeable you are prior to writing your research proposal, the more likely you are to succeed.
One of the top reasons scientific research proposals are rejected is due to poor logic and flow. Check out our Language Editing Services to ensure a great proposal , that’s clear and concise, and properly referenced. Check our video for more information, and get started today.

Research Fraud: Falsification and Fabrication in Research Data

Research Team Structure
You may also like.

Descriptive Research Design and Its Myriad Uses

Five Common Mistakes to Avoid When Writing a Biomedical Research Paper

Making Technical Writing in Environmental Engineering Accessible

To Err is Not Human: The Dangers of AI-assisted Academic Writing

When Data Speak, Listen: Importance of Data Collection and Analysis Methods

Choosing the Right Research Methodology: A Guide for Researchers

Why is data validation important in research?

Writing a good review article
Input your search keywords and press Enter.
- Chicago Cafe: California’s Oldest Chinese Restaurant
- Surprising Benefits of Using Sheep as Lawn Mowers
- California Families Project: Resilience and Community
This In Focus story is a part of Driven by Curiosity series.
LZ Experiment Sets New Record in Search for Dark Matter
New results leave fewer places for elusive dark matter particles to hide.
- by Lauren Biron
- August 26, 2024

Figuring out the nature of dark matter, the invisible substance that makes up most of the mass in our universe, is one of the greatest puzzles in physics. New results from the world’s most sensitive dark matter detector, LUX-ZEPLIN (LZ), have narrowed down possibilities for one of the leading dark matter candidates: weakly interacting massive particles, or WIMPs.
LZ, led by the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab), hunts for dark matter from a cavern nearly one mile underground at the Sanford Underground Research Facility in South Dakota. Mani Tripathi, Distinguished Professor in the UC Davis Department of Physics and Astronomy, is a member of the LZ project team.
The experiment’s new results explore weaker dark matter interactions than ever searched before and further limit what WIMPs could be.
“These are new world-leading constraints by a sizable margin on dark matter and WIMPs,” said Chamkaur Ghag, spokesperson for LZ and a professor at University College London (UCL). He noted that the detector and analysis techniques are performing even better than the collaboration expected. “If WIMPs had been within the region we searched, we’d have been able to robustly say something about them. We know we have the sensitivity and tools to see whether they’re there as we search lower energies and accrue the bulk of this experiment’s lifetime.”
Fewer places for WIMPs to hide
The collaboration found no evidence of WIMPs above a mass of 9 gigaelectronvolts/c 2 (GeV/c 2 ). (For comparison, the mass of a proton is slightly less than 1 GeV/c 2 .) The experiment's sensitivity to faint interactions helps researchers reject potential WIMP dark matter models that don't fit the data, leaving significantly fewer places for WIMPs to hide. The new results were presented at two physics conferences on August 26: TeV Particle Astrophysics 2024 in Chicago, Illinois, and LIDINE 2024 in São Paulo, Brazil. A scientific paper will be published in the coming weeks.
The results analyze 280 days’ worth of data: a new set of 220 days (collected between March 2023 and April 2024) combined with 60 earlier days from LZ’s first run. The experiment plans to collect 1,000 days’ worth of data before it ends in 2028.
“If you think of the search for dark matter like looking for buried treasure, we’ve dug almost five times deeper than anyone else has in the past,” said Scott Kravitz, LZ’s deputy physics coordinator and a professor at the University of Texas at Austin. “That’s something you don’t do with a million shovels – you do it by inventing a new tool.”
LZ’s sensitivity comes from the myriad ways the detector can reduce backgrounds, the false signals that can impersonate or hide a dark matter interaction. Deep underground, the detector is shielded from cosmic rays coming from space. To reduce natural radiation from everyday objects, LZ was built from thousands of ultraclean, low-radiation parts. The detector is built like an onion, with each layer either blocking outside radiation or tracking particle interactions to rule out dark matter mimics. And sophisticated new analysis techniques help rule out background interactions, particularly those from the most common culprit: radon.
This result is also the first time that LZ has applied “salting”– a technique that adds fake WIMP signals during data collection. By camouflaging the real data until “unsalting” at the very end, researchers can avoid unconscious bias and keep from overly interpreting or changing their analysis.
“We’re pushing the boundary into a regime where people have not looked for dark matter before,” said Scott Haselschwardt, the LZ physics coordinator and a recent Chamberlain Fellow at Berkeley Lab who is now an assistant professor at the University of Michigan. “There’s a human tendency to want to see patterns in data, so it’s really important when you enter this new regime that no bias wanders in. If you make a discovery, you want to get it right.”
The invisible 85 percent
Dark matter, so named because it does not emit, reflect, or absorb light, is estimated to make up 85% of the mass in the universe but has never been directly detected, though it has left its fingerprints on multiple astronomical observations. We wouldn’t exist without this mysterious yet fundamental piece of the universe; dark matter’s mass contributes to the gravitational attraction that helps galaxies form and stay together.
LZ uses 10 tonnes of liquid xenon to provide a dense, transparent material for dark matter particles to potentially bump into. The hope is for a WIMP to knock into a xenon nucleus, causing it to move, much like a hit from a cue ball in a game of pool. By collecting the light and electrons emitted during interactions, LZ captures potential WIMP signals alongside other data.
“We’ve demonstrated how strong we are as a WIMP search machine, and we’re going to keep running and getting even better – but there’s lots of other things we can do with this detector,” said Amy Cottle, lead on the WIMP search effort and an assistant professor at UCL. “The next stage is using these data to look at other interesting and rare physics processes, like rare decays of xenon atoms, neutrinoless double beta decay, boron-8 neutrinos from the sun, and other beyond-the-Standard-Model physics. And this is in addition to probing some of the most interesting and previously inaccessible dark matter models from the last 20 years.”
LZ is a collaboration of roughly 250 scientists from 38 institutions in the United States, United Kingdom, Portugal, Switzerland, South Korea, and Australia; much of the work building, operating, and analyzing the record-setting experiment is done by early career researchers. The collaboration is already looking forward to analyzing the next data set and using new analysis tricks to look for even lower-mass dark matter. Scientists are also thinking through potential upgrades to further improve LZ, and planning for a next-generation dark matter detector called XLZD.
“Our ability to search for dark matter is improving at a rate faster than Moore’s Law,” Kravitz said. “If you look at an exponential curve, everything before now is nothing. Just wait until you see what comes next.”
LZ is supported by the U.S. Department of Energy, Office of Science, Office of High Energy Physics and the National Energy Research Scientific Computing Center, a DOE Office of Science user facility. LZ is also supported by the Science & Technology Facilities Council of the United Kingdom; the Portuguese Foundation for Science and Technology; the Swiss National Science Foundation, and the Institute for Basic Science, Korea. Over 38 institutions of higher education and advanced research provided support to LZ. The LZ collaboration acknowledges the assistance of the Sanford Underground Research Facility.
Media Resources
News release from Lawrence Berkeley Lab
LUX-ZEPLIN Dark Matter Detector Starts Up (2022)
Media Contacts
- Mani Tripathi, Physics and Astronomy, [email protected]
- Andy Fell, UC Davis News and Media Relations, 530-304-8888, [email protected]
Lauren Biron is a science writer at the Lawrence Berkeley Laboratory.
Primary Category
Secondary categories.
Kaptur Leads Dozen House Colleagues In Letter To President Biden Calling On DOE To Expedite Review Of LNG Projects For Ukraine And Other Eastern European Allies
Washington, DC – Today, Congresswoman Marcy Kaptur (OH-09), Co-Chair and Co-Founder of the Congressional Ukraine Caucus led a dozen colleagues in a letter to President Biden requesting that the Department of Energy (DOE) prioritize and expedite review of projects that will supply liquefied natural gas (LNG) to Ukrainian and Eastern European allies as it recommences the processing of applications for authorization to export LNG to countries where the US does not have existing free trade agreements (non-FTA nations). This request was made with a focus on maintaining both US national security and energy security for our European allies. Other signers of the letter include Representatives Lou Correa (CA-46), Jim Costa (CA-21), Don Davis (NC-01), Chris Deluzio (PA-17), Sylvia Garcia (TX-29), Vicente Gonzalez (TX-15), Chrissy Houlahan (PA-06), Mary Peltola (AK-AL), Marie Gluesenkamp Perez (WA-03), Marc Veasey (TX-33), and Susan Wild (PA-07).
“We must ensure that new exports do not impact energy prices for American consumers and businesses. However, the public interest also requires consideration of the extent to which LNG exports promote geopolitical stability and serve our national security interests. Russia's increasingly aggressive actions towards Ukrainian infrastructure, including electricity and gas storage facilities, highlight the urgent need to assist Ukraine in recovering and rebuilding and for Ukraine to diversify and secure its energy supply,” said the Members.
A full copy of the letter can be found by clicking here , or reading below:
Dear President Biden:
As members of Congress, we write to request that the Department of Energy (DOE) prioritize and expedite review of projects that will supply liquefied natural gas (LNG) to Ukrainian and Eastern European allies as it recommences the processing of applications for authorization to export LNG to countries where the US does not have existing free trade agreements (non-FTA nations). This request is made with a focus on maintaining both US national security and energy security for our European allies.
DOE performs a critical function when it reviews applications for new LNG exports to non-FTA nations for consistency with the public interest. We must ensure that new exports do not impact energy prices for American consumers and businesses. However, the public interest also requires consideration of the extent to which LNG exports promote geopolitical stability and serve our national security interests. Russia's increasingly aggressive actions towards Ukrainian infrastructure, including electricity and gas storage facilities, highlight the urgent need to assist Ukraine in recovering and rebuilding and for Ukraine to diversify and secure its energy supply. The Administration’s recent announcement of over $800 Million towards emergency energy needs in Ukraine to help “repair energy infrastructure damaged in the war, expand power generation, encourage private sector investment and protect energy infrastructure” will be vital to helping Ukraine recover and rebuild.
Equally important will be allowing Ukraine the ability to replace its natural gas supply when its contract with Gazprom expires at the end of this year. We believe that reducing Ukraine’s dependence on Russian energy will strengthen Ukraine's energy security and align with the broader strategic goals of diminishing Russia's influence in the region and reducing the leverage that hostile actors like Russia have over our allies.
Any delays to providing additional supplies of LNG to Ukraine and our Eastern European allies could jeopardize European energy security and market stability in the long-term. Typical gas offtake contracts are measured in years, not months, and are underpinned by certainty. We should not send mixed signals to our allies who want to eliminate their reliance on Vladimir Putin for good. We believe that the United States must demonstrate its commitment to supporting Ukraine's sovereignty and resilience amidst ongoing threats by prioritizing and expediting review of projects that will supply LNG to Ukraine and Eastern Europe.
Additionally, American LNG is produced with some of the strongest environmental protections globally.1 Rigorous regulations and oversight ensure that our LNG exports are reliable and adhere to high environmental standards. We believe that these environmental standards, in combination with assistance made available through Inflation Reduction Act programs, such as the GHG Reporting and the Methane Emissions Reduction Programs, will ensure industry and this Administration work to continue reducing emissions from natural gas. By prioritizing and expediting review of LNG projects that will supply LNG to vulnerable nations, we believe DOE would enable our allies to benefit from cleaner LNG sources that have been shown to reduce emissions compared to foreign supplies and coal,2 thus supporting their transition to more sustainable energy systems.
The United States has already shown a strong commitment to supporting Ukraine. Extending and expanding support to the energy sector is a natural and necessary step. We must continue to lead by example, showing that we can balance our environmental commitments with the need to provide reliable energy to our European allies. We believe that, if US LNG producers adhere to increasingly stringent environmental standards, then this balance is maintained, promoting both energy security and environmental stewardship.
In conclusion, we believe that prioritizing and expediting review of LNG projects that will supply Ukraine and Eastern Europe will support geopolitical stability and advance the national security interests of the United States. Thank you in advance for your consideration of this request.
An official website of the United States government
Here's how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS. A lock ( Lock Locked padlock ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
Dear Colleague Letter: ECosystem for Leading Innovation in Plasma Science and Engineering (ECLIPSE): Special Focus on PFAS and Microelectronics
September 16, 2024
Dear Colleagues:
With this Dear Colleague Letter (DCL), the US National Science Foundation (NSF) encourages submission of interdisciplinary proposals that capitalize on opportunities for bringing fundamental plasma science and engineering investigations to bear on two focus areas of societal and technological need:
- removal of per- and polyfluoroalkyl substances (PFAS) from the environment; and
- novel and more efficient methods for fabrication of microelectronics.
Per- and polyfluoroalkyl substances (PFAS) are a large group of synthetic chemicals that have been used for decades in consumer products and manufacturing processes. Because of the strength of the carbon-fluorine bonds, PFAS do not degrade easily and are persistent in water and soil. These "forever chemicals" are now widely distributed in the environment. Growing evidence shows that environmental PFAS bioaccumulate in fish, wildlife, and humans and may contribute to a wide range of adverse health effects. Limited methods are available for the destruction of PFAS in water and soil, either directly or after concentration. Plasmas generate highly reactive species, which may be effective at breaking down PFAS, particularly long-chain PFAS.
Semiconductors are also manufactured using many steps that involve plasmas during their fabrication. Many semiconductor devices are made in a low temperature plasma environment where the plasma is used in key parts of the workflow in semiconductor manufacturing to etch/deposit material, and in clean, dope or ash steps, etc. Plasmas are used in process steps that may produce or reduce PFAS. At the same time, lithography also plays a critical role in semiconductor manufacture and, as the feature sizes get smaller, tools using shorter wavelengths into the extreme ultraviolet (EUV) are entering the manufacturing process. Here plasmas can play an important role in generating the EUV light needed for lithography.
Proposals submitted in response to this DCL should be responsive to and will be considered within the ECosystem for Leading Innovation in Plasma Science and Engineering (ECLIPSE) meta-program, PD 24-110Z .
This DCL does not constitute a new competition or program. Proposals submitted in response to this DCL should be prepared and submitted in accordance with guidelines in the NSF Proposal & Award Policies & Procedures Guide (PAPPG) and should clearly articulate:
- the fundamental scientific and/or engineering challenge in plasma science and engineering that is proposed to be overcome; and
- how the proposed resolution of the stated scientific and/or engineering challenge will address either of the two focus areas under this DCL.
This DCL also encourages workforce development towards careers associated with the two focus areas through participation in plasma science and engineering research by the full spectrum of diverse talent that society has to offer, which includes underrepresented and underserved communities.
For consideration in the FY2025 funding cycle, proposals responsive to this DCL should be submitted directly to the ECLIPSE program description PD 24-110Z by 5 p.m. submitter's local time on November 18, 2024 .
Proposal titles should begin with (1) " ECLIPSE-PFAS: " or (2) " ECLIPSE-CHIPS: " followed by any other relevant prefixes and the project title.
Proposals addressing DCL’s two focus areas may also be submitted in response to:
- Solicitation NSF 24-575 : EPSCoR Centers of Research Excellence in Science and Technology (EPSCoR CREST Centers). Titles for proposals submitted to NSF 24-575 should begin with "EPSCoR CREST ECLIPSE Phase I Proposal: Center for ". CREST Partnership Supplements may also be submitted by CREST Center awardees to support collaborative research on topics within either of the two focus areas.
- Solicitation NSF 23-563 : Historically Black Colleges and Universities – Undergraduate Program (HBCU-UP). Titles for proposals submitted to NSF 23-563 should begin with "ECLIPSE [Insert HBCU-UP track]: Project Title."
- Solicitation NSF 23-598 : Historically Black Colleges and Universities – Excellence in Research (HBCU-EiR). Titles for proposals submitted to NSF 23-598 should begin with "Excellence in Research ECLIPSE: Project Title."
All correspondence and inquiries regarding this DCL should be submitted to [email protected] .
David B. Berkowitz, Assistant Director Directorate for Mathematical and Physical Sciences (MPS) Susan S. Margulies, Assistant Director Directorate for Engineering (ENG) James L. Moore III, Assistant Director Directorate for STEM Education (EDU) Alicia Knoedler, Office Head Office of Integrative Activities

IMAGES
VIDEO
COMMENTS
Q: What is a permission letter to conduct research? Answer: A permission letter to conduct research is a formal request to obtain permission from an organization or individual to conduct research on a particular topic. This type of letter is commonly used by students, researchers, and scholars who require permission to carry out their research.
A Letter of Permission to Conduct Research is a formal request to an individual or organization seeking permission to conduct research. It outlines the purpose, methodology, and ethical considerations of the research project.
When writing a letter requesting permission to conduct research, it's essential to be clear, polite, and include all necessary details. Address the letter to the appropriate supervisor or authority, state your name, department, and the purpose of your research. Clearly outline the scope of your research and any materials or fields involved. Express gratitude for their consideration and approval.
A written permission letter is a vital document that grants access to laboratory facilities, and it is frequently required of students and researchers for their projects, experiments, and research studies. This article aims to simplify the concept of permission letters, shedding light on their importance, the typical addressees, and the process of crafting an effective one. A permission letter ...
Y - Yeast experiment - Help your preschooler understand how yeast makes bread rise with this yeast experiment from Steam Powered Family. Z - FiZZy science - Just put a layer of baking soda in a rimmed baking sheet and give your child some food-colored vinegar in a spray or squirt bottle. Let the fizzing begin!
These four templates serve as formal requests for permission to conduct research in a laboratory setting, addressing various scenarios and academic levels. …
When composing a permission letter to use a laboratory, maintain a polite and respectful tone. Clearly state your name, batch, roll/ID number, and the specific laboratory you wish to use. Provide details such as the date and time of intended use, as well as the purpose for which you require access to the laboratory. Attach a list of equipment and materials you plan to utilize. End the letter ...
When writing a request letter for permission to use the laboratory, it is important to be clear and polite. Introduce yourself and provide detailed information about the purpose of your request. Clearly state why you need access to the laboratory and how it will benefit your studies or projects. Always thank the recipient for considering […]
The purpose of my research is to [briefly explain the main objectives of your research and its potential benefits or contributions to the field]. The study will involve [describe the research methodology, such as surveys, interviews, observations, experiments, etc.] and is expected to be conducted from [start date] to [end date], though the duration may vary depending on the scope of the research.
lly-sponsored AND participants are adultsStandard Informed Consent Template for Research. se this template if your research is NOT. derally-sponsore. A. D participants are adults.Avoid Common Problems with Consent Forms. Read these tips!1. ustomize this template to reflect the specifics of your study and participan.
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study.
Sample consent and permission forms. General consent form to participate in research (DOC) Two stage project consent form (DOC) Parent permission form for research with child (DOC) Child assent form (DOC) Multiple consent form including audio-recording and quotations (DOC) Photo and video consent form (DOC)
Informed Consent Templates in Research. Here is an example of an informed consent template that can be used in research studies: Title of Study: [Insert Title of Study] Investigator (s): [Insert Name (s) of Investigator (s)] Introduction. You are being invited to participate in a research study.
Sample Informed Consent Form (HERB) Informed Consent Form. The Department of Psychology at Wagner College supports the practice of protection of human participants in research. The following will provide you with information about the experiment that will help you in deciding whether or not you wish to participate.
Informed consent is the process of telling potential research participants about the key elements of a research study and what their participation will involve. The informed consent process is one of the central components of the ethical conduct of research with human subjects. The consent process typically includes providing a written consent ...
Please see the table below for instructions on when and how to use these forms. COVID-19 Screening Information Sheet. COVID-19 Testing Assent/Consent Form Addendum: Adults, Adolescents (13+), and Parents of Minors. COVID-19 Testing Assent Form Addendum: Children Aged 7-12.
Consent Form Templates These consent form templates have been posted for your reference. When completing and IRB submission in IRBIS, please fill in the application and use the consent form builder specific to your project. For more information, please find … Read more
A lab report conveys the aim, methods, results, and conclusions of a scientific experiment. The main purpose of a lab report is to demonstrate your
SciComm Example Letters Letter Enhancements Student Engagement Example Letters These examples reflect the wide range of formats, reading/writing levels, topics, and interest topics and levels in science. In sharing these ...
In order that research without consent is considered justifiable, the following three conditions have to be met: 1) it is impracticable to obtain consent, 2) the research does not infringe the principle of self-determination, and 3) the research provides significant clinical relevance [8].
For your information I have enclosed an information booklet with this letter to explain this stage of the study in more detail together with a copy of the letter that will be sent out to the individuals concerned.
I have put aside all political feelings and intend to vote in a scientific way, the science being sociology.
Lab reports are an essential part of all laboratory courses and a significant part of your grade. Here's a template for how to write a lab report.
Read about the basics of writing an effective scientific research proposal, and the differences between research proposals, grants and cover letters here.
Crafting a compelling letter of inquiry is a crucial step in securing funding for your organization's project. This art form requires precision and strategy, as it serves as the gateway to captivate potential funders and pave the way for a comprehensive grant proposal. ... where projects are leveraging novel computational approaches and ...
The experiment plans to collect 1,000 days' worth of data before it ends in 2028. "If you think of the search for dark matter like looking for buried treasure, we've dug almost five times deeper than anyone else has in the past," said Scott Kravitz, LZ's deputy physics coordinator and a professor at the University of Texas at Austin.
Washington, DC - Today, Congresswoman Marcy Kaptur (OH-09), Co-Chair and Co-Founder of the Congressional Ukraine Caucus led a dozen colleagues in a letter to President Biden requesting that the Department of Energy (DOE) prioritize and expedite review of projects that will supply liquefied natural gas (LNG) to Ukrainian and Eastern European allies a
September 16, 2024. Dear Colleagues: With this Dear Colleague Letter (DCL), the US National Science Foundation (NSF) encourages submission of interdisciplinary proposals that capitalize on opportunities for bringing fundamental plasma science and engineering investigations to bear on two focus areas of societal and technological need: