- Search Menu
- Sign in through your institution
- Volume 16, Issue 4, July 2024 (In Progress)
- Volume 16, Issue 3, June 2024
- Advance articles
- Editor's Choice
- Special Issues
- Author Guidelines
- Article Metrics
- Review Procedures and Criteria
- Open Access Policy
- Publication Charges
- Submission Site
- Call for Papers
- Why Publish?
- About AoB PLANTS
- Impact Factor
- Editorial Board
- About the Annals of Botany Company
- Advertising and Corporate Services
- Journals Career Network
- Self-Archiving Policy
- Journals on Oxford Academic
- Books on Oxford Academic

Article Contents
Introduction, conclusions, sources of funding, contributions by the authors, conflicts of interest statement, acknowledgements, literature cited.
- < Previous
Effect of saline water on seed germination and early seedling growth of the halophyte quinoa
Guest Editor: Tim J. Flowers
- Article contents
- Figures & tables
- Supplementary Data
M. R. Panuccio, S. E. Jacobsen, S. S. Akhtar, A. Muscolo, Effect of saline water on seed germination and early seedling growth of the halophyte quinoa, AoB PLANTS , Volume 6, 2014, plu047, https://doi.org/10.1093/aobpla/plu047
- Permissions Icon Permissions
Salinization is increasing on a global scale, decreasing average yields for most major crop plants. Investigations into salt resistance have, unfortunately, mainly been focused on conventional crops, with few studies screening the potential of available halophytes as new crops. This study has been carried out to investigate the mechanisms used by quinoa, a facultative halophytic species, in order to cope with high salt levels at various stages of its development. Quinoa is regarded as one of the crops that might sustain food security in this century, grown primarily for its edible seeds with their high protein content and unique amino acid composition. Although the species has been described as a facultative halophyte, and its tolerance to salt stress has been investigated, its physiological and molecular responses to seawater (SW) and other salts have not been studied. We evaluated the effects of SW and different salts on seed germination, seedling emergence and the antioxidative pathway of quinoa. Seeds were germinated in Petri dishes and seedlings grown in pots with SW solutions (25, 50, 75 and 100 %) and NaCl, CaCl 2 , KCl and MgCl 2 individually, at the concentrations in which they are present in SW. Our results demonstrated that all salts, at lower concentrations, increased the germination rate but not the germination percentages, compared with control (pure water). Conversely, seedlings were differently affected by treatments in respect to salt type and concentration. Growth parameters affected were root and shoot length, root morphology, fresh and dry weight, and water content. An efficient antioxidant mechanism was present in quinoa, activated by salts during germination and early seedling growth, as shown by the activities of antioxidant enzymes. Total antioxidant capacity was always higher under salt stress than in water. Moreover, osmotic and ionic stress factors had different degrees of influence on germination and development.
Soil salinity and sodicity cause severe problems in agriculture worldwide, and salt tolerance in crops is an extremely important trait and a major focus of research. Detrimental effects of high salinity on crops are multifaceted and affect plants in several ways: drought stress, ion toxicity, nutritional disorders, oxidative stress, alteration of metabolic processes, membrane disorganization and reduction of cell division and expansion ( Hasegawa et al. 2000 ; Munns 2002 ; Muscolo et al. 2007 , 2013 ; Zhu 2007 ; Sidari et al. 2008 ). As a result, plant growth, development and survival are reduced ( Muscolo et al. 2011 ; Schleiff and Muscolo 2011 ). Two major stresses affecting plants under salinity are osmotic and ionic stresses. Osmotic stress, occurring immediately in the root medium on exposure to salts, can result in inhibition of water uptake, cell expansion and lateral bud development ( Munns and Tester 2008 ). Ionic stress develops when toxic ions (e.g. Na + ) accumulate in cells causing increase in leaf mortality, chlorosis, necrosis and decrease in the activity of cellular metabolism including photosynthesis ( Yeo and Flowers 1986 ; Glenn and Brown 1999 ). In fact, excess Na + and Cl − have the potential to affect plant enzymes, resulting in reduced energy production and other physiological processes ( Larcher 1980 ; Morais et al. 2012 a , b ). Ionic stress results in premature senescence of older leaves and in toxicity symptoms (chlorosis, necrosis) in mature leaves due to high Na + and Cl − which affect plants by disrupting protein synthesis and by interfering with enzyme activity ( Munns and Termaat 1986 ; Hasegawa et al. 2000 ; Munns 2002 ).
In order to counteract the detrimental effects of salinity on agricultural production, extensive research on plant screening for salt tolerance has been conducted, with the aim of providing more tolerant cultivars. However, these studies have mainly focused on conventional crops, screening criteria and investigating how plants tolerate salts ( Shannon and Noble 1990 ; Chen et al. 2005 ; Sevengor et al. 2011 ). Unfortunately, there are few investigations on screening of available halophytes and their responses to saline conditions ( Flowers et al. 2010 ). The seed crop quinoa is a facultative halophyte native to the Andean region of Bolivia and Peru, and a member of the Amaranthaceae: quinoa is traditionally cultivated across a range of extreme environments. Due to its huge genetic variability, the species can be grown under unfavourable soil and climatic conditions ( Ruiz-Carrasco et al. 2011 ), showing a diverse tolerance to a wide range of abiotic stresses such as frost, salinity and drought, as well as an ability to grow on marginal soils ( Jacobsen et al. 2005 , 2007 , 2009 ; Maughan et al. 2009 ; Sun et al. 2014 ). Some varieties can grow in salt concentrations similar to those found in seawater (SW, 40 dS m −1 ) and even higher ( Jacobsen et al. 2001 ; Adolf et al. 2012 , 2013 ; Shabala et al. 2012 , 2013 ), well above the threshold for any known crop species.
Quinoa is considered a major alternative crop to meet food shortages in this century ( Jensen et al. 2000 ; Jacobsen et al. 2003 ; Sanchez et al. 2003 ; Trognitz 2003 ; Ruiz et al. 2014 ), for its gluten-free seeds and also as its grains provide a rich source of a wide range of minerals (Ca, P, Mg, Fe and Zn), vitamins (B 1 , B 9 , C and E), linolenate, natural antioxidants and high-quality protein, containing ample amounts of essential amino acids such as lysine and methionine ( Abugoch et al. 2008 ; Koyro and Eisa 2008 ). Quinoa's tolerance to high salinity at the primary stages of seed germination is based upon alterations in the levels of primary metabolites and enzyme activity ( González and Prado 1992 ; Adolf et al. 2013 ). Most of the studies on the effect of salinity on seed germination of halophytes have, however, been conducted using NaCl solutions. Such investigations may not provide information on germination under field conditions, because soils contain different salts, which may collectively influence germination in different ways from their individual effects ( Ungar 1996 ). Sea salt mimics the composition of saline soil solutions and can be used to study the synergistic effect of different salts on seed germination ( Liu et al. 2006 ). Therefore, the work presented here was carried out to examine the effects of SW and its component salts on seed germination, seedling emergence and the antioxidative pathway of quinoa cv. Titicaca, as well as the relative importance of two components (ionic and osmotic) of salinity stress.
Quinoa cultivars have been shown to differ in salt tolerance ( Bonales-Alatorre et al. 2013 ). In general, varieties originating from salt-affected areas are adapted to saline conditions and hence are less affected by salinity ( Adolf et al. 2012 ; Shabala et al. 2013 ) than those from non-saline areas. In this study, we used the Danish-bred quinoa cv. Titicaca ( Jacobsen et al. 2010 ; Adolf et al. 2012 ) to verify the salinity tolerance of a variety well adapted to European climatic conditions. Quinoa production may be a viable option for farmers interested in a high-value crop with regional production and local markets in Mediterranean countries where saline water and soil salinity are major risks for the future of agricultural development. Here fresh water resources are limited, while food requirements and pressure from climate change are still growing. The use of saline water resources may constitute a remedy for the current water scarcity. For these reasons, quinoa offers the possibility of an alternative, promising, cash crop to be cultivated in arid and semiarid environments that are prohibitive for other species and so may be able to utilize saline soils in a sustainable and productive way.
Plant material
Mature seeds of the Danish-bred quinoa ( Chenopodium quinoa cv. Titicaca) (provided by Department of Plant Environmental Science, University of Copenhagen) were stored at 5 °C until the start of experiments. Two different experiments were carried out in a growth chamber (Green line WRS 96-85, KW, Scientific Equipment, Italy) (temperature of 25 ± 1 °C in the dark with a relative humidity of 70 %) to characterize the responses of quinoa to salt stress. Seed germination and biochemical responses were studied in the first experiment, while morphological, physiological and biochemical responses of seedlings were studied in the second experiment.
Experiment 1: seed germination
Germination conditions and experimental design.
Seeds were surface-sterilized for 20 min in 20 % (v/v) sodium hypochlorite, rinsed and soaked for 1 h in distilled water. The sterilization procedure is required to eliminate saponine from seeds and to avoid contamination by microorganisms during the germination process. The entire sterilization procedure, including soaking, took 1 h and did not affect the germination process ( Ruiz-Carrasco et al. 2011 ; Burrieza et al. 2012 ). For the germination tests, five 50-seed replicates were used with either Mediterranean SW collected from the Tirreno sea (Calabria Southern Italy) with a salinity of 38 % ( Cotruvo 2005 ) or solutions of NaCl, CaCl 2 , KCl or MgCl 2 at the concentration in which they were in the SW and at various dilutions. In the experiment, five different concentrations of NaCl (0, 100, 200, 300 and 400 mM); KCl (0, 2.54, 5.08, 7.62 and 10.2 mM); CaCl 2 (0, 2.54, 5.08, 7.62 and 10.2 mM) and MgCl 2 (0, 13.4, 26.7, 40.1 and 53.5 mM) were used to test whether the various ions differently affected germination indexes and to verify possible antagonistic or synergic ion effects on seed germination. Seeds were placed on filter paper in 9 cm diameter Petri dishes containing 3 mL of each solution. The Petri dishes were hermetically sealed with Parafilm to prevent evaporation and kept in the growth chamber at a temperature of 25 ± 1 °C in the dark with a relative humidity of 70 %. Seeds were considered germinated when the radicle had extended at least 2 mm.
Germination indexes
Determination of ionic and osmotic effect, determination of enzyme activities.
Seeds (0.5 g) that had been soaked for 3 days in the test solutions were ground using a chilled mortar and pestle and homogenized in 0.1 M phosphate buffer solution (pH 7.0) containing 100 mg soluble polyvinylpolypyrrolidone and 0.1 mM ethylenediamine tetra acetic acid (EDTA). The homogenate was filtered through two layers of muslin cloth and centrifuged at 15 000 g for 15 min at 4 °C. The resulting supernatant was used to evaluate the activity of catalase (CAT, EC 1.11.1.6), peroxidase (POX, EC 1.11.1.7), ascorbate peroxidase (APX, EC 1.11.1.11) and superoxide dismutase (SOD EC 1.15.1.1). All enzyme activities were measured at 25 °C by a UV–visible light spectrophotometer (UV-1800 CE, Shimadzu, Japan).
Catalase activity was determined by monitoring the disappearance of H 2 O 2 at 240 nm, calculated using its extinction coefficient ( ε ) = 0.036 mM −1 cm −1 . The reaction mixture contained 1 mL of potassium phosphate buffer (50 mM, pH 7.0), 40 μL of enzyme extract and 5 μL of H 2 O 2 ( Beaumont et al. 1990 ).
Ascorbate peroxidase activity was assayed according to Nakano and Asada (1981) . The reaction mixture (1.5 mL) contained 50 mM phosphate buffer (pH 6.0), 0.1 μM EDTA, 0.5 mM ascorbate, 1.0 mM H 2 O 2 and 50 μL enzyme extract. The reaction was started by the addition of H 2 O 2 and ascorbate oxidation measured at 290 nm for 1 min. Enzyme activity was quantified using the molar extinction coefficient for ascorbate (2.8 mM −1 cm −1 ).
Peroxidase activity was measured on the basis of determination of guaiacol oxidation at 436 nm for 90 s ( Panda et al. 2003 ). The reaction mixture contained 1 mL of potassium phosphate buffer (0.1 M, pH 7.0), 20 μL of guaiacol, 40 μL of enzyme extract and 15 μL of H 2 O 2 . Peroxidase activity was quantified by the amount of tetraguaiacol formed using its extinction coefficient ( ε ) = 25.5 mM −1 cm −1 .
Superoxide dismutase activity was estimated by recording the decrease in the absorbance of superoxide nitro-blue tetrazolium complex by the enzyme ( Gupta et al. 1993 ). The reaction mixture (3 mL) contained 0.1 mL of 200 mM methionine, 01 mL of 2.25 mM nitro-blue tetrazolium, 0.1 mL of 3 mM EDTA, 1.5 mL of 100 mM potassium phosphate buffer, 1 mL of distilled water and 0.05 mL of enzyme extract. The assay was performed in duplicate for each sample. Two tubes without enzyme extract were used as a background control. The reaction was started by adding 0.1 mL of riboflavin (60 μM) and placing the tubes below a light source of two 15 W florescent lamps for 15 min. The reaction was stopped by switching off the light and covering the tubes with black cloth. Tubes without enzyme developed maximum colour. A non-irradiated complete reaction mixture which did not develop colour served as the blank. Absorbance was recorded at 560 nm and one unit of enzyme activity was taken as the quantity of enzyme which reduced the absorbance of samples to 50 % in comparison with tubes lacking enzymes.
For CAT, APX, SOD and POX activities, the results were expressed as enzyme units (U) per mg fresh weight. One unit of enzyme was defined as the amount of enzyme necessary to decompose 1 μmol of substrate per min at 25 °C.
Determination of total antioxidant capacity
Seeds (treated with different salt solutions for 3 days) were homogenized in a chilled mortar with distilled water at a ratio of 1 : 4 (seeds/water; w/v) and centrifuged at 14 000 g for 30 min. All steps were performed at 4 °C. The supernatants were filtered through two layers of muslin cloth and were used to determine the total antioxidant capacity by the spectrophotometric method of Prieto et al. (1999) . Aqueous extracts of the seeds were mixed in Eppendorf tubes with 1 mL of reagent solution (0.6 M H 2 SO 4 , 28 mM sodium phosphate, 4 mM ammonium molybdate mixture). The tubes were incubated for 90 min at 95 °C, then cooled to room temperature, and the absorbance read at 695 nm against a blank (mixture without seed extract). The assay was conducted in triplicate and the total antioxidant activity expressed as the absorbance of the sample at 695 nm. The higher the absorbance value, the higher the antioxidant activity ( Prasad et al. 2009 ).
Determination of total phenolic content
Total phenolic content was determined with the Folin–Ciocalteu reagent according to a modified procedure described by Singleton and Rossi (1965) . Briefly, 0.50 mL of the aqueous extract of the seeds was reacted with 2.5 mL of Folin–Ciocalteu reagent (1 : 10 diluted with distilled water) for 4 min, and then 2 mL of saturated sodium carbonate solution (∼75 g/L) was added to the reaction mixture. The absorbance readings were taken at 760 nm after incubation at room temperature for 2 h. Tannic acid was used as a reference standard, and the results were expressed as milligram tannic acid equivalent (mg TAET/g fresh weight).
Experiment 2: morphological, physiological and biochemical responses of seedlings
Plantlet growth in pots.
Seeds were germinated in Petri dishes. After 3 days from the beginning of germination, germinated seeds were grown for 21 days in plastic pots (10 cm diameter × 7 cm height), in a growth chamber (Green line WRS 96-85, KW apparecchi scientifici, Italy), under white light (80 W m −2 , Osram HQI halogen vapor W lamp, PAR 1055 μmol m −2 s−1) in a 16/8-h photoperiod, 70 % relative humidity and at 21 °C. All pots were filled with Perlite that had been equilibrated, before transplanting the germinated seeds, with one of the different salts or SW solutions at the desired concentration. All reagents used were of the highest analytical grade and were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All pots were watered with a one-fourth strength Murashige and Skoog medium (MS /4 ) containing macro and micronutrients at pH 5.8: the pots were weighed daily, and watered when their weight decreased by 30 % (corresponding to water that was lost by evapotranspiration). The control pots were watered with MS /4 alone. Leaf and root length were evaluated 21 days after the beginning of the stress, using six plants for each treatment.
Measurement of enzyme activities
After 21 days in pots under different salinity treatments, plantlet material was ground with a mortar and pestle in 100 mM HEPES–NaOH (pH 7.5), 5 mM MgCl 2 and 1 mM dithiothreitol . The ratio of plant material to buffer was 1 : 3. The extract was filtered through two layers of muslin and clarified by centrifugation at 15 000 g for 15 min. The supernatant was used for CAT, APX, POX, SOD analyses and total antioxidant capacity as described above. All steps were performed at 4 °C.
Cations (Na + , K + , Ca 2+ Mg 2+ and NH 4 + ) and anions (Cl − and SO 4 2− ) were determined in the water extracts of treated seedlings by ion chromatography (DIONEX ICS-1100).
Measurement of root morphology
Seedlings were harvested and root weight was recorded. Roots were scanned using an Epson Expression/STD 1600 scanner and personal computer with Intel Pentium III/500 CPU, 128 MB RAM, optimized for root analyses by Regent Instrument, Inc., and their length was analysed using the WinRHIZO image analysis system (Regent Instruments, Quebec, Canada). When scanning, each root sample was placed in a rectangular glass dish (300 × 200 mm) with ∼4–5 mm of water to untangle the roots and minimize root overlap. Three replicated roots were analysed for each treatment.
Statistical analysis
All data were analysed by one-way analysis of variance (ANOVA) with the salt concentration as the grouping factor. Separate ANOVAs were performed for each of four salt types and concentrations: NaCl (0, 100, 200, 300, 400 mM); KCl (0, 2.54, 5.08, 7.62, 10.16 mM); CaCl 2 (0, 2.54, 5.08, 7.62, 10.16 mM) and MgCl 2 (0, 13.36, 26.72, 40.09, 53.46 mM). The response variables for these ANOVAs were: seed germination, seedling growth, enzyme activities, ion contents and root morphology. Since salt concentration had five levels, on all significant ANOVAs we performed Tukey's multiple comparison tests to compare all pairs of means. The germination percentage data were previously subjected to arcsine transformation but are reported in tables as untransformed values. All data collected were statistically analysed using SYSTAT 8.0 software (SPSS Inc.).
Experiment 1: Germination under saline conditions
In water, all (100 %) seeds germinated (Table 1 ). At the lower concentrations, individual salts (NaCl, CaCl 2 , KCl and MgCl 2 ) did not have any significant effects on the germination percentage of quinoa seeds. Conversely, dilute SW significantly lowered germination (Table 1 ). With increasing salt concentration, the germination percentage decreased, irrespective of the treatment, except for MgCl 2 . The strongest reduction of germination was observed in the presence of 75 and 100 % SW in comparison to the other salts. The inhibition of different salt solutions on seed germination was in the order of SW > NaCl > KCl > CaCl 2 > MgCl 2 (Table 1 ). There were no significant differences among the treatments in germination rapidity (CVG), except in the SW (Table 1 : with increasing SW concentration, the CVG decreased, with a reduction of 53 % at 75 % SW). The GRI, reflecting the percentage of germination on each day of the germination period, decreased under NaCl and SW. The strongest decrease was observed in SW. No significant differences were observed among NaCl, CaCl 2 , KCl and MgCl 2 and the control, in terms of MGT (MGT, Table 1 ). Conversely, with increasing SW percentage, the MGT increased, reaching values 10 times greater than the control and of the other treatments. The strong significant inverse relationship between SW concentrations and germination indexes confirmed the detrimental effects of the SW on seed germination (Table 1 ).
Germination indices: total germination; CVG, GRI and MGT determined for quinoa seeds after 7 days of germination in the presence of NaCl, CaCl 2 , KCl, MgCl 2 and SW at different concentrations. Data are expressed as percentage in respect to control. Data are the means of five replicates. *** P < 0.001; ** P < 0.01: * P < 0.05.
| . | Total germination (%) . | CVG (%) . | GRI (%) . | MGT (days) . | |
|---|---|---|---|---|---|
| Control | 100 | 26.8 | 26.7 | 3.7 | |
| NaCl | 100 mM | 100 | 26.2 | 26.4 | 3.8 |
| NaCl | 200 mM | 100 | 27.0 | 28.4 | 3.7 |
| NaCl | 300 mM | 95 | 26.0 | 24.7* | 3.8 |
| NaCl | 400 mM | 80** | 26.7 | 22.2* | 3.7 |
| KCl | 2.54 mM | 96 | 26.8 | 26.7 | 3.8 |
| KCl | 5.08 mM | 95 | 26.8 | 26.7 | 3.8 |
| KCl | 7.62 mM | 93* | 26.5 | 25.3 | 3.8 |
| KCl | 10.16 mM | 86* | 26.9 | 23.9* | 3.7 |
| MgCl | 13.36 mM | 100 | 26.1 | 26.1 | 3.7 |
| MgCl | 26.73 mM | 100 | 26.9 | 28.3 | 3.7 |
| MgCl | 40.00 mM | 100 | 26.3 | 26.6 | 3.7 |
| MgCl | 53.46 mM | 100 | 27.0 | 29.1* | 3.6 |
| CaCl | 2.54 mM | 98 | 26.6 | 26.8 | 3.8 |
| CaCl | 5.08 mM | 95 | 26.4 | 25.4 | 3.8 |
| CaCl | 7.62 mM | 93* | 26.4 | 24.9 | 3.8 |
| CaCl | 10.16 mM | 93* | 27.0 | 26.8 | 3.7 |
| SW | 25 % | 85* | 25.8* | 21.4** | 3.9 |
| SW | 50 % | 65*** | 19.6** | 6.6*** | 5.1* |
| SW | 75 % | 10*** | 14.3** | 0.28*** | 35*** |
| SW | 100 % | 0 | nd | nd | nd |
| . | Total germination (%) . | CVG (%) . | GRI (%) . | MGT (days) . | |
|---|---|---|---|---|---|
| Control | 100 | 26.8 | 26.7 | 3.7 | |
| NaCl | 100 mM | 100 | 26.2 | 26.4 | 3.8 |
| NaCl | 200 mM | 100 | 27.0 | 28.4 | 3.7 |
| NaCl | 300 mM | 95 | 26.0 | 24.7* | 3.8 |
| NaCl | 400 mM | 80** | 26.7 | 22.2* | 3.7 |
| KCl | 2.54 mM | 96 | 26.8 | 26.7 | 3.8 |
| KCl | 5.08 mM | 95 | 26.8 | 26.7 | 3.8 |
| KCl | 7.62 mM | 93* | 26.5 | 25.3 | 3.8 |
| KCl | 10.16 mM | 86* | 26.9 | 23.9* | 3.7 |
| MgCl | 13.36 mM | 100 | 26.1 | 26.1 | 3.7 |
| MgCl | 26.73 mM | 100 | 26.9 | 28.3 | 3.7 |
| MgCl | 40.00 mM | 100 | 26.3 | 26.6 | 3.7 |
| MgCl | 53.46 mM | 100 | 27.0 | 29.1* | 3.6 |
| CaCl | 2.54 mM | 98 | 26.6 | 26.8 | 3.8 |
| CaCl | 5.08 mM | 95 | 26.4 | 25.4 | 3.8 |
| CaCl | 7.62 mM | 93* | 26.4 | 24.9 | 3.8 |
| CaCl | 10.16 mM | 93* | 27.0 | 26.8 | 3.7 |
| SW | 25 % | 85* | 25.8* | 21.4** | 3.9 |
| SW | 50 % | 65*** | 19.6** | 6.6*** | 5.1* |
| SW | 75 % | 10*** | 14.3** | 0.28*** | 35*** |
| SW | 100 % | 0 | nd | nd | nd |
Separation of ionic and osmotic components
Calculating the relative importance of the osmotic and ionic component stresses showed that the two stressful factors made a different contribution to the deterioration of germination depending on the salts used. In the presence of MgCl 2 , the two stressful factors (ionic and osmotic) had a proportional effect on the reduction of seed germination as shown by the value of the IE/OE ratio (1.0, Table 2 ). Regarding NaCl, the osmotic effect prevailed (IE/OE ratio = 0.53). In CaCl 2 and KCl, at LD 50 concentrations, seed germination decreased, mainly due to osmotic factors, as suggested by the IE/OE ratios that were always <1.0 and by IE values that were under 50 (Table 2 ). Seawater (the most toxic) affected seed germination mainly through its IE as evidenced by the IE/OE ratio >1.0 (Table 2 ).
Influence of osmotic and ionic factors on seed germination of Titicaca quinoa seeds in the presence of NaCl, KCl, MgCl 2 , CaCl 2 and SW at LD 50max concentration. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). The values correspond to the average of five replicates.
| Treatments . | OE . | TE (OE + IE) (%) . | IE . | IE/OE . | IE/TE . |
|---|---|---|---|---|---|
| NaCl | 36 | 55 | 19 | 0.53 | 35 |
| KCl | 29 | 52 | 23 | 0.79 | 44 |
| MgCl | 27 | 54 | 27 | 1.0 | 50 |
| CaCl | 33 | 58 | 25 | 0.76 | 43 |
| SW | 20 | 60 | 40 | 2.0 | 67 |
| Treatments . | OE . | TE (OE + IE) (%) . | IE . | IE/OE . | IE/TE . |
|---|---|---|---|---|---|
| NaCl | 36 | 55 | 19 | 0.53 | 35 |
| KCl | 29 | 52 | 23 | 0.79 | 44 |
| MgCl | 27 | 54 | 27 | 1.0 | 50 |
| CaCl | 33 | 58 | 25 | 0.76 | 43 |
| SW | 20 | 60 | 40 | 2.0 | 67 |
Enzyme activities, phenols and antioxidants
With increasing salt concentrations, POX activity decreased, with respect to the control in the presence of NaCl, CaCl 2 and SW. Conversely, an increase in POX activity was observed with MgCl 2 and particularly KCl (Fig. 1 A). Ascorbate peroxidase, CAT and SOD activities were always lower in control seeds compared with treated seeds; the highest concentrations of KCl and SW increased APX activity five and four times, respectively, compared with control. In NaCl and MgCl 2 , APX activity was higher at the lower, than at the higher, concentrations, and it was unaffected by CaCl 2 treatment (Fig. 1 B). Catalase activity increased with increasing concentration of CaCl 2 and SW. In contrast, in the presence of KCl and MgCl 2 , CAT activity decreased when the concentration increased (Fig. 1 C). Superoxide dismutase activity decreased as the concentrations of NaCl and CaCl 2 increased. Conversely, in the presence of increasing concentrations of KCl, MgCl 2 and SW, SOD activity increased, but to different extents. The highest values of SOD were observed in the presence of SW and KCl (Fig. 1 D).

Effect of different salts on POX, APX, CAT, SOD of quinoa seeds 3 days after sowing. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
The amount of total phenols and the total antioxidant capacity of seeds varied with the salt used. Total phenols increased in seeds treated with NaCl and SW, but the greatest increase was observed in the presence of SW (Table 3 ). Increasing the concentrations of KCl and MgCl 2 decreased total phenols; no significant differences were instead observed with increasing the concentration of CaCl 2 with respect to control and the other treatments. Total antioxidant capacity increased in all treated seeds compared with control. The highest antioxidant capacity was detected in the presence of SW (Table 3 ).
Total antioxidant activity and total phenol content in quinoa seeds 3 days after sowing with different salt treatments: A= control; B= 100 mM NaCl, 2.54 mM KCl, 2.54 mM CaCl 2 , 13.38 mM MgCl 2 , 25 % SW; C= 200 mM NaCl, 5.08 mM KCl, 5.08 mM CaCl 2 , 26.76 mM MgCl 2 , 50 % SW; D = 300 mM NaCl, 7.62 mM KCl, 7.62 mM CaCl 2 , 40.1 mM MgCl 2 , 75 % SW; E = 400 mM NaCl, 10.16 mM KCl, 10.16 mM CaCl 2 , 53.52 mM MgCl 2 , 100 % SW. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
| . | NaCl . | KCl . | CaCl . | MgCl . | SW . |
|---|---|---|---|---|---|
| Total antioxidant activity (µmol α-tocopherol/g FW) | |||||
| A | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.65 ± 0.02 |
| B | 3.15 ± 0.02 | 3.06 ± 0.04 | 1.91 ± 0.10 | 1.95 ± 0.10 | 2.24 ± 0.08 |
| C | 2.98 ± 0.02 | 2.91 ± 0.09 | 2.62 ± 0.03 | 2.69 ± 0.02 | 4.13 ± 0.15 |
| D | 3.06 ± 0.04 | 2.89 ± 0.1 | 2.50 ± 0.02 | 2.52 ± 0.08 | 3.24 ± 0.03 |
| E | 2.44 ± 0.05 | 2.94 ± 0.05 | 2.51 ± 0.03 | 2.75 ± 0.10 | 3.17 ± 0.02 |
| Total phenols (mg TAET/g DW) | |||||
| A | 209 ± 10 | 209 ± 10 | 209 ± 10 | 209 ± 10 | 209 ± 10 |
| B | 285 ± 10 | 200 ± 15 | 198 ± 10 | 167 ± 8 | 555 ± 25 |
| C | 307 ± 8 | 180 ± 10 | 223 ± 20 | 169 ± 5 | 521 ± 10 |
| D | 370 ± 12 | 181 ± 12 | 223 ± 18 | 171 ± 10 | 625 ± 20 |
| E | 347 ± 9 | 180 ± 13 | 224 ± 22 | 163 ± 6 | 568 ± 10 |
| . | NaCl . | KCl . | CaCl . | MgCl . | SW . |
|---|---|---|---|---|---|
| Total antioxidant activity (µmol α-tocopherol/g FW) | |||||
| A | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.65 ± 0.02 |
| B | 3.15 ± 0.02 | 3.06 ± 0.04 | 1.91 ± 0.10 | 1.95 ± 0.10 | 2.24 ± 0.08 |
| C | 2.98 ± 0.02 | 2.91 ± 0.09 | 2.62 ± 0.03 | 2.69 ± 0.02 | 4.13 ± 0.15 |
| D | 3.06 ± 0.04 | 2.89 ± 0.1 | 2.50 ± 0.02 | 2.52 ± 0.08 | 3.24 ± 0.03 |
| E | 2.44 ± 0.05 | 2.94 ± 0.05 | 2.51 ± 0.03 | 2.75 ± 0.10 | 3.17 ± 0.02 |
| Total phenols (mg TAET/g DW) | |||||
| A | 209 ± 10 | 209 ± 10 | 209 ± 10 | 209 ± 10 | 209 ± 10 |
| B | 285 ± 10 | 200 ± 15 | 198 ± 10 | 167 ± 8 | 555 ± 25 |
| C | 307 ± 8 | 180 ± 10 | 223 ± 20 | 169 ± 5 | 521 ± 10 |
| D | 370 ± 12 | 181 ± 12 | 223 ± 18 | 171 ± 10 | 625 ± 20 |
| E | 347 ± 9 | 180 ± 13 | 224 ± 22 | 163 ± 6 | 568 ± 10 |
Ion contents
In seeds 3 days after sowing, the total quantity of ions increased with increasing concentration of NaCl. A similar response was observed in the presence of SW, the only exception being at the higher concentrations (mainly ungerminated seeds) (Fig. 2 ). In the presence of KCl and CaCl 2 , the total ionic concentration gradually decreased with increasing concentrations of salts due to the increased number of non-germinated seeds (Fig. 2 ). On increasing MgCl 2 concentrations, the reduction in total ion concentration compared with control is likely due to the greater seed dry weight observed (+20 %). The ratio of cations/anions was unchanged in CaCl 2 and MgCl 2 and in NaCl up to a concentration of 400 mM. Increasing the concentration of KCl caused an increase in cations and a concomitant decrease in anion percentage (Fig. 2 ). Seawater, at the lowest concentrations (25 and 50 %), increased the total ions, lowering the amount of cations (33 %) with respect to the anions. Conversely, at the highest concentrations (75 and 100 %), SW decreased the number of germinated seeds and consequently the quantity of total ions but did not affect the cation–anion ratio (Fig. 2 ).

Total ion content, cation and anion percentages in seeds of quinoa after 3 days of different salt treatments. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
The ratio of Na + to cations and of Cl − to anions changed significantly depending on the salts used (Table 4 ). The ratio of Na + /cations increased significantly in comparison to the control with increasing the concentration of NaCl and SW. No differences were observed in the presence of MgCl 2 , while with CaCl 2 a slight decline was observed with respect to the control. The greatest significant decrease in Na + /cations ratio (ranging from 30 to 22 %) was observed in seeds under KCl treatment. For the Cl − /anions ratio, the lowest values were observed in the presence of KCl and the highest with NaCl. Increasing the concentration of SW and NaCl, increased the Na + /Cl − ratio with respect to the control, while this ratio decreased in the presence of other salts when their concentrations increased (Table 4 ). The greatest decrease in K + /Cl − ratio was observed in the presence of NaCl with a reduction ranging from 49 to 87 %. Mg 2+ /Cl − and NH 4 + /Cl − ratios decreased with respect to the control, mainly with increasing salt concentrations (Table 4 ). The Ca 2+ /Cl − ratio decreased in each treatment except for CaCl 2 and KCl. The PO 4 3− /Cl − ratio was significantly reduced compared with control in the presence of SW, NaCl and MgCl 2 (Table 4 ). The highest SO 4 2− /Cl − ratios were observed in the presence of SW and the lowest under NaCl treatment.
Cation and anion content against chloride, in seeds of quinoa treated with different salts, expressed as percentages. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
| Treatments | Na /cations . | Cl /anions . | Na /Cl . | PO /Cl . | SO /Cl . | K /Cl . | NH /Cl . | Mg /Cl . | Ca /Cl . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 5.0 | 13 | 29 | 635 | 23 | 349 | 76 | 69 | 28 | |
| NaCl | 100 mM | 41 | 49 | 63 | 102 | 1.8 | 51 | 23 | 15 | 3.2 |
| NaCl | 400 mM | 72 | 64 | 76 | 54 | 0 | 13 | 5.8 | 10 | 1.9 |
| MgCl | 13.38 mM | 5.0 | 33 | 16 | 202 | 6.5 | 185 | 34 | 53 | 19 |
| MgCl | 53.52 mM | 5.0 | 63 | 6.1 | 58 | 0 | 53 | 9.6 | 44 | 6.8 |
| CaCl | 2.54 mM | 6.0 | 16 | 30 | 530 | 15 | 345 | 45 | 55 | 14 |
| CaCl | 10.16 mM | 4.3 | 37 | 11 | 170 | 0.82 | 130 | 15 | 43 | 43 |
| KCl | 2.54 mM | 3.5 | 13 | 24 | 500 | 100 | 485 | 7.4 | 62 | 29 |
| KCl | 10.16 mM | 3.2 | 45 | 18 | 120 | 2.6 | 273 | 7.3 | 29 | 45 |
| SW | 25 % | 37 | 35 | 51 | 58 | 128 | 51 | 13 | 14 | 9.3 |
| SW | 100 % | 55 | 57 | 63 | 41 | 19 | 36 | 0 | 13 | 3.2 |
| Treatments | Na /cations . | Cl /anions . | Na /Cl . | PO /Cl . | SO /Cl . | K /Cl . | NH /Cl . | Mg /Cl . | Ca /Cl . | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 5.0 | 13 | 29 | 635 | 23 | 349 | 76 | 69 | 28 | |
| NaCl | 100 mM | 41 | 49 | 63 | 102 | 1.8 | 51 | 23 | 15 | 3.2 |
| NaCl | 400 mM | 72 | 64 | 76 | 54 | 0 | 13 | 5.8 | 10 | 1.9 |
| MgCl | 13.38 mM | 5.0 | 33 | 16 | 202 | 6.5 | 185 | 34 | 53 | 19 |
| MgCl | 53.52 mM | 5.0 | 63 | 6.1 | 58 | 0 | 53 | 9.6 | 44 | 6.8 |
| CaCl | 2.54 mM | 6.0 | 16 | 30 | 530 | 15 | 345 | 45 | 55 | 14 |
| CaCl | 10.16 mM | 4.3 | 37 | 11 | 170 | 0.82 | 130 | 15 | 43 | 43 |
| KCl | 2.54 mM | 3.5 | 13 | 24 | 500 | 100 | 485 | 7.4 | 62 | 29 |
| KCl | 10.16 mM | 3.2 | 45 | 18 | 120 | 2.6 | 273 | 7.3 | 29 | 45 |
| SW | 25 % | 37 | 35 | 51 | 58 | 128 | 51 | 13 | 14 | 9.3 |
| SW | 100 % | 55 | 57 | 63 | 41 | 19 | 36 | 0 | 13 | 3.2 |
Growth parameters
Seawater and NaCl, at the highest concentrations, affected the dry weights of the whole seedlings, as shown by the highest fresh weight/dry weight (FW/DW) ratio (Table 5 ), and additionally they reduced the root mass ratio (RMR). These findings suggest that the reduction of root mass may be the cause of the decrease in the total dry matter of the seedlings (Table 5 ). Investigating the root morphology showed that the total root length in all treatments was the most affected root parameter, as shown by F -ratios (Table 6 ). The plants irrigated with SW (50 %) had root lengths, surface areas and root volumes significantly lower than control (Table 6 ).
Total FW/DW ratio, LMR (leaf mass ratio = leaf dry weight/plant dry weight) and RMR (root mass ratio = root dry weight/plant dry weight) of quinoa seedlings after 21 days under different salt treatments. Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
| . | FW/DW (g plant ) . | LMR (g plant ) . | RMR (g plant ) . | |
|---|---|---|---|---|
| Control | 9.7 ± 0.2 | 0.81 ± 0.02 | 0.19 ± 0.01 | |
| SW | 50 % | 11.8 ± 0.2 | 0.89 ± 0.01 | 0.11 ± 0.02 |
| KCl | 5.08 mM | 8.3 ± 0.7 | 0.83 ± 0.02 | 0.17 ± 0.01 |
| KCl | 10.16 mM | 8.5 ± 0.4 | 0.84 ± 0.02 | 0.16 ± 0.01 |
| CaCl | 5.08 mM | 9.0 ± 0.5 | 0.82 ± 0.03 | 0.18 ± 0.02 |
| CaCl | 10.16 mM | 8.8 ± 0.3 | 0.83 ± 0.01 | 0.17 ± 0.01 |
| NaCl | 200 mM | 9.8 ± 0.2 | 0.82 ± 0.02 | 0.18 ± 0.02 |
| NaCl | 400 mM | 10.8 ± 0.2 | 0.90 ± 0.02 | 0.10 ± 0.01 |
| MgCl | 26.76 mM | 9.2 ± 0.3 | 0.78 ± 0.02 | 0.22 ± 0.03 |
| MgCl | 53.52 mM | 9.5 ± 0.5 | 0.82 ± 0.01 | 0.18 ± 0.02 |
| . | FW/DW (g plant ) . | LMR (g plant ) . | RMR (g plant ) . | |
|---|---|---|---|---|
| Control | 9.7 ± 0.2 | 0.81 ± 0.02 | 0.19 ± 0.01 | |
| SW | 50 % | 11.8 ± 0.2 | 0.89 ± 0.01 | 0.11 ± 0.02 |
| KCl | 5.08 mM | 8.3 ± 0.7 | 0.83 ± 0.02 | 0.17 ± 0.01 |
| KCl | 10.16 mM | 8.5 ± 0.4 | 0.84 ± 0.02 | 0.16 ± 0.01 |
| CaCl | 5.08 mM | 9.0 ± 0.5 | 0.82 ± 0.03 | 0.18 ± 0.02 |
| CaCl | 10.16 mM | 8.8 ± 0.3 | 0.83 ± 0.01 | 0.17 ± 0.01 |
| NaCl | 200 mM | 9.8 ± 0.2 | 0.82 ± 0.02 | 0.18 ± 0.02 |
| NaCl | 400 mM | 10.8 ± 0.2 | 0.90 ± 0.02 | 0.10 ± 0.01 |
| MgCl | 26.76 mM | 9.2 ± 0.3 | 0.78 ± 0.02 | 0.22 ± 0.03 |
| MgCl | 53.52 mM | 9.5 ± 0.5 | 0.82 ± 0.01 | 0.18 ± 0.02 |
Analysis of variance of the effect of different salt treatments on root morphology parameters of quinoa seedlings 21 days old. *** P < 0.001; ** P < 0.01; * P < 0.05.
| Treatment . | Total root length . | Surface area . | Volume . | . |
|---|---|---|---|---|
| SW | 2309.20*** | 200.82*** | 132.25*** | -ratio |
| 0.99 | 0.99 | 0.98 | ||
| KCl | 56.11*** | 2.98 | 8.22* | -ratio |
| 0.97 | 0.71 | 0.86 | ||
| CaCl | 49.18*** | 27.73** | 8.33* | -ratio |
| 0.97 | 0.95 | 0.86 | ||
| MgCl | 95.77*** | 21.25** | 11.27** | -ratio |
| 0.98 | 0.94 | 0.89 | ||
| NaCl | 42.67*** | 3.94 | 7.00* | -ratio |
| 0.97 | 0.75 | 0.84 |
| Treatment . | Total root length . | Surface area . | Volume . | . |
|---|---|---|---|---|
| SW | 2309.20*** | 200.82*** | 132.25*** | -ratio |
| 0.99 | 0.99 | 0.98 | ||
| KCl | 56.11*** | 2.98 | 8.22* | -ratio |
| 0.97 | 0.71 | 0.86 | ||
| CaCl | 49.18*** | 27.73** | 8.33* | -ratio |
| 0.97 | 0.95 | 0.86 | ||
| MgCl | 95.77*** | 21.25** | 11.27** | -ratio |
| 0.98 | 0.94 | 0.89 | ||
| NaCl | 42.67*** | 3.94 | 7.00* | -ratio |
| 0.97 | 0.75 | 0.84 |
Root parameters
Root length to mass ratio (SRL) and root fineness (RF), under SW, were not different from control while the ratio of root mass to volume (RTD) was lower. In seedlings irrigated with 400 mM NaCl, a higher SRL value indicated longer roots per unit root mass, while RTD and RF ratios were significantly reduced (Table 7 ), suggesting a decrease in root length and dry weight of seedlings treated with NaCl (200 mM) or MgCl 2 (26, 76 mM). Root morphology parameters were significantly changed by CaCl 2 and KCl compared with control but to different extents, depending on salt type (Table 7 ). NaCl, MgCl 2 and CaCl 2 , at lower concentrations, significantly increased RTD and RF ratios. No differences were observed when CaCl 2 and NaCl concentrations increased (Table 7 ). KCl, at all concentrations, significantly increased RTD and RF ratios, inducing a root system with thinner roots in comparison with control.
Specific root length (SRL = root length/root DW), root tissue density (RTD = root DW/root volume), root fineness (RF = root length/root volume) of quinoa seedlings after 21 days of different salt treatments. *** P < 0.001; ** P < 0.01; * P < 0.05.
| . | SRL (cm/mg DW) . | RTD (mg DW/cm ) . | RF (cm/cm ) . | |
|---|---|---|---|---|
| Control | 18.7 | 28 | 590 | |
| SW | 50 % | 21 | 23.1* | 613 |
| SW | 100 % | – | – | – |
| KCl | 5.08 mM | 19.3 | 35** | 675* |
| KCl | 10.16 mM | 21 | 31.3* | 690** |
| CaCl | 5.08 mM | 18.6 | 31.2* | 687** |
| CaCl | 10.16 mM | 20.0 | 27.5 | 520.9* |
| MgCl | 26.76 mM | 16.8 | 33.4* | 636* |
| MgCl | 53.52 mM | 20 | 29.0 | 601 |
| NaCl | 200 mM | 18.35 | 36.6* | 659* |
| NaCl | 400 mM | 24* | 19.6** | 522* |
| . | SRL (cm/mg DW) . | RTD (mg DW/cm ) . | RF (cm/cm ) . | |
|---|---|---|---|---|
| Control | 18.7 | 28 | 590 | |
| SW | 50 % | 21 | 23.1* | 613 |
| SW | 100 % | – | – | – |
| KCl | 5.08 mM | 19.3 | 35** | 675* |
| KCl | 10.16 mM | 21 | 31.3* | 690** |
| CaCl | 5.08 mM | 18.6 | 31.2* | 687** |
| CaCl | 10.16 mM | 20.0 | 27.5 | 520.9* |
| MgCl | 26.76 mM | 16.8 | 33.4* | 636* |
| MgCl | 53.52 mM | 20 | 29.0 | 601 |
| NaCl | 200 mM | 18.35 | 36.6* | 659* |
| NaCl | 400 mM | 24* | 19.6** | 522* |
In 21-day-old seedlings, total percentage of ions increased in the presence of NaCl, SW and KCl at all concentrations (Fig. 3 ) and at the highest concentrations of CaCl 2 and MgCl 2 . Total cations (Fig. 3 ) decreased in the presence of NaCl at all concentrations and at the highest concentrations of SW, MgCl 2 and CaCl 2 , with a concomitant increase in anion percentages (Fig. 3 ). No significant differences, in comparison to control, were observed in the presence of KCl.

Total ion content, cation and anion percentages in quinoa seedlings after 21 days of different salt treatments. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
Different salts caused a different distribution of cations and anions between root and shoot (Fig. 4 ). More cations were accumulated in shoots than in roots, decreasing in shoots when NaCl and MgCl 2 concentrations increased, while roots accumulated more anions than cations. The highest accumulation of anions was observed with CaCl 2 and KCl but with a different trend. In CaCl 2 , the anions increased in a concentration-dependent manner; in contrast increasing KCl concentrations lowered the anion percentage (Fig. 4 ). NaCl and MgCl 2 increased the cation concentration in roots as their external concentrations increased (Fig. 4 ).

Cation and anion percentages in root and shoot of quinoa seedlings after 21 days of different salt treatments.
The ratios of Na + /total cations and of Cl − /anions changed significantly depending on the salts used (Table 8 ). The Na + /cations ratio increased in comparison to the control with increasing the concentration of NaCl and SW. In contrast, Na + /cations ratio decreased with increasing the concentration of KCl, MgCl 2 and CaCl 2 . Cl − /anions ratios increased in the different salts at all concentrations, the highest value being observed with NaCl treatment. Increasing the concentration of SW and NaCl increased the Na + /Cl − ratio, while it was lowered in the other salts as their concentration increased. The K + /Cl − ratio decreased in the presence of all salts except for KCl, the greatest decrease being observed in NaCl. The Mg 2+ /Cl − ratio decreased with increasing concentrations of salts, other than for MgCl 2 . A similar situation was seen for the Ca 2+ /Cl − ratio, which decreased in each treatment except for CaCl 2 . The NH 4 + /Cl − ratio decreased in all situations as did SO 4 2− /Cl − ratios, where the highest values were detected in SW (Table 8 ).
Cation and anion content against chloride, in seedlings of quinoa treated with different salts, expressed as percentages. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). The values correspond to the average of five replicates.
| Treatments | Na /cations . | Cl /anions . | SO /Cl . | K /Cl . | NH /Cl . | Mg /Cl . | Ca /Cl . | Na /Cl . | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 15 | – | – | – | – | – | – | – | |
| NaCl | 200 mM | 55 | 83 | 18 | 18 | 3 | 15 | 24 | 1.9 |
| NaCl | 400 mM | 65 | 87 | 15 | 11 | 2.9 | 5 | 21 | 1.2 |
| MgCl | 26.73 mM | 17 | 41 | 19 | 26 | 36 | 46 | 63 | 62 |
| MgCl | 53.46 mM | 11 | 85 | 18 | 21 | 18 | 49 | 50 | 4 |
| CaCl | 5.08 mM | 9 | 42 | 16 | 37 | 54 | 23 | 70 | 110 |
| CaCl | 10.16 mM | 2.6 | 78 | 4.7 | 13 | 20 | 9 | 78 | 149 |
| KCl | 5.08 mM | 12 | 13 | 6.4 | 345 | 14 | 23 | 56 | 27 |
| KCl | 10.16 mM | 6 | 71 | 4.0 | 654 | 5 | 21 | 52 | 26 |
| SW | 50 % | 43 | 67 | 48 | 30 | 1.9 | 54 | 11 | 11 |
| Treatments | Na /cations . | Cl /anions . | SO /Cl . | K /Cl . | NH /Cl . | Mg /Cl . | Ca /Cl . | Na /Cl . | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 15 | – | – | – | – | – | – | – | |
| NaCl | 200 mM | 55 | 83 | 18 | 18 | 3 | 15 | 24 | 1.9 |
| NaCl | 400 mM | 65 | 87 | 15 | 11 | 2.9 | 5 | 21 | 1.2 |
| MgCl | 26.73 mM | 17 | 41 | 19 | 26 | 36 | 46 | 63 | 62 |
| MgCl | 53.46 mM | 11 | 85 | 18 | 21 | 18 | 49 | 50 | 4 |
| CaCl | 5.08 mM | 9 | 42 | 16 | 37 | 54 | 23 | 70 | 110 |
| CaCl | 10.16 mM | 2.6 | 78 | 4.7 | 13 | 20 | 9 | 78 | 149 |
| KCl | 5.08 mM | 12 | 13 | 6.4 | 345 | 14 | 23 | 56 | 27 |
| KCl | 10.16 mM | 6 | 71 | 4.0 | 654 | 5 | 21 | 52 | 26 |
| SW | 50 % | 43 | 67 | 48 | 30 | 1.9 | 54 | 11 | 11 |
The activity of the antioxidant enzymes depended on the salt and on the concentrations used (Fig. 5 ). Ascorbate peroxidase activity significantly decreased in the presence of MgCl 2 and KCl. In contrast, it increased in CaCl 2 -, SW- and NaCl-treated seedlings compared with control. POX activity increased in all treatments except for MgCl 2 and KCl. The most significant increase in catalase activity was in NaCl and SW. The same trend was observed for the SOD activity, with the highest values seen in the presence of SW and NaCl.

Effect of different salts on antioxidant enzymatic activities of quinoa seedlings after 21 days of different salt treatments. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
A significant increase in total phenols was observed in seedlings grown with NaCl and SW (Table 9 ). The SW was the most damaging agent, causing a 2-fold increase in the concentration of phenols. The total antioxidant capacity was doubled by NaCl and tripled by SW in respect to the control (Table 9 ).
Total antioxidant activity and total phenol content in quinoa seedlings after 21 days with different salt treatments: A = 200 mM NaCl, 5.08 mM KCl, 5.08 mM CaCl 2 , 26.76 mM MgCl 2 , 50 % SW; B = 400 mM NaCl, 10.16 mM KCl, 10.16 mM CaCl 2 , 53.52 mM MgCl 2 , 100 % SW. Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
| . | NaCl . | KCl . | CaCl . | MgCl . | SW . |
|---|---|---|---|---|---|
| Total antioxidant activity (mmol α-tocopherol/g FW) | |||||
| Control | 2.07 ± 0.03 | 2.07 ± 0.03 | 2.07 ± 0.03 | 2.07 ± 0.03 | 2.07 ± 0.03 |
| A | 4.35 ± 0.05 | 1.76 ± 0.02 | 2.06 ± 0.02 | 2.16 ± 0.07 | 6.20 ± 0.03 |
| B | 6.01 ± 0.06 | 1.56 ± 0.02 | 1.82 ± 0.04 | 1.58 ± 0.04 | – |
| Total phenols (mg TAET/g DW) | |||||
| Control | 272 ± 40 | 272 ± 40 | 272 ± 40 | 272 ± 40 | 272 ± 40 |
| A | 635 ± 20 | 322 ± 30 | 252 ± 30 | 222 ± 30 | 1075 |
| B | 840 ± 30 | 312 ± 10 | 240 ± 20 | 239 ± 20 | – |
| . | NaCl . | KCl . | CaCl . | MgCl . | SW . |
|---|---|---|---|---|---|
| Total antioxidant activity (mmol α-tocopherol/g FW) | |||||
| Control | 2.07 ± 0.03 | 2.07 ± 0.03 | 2.07 ± 0.03 | 2.07 ± 0.03 | 2.07 ± 0.03 |
| A | 4.35 ± 0.05 | 1.76 ± 0.02 | 2.06 ± 0.02 | 2.16 ± 0.07 | 6.20 ± 0.03 |
| B | 6.01 ± 0.06 | 1.56 ± 0.02 | 1.82 ± 0.04 | 1.58 ± 0.04 | – |
| Total phenols (mg TAET/g DW) | |||||
| Control | 272 ± 40 | 272 ± 40 | 272 ± 40 | 272 ± 40 | 272 ± 40 |
| A | 635 ± 20 | 322 ± 30 | 252 ± 30 | 222 ± 30 | 1075 |
| B | 840 ± 30 | 312 ± 10 | 240 ± 20 | 239 ± 20 | – |
In the Mediterranean region, besides water scarcity or high coastal soil salinity, it is mainly where saline water is used for irrigation that adverse effects are seen on crops, delaying or preventing germination and seedling growth ( Hegarty 1978 ; Almodares et al. 2007 ). Utilization of halophytes as crops would help in highly salinized zones, where only poor quality water, unsuitable for most agriculture, is available ( Rozema and Flowers 2008 ).
In this context, quinoa a facultative halophyte with exceptional nutritional quality could be useful to recover salinized land and to increase the poor agricultural economy of semiarid regions of the Mediterranean area. Our study focused on germination and seedling growth, because crop establishment depends on a successful germination and seedling emergence. Optimal germination for most halophytes has been reported in non-saline conditions ( Khan et al. 2002 ; Gul et al. 2013 ), and our data conform to these findings, showing toxicity of different salts. Results provided evidence for the existence of both ionic and osmotic effects by different treatments on seeds, depending on the salts used.
Our data clearly demonstrated that SW was the most detrimental solution affecting seed germination and seedling emergence of quinoa, mainly through its IE, confirming previous work showing that germination of halophytes was inhibited more by SW than different chlorides of Na, K, Mg ( Joshi et al. 1995 ). There is little information available on comparative influence of single salts and SW on seed germination of other halophytes ( Joshi et al. 1995 ; Baskin and Baskin 1998 ; Houle et al. 2001 ; Zia and Khan 2002 ; Atia et al. 2006 ; Liu et al. 2006 ). Some authors found NaCl more detrimental than SW and others the opposite ( Tirmizi et al. 1993 ; Zia and Khan 2002 ; Duan et al. 2003 ). Our data showed that the inhibition of different salt solutions on seed germination was in the order of SW > NaCl > KCl > CaCl 2 > MgCl 2 with no significant differences among the treatments in germination rapidity, except for the SW. The greatest negative effects of SW may be due to ion toxicity on germination, as a consequence of a coincident increase in cations and anions. Ion toxicity during germination has been previously demonstrated by Zehra et al. (2013) for the halophytic reed Phragmites karka : the inhibitory effect of different salts was interpreted mainly as an IE.
Although NaCl is the predominant salt in SW, its effects on seed germination and seedling growth were less detrimental than SW itself. The negative effects of SW on seedling growth may be ascribed to the induced accumulation of SO 4 2− (7.67 mmol g −1 DW, at least five times more than the other treatments) in leaves and of SO 4 2− (0.88 mmol g −1 DW) and Cl − (47.97 mmol g −1 DW) in roots. Sulfate is one of the components of sulfur-containing amino acids (cysteine and methionine) and many other compounds (e.g. glutathione or ferredoxin), which play important physiological functions, but when SO 4 2− is present in high concentration, it may affect plant development and crop yield, becoming injurious to plants ( Lianes et al. 2013 ). Lianes et al. (2013) previously showed that when the SO 4 2− is present in the medium, the capacities for ion compartmentalization and osmotic adjustment were reduced in the halophyte Prosopis strombulifera , resulting in water imbalance and symptoms of toxicity due to altered carbon metabolism (e.g. synthesis of sorbitol instead of mannitol, reduced sucrose production and protein content). This inhibition was partially mitigated when SO 4 2− and Cl − were present together in the solution, demonstrating a detrimental effect of the sulphate ion on plant growth ( Reginato et al. 2013 ).
According to Munns (2002) , the time scales for the osmotic and specific ionic component of salinity stress differ significantly, with the osmotic component dominating the first several days. Interestingly, however, comparing seed germination and seedling growth in the different salts, the results suggest that most probably ion toxicity is more detrimental to seedlings compared with the osmotic component of salt stress, as evidenced by the effect of SW treatment. This high salinity tolerance of quinoa, during germination and early seedling growth, may be explained by the existence of a significant gradient in the accumulation of potentially toxic (Na and Cl) and non-toxic essential (K, Mg, Ca, P and S) elements in seeds and also in the different distribution between shoot and root in salt-treated seedlings, as already demonstrated by Koyro and Eisa (2008) . Hence, we suggest that, once the seed's ability to exclude toxic Na + from the developing embryo fails, ion toxicity occurs, and seeds become unviable. The details of the distributions of ions between root and shoot showed differences among treatments; specifically with NaCl in shoot, we observed a significant accumulation of Na + , and little Cl − . In accordance with previous investigations ( Eisa et al. 2000 ), Na + was shown to be preferentially accumulated in shoots thereby the plants avoid excessive ion accumulation in the root tissues ( Koyro 2000 ; Ashraf et al. 2006 ).
Seawater caused an accumulation of Na + and SO 4 2− both in roots and in shoots, and an accumulation of Cl − in roots. Excessive accumulation of ions in halophytes (salt includers) under high substrate salinities (such a full strength SW) can lead to toxic effects in plants ( Munns 2005 ). The cause of injury is probably the salt load exceeding the ability of cells to compartmentalize salts in the vacuole. Salts might then build up rapidly in the cytoplasm inhibiting enzyme activity or alternatively, they might build up in cell walls, dehydrating the cell.
Considering the high energy cost of de novo synthesis of organic osmolytes ( Raven 1985 ), we can suppose that the seedlings tend to use Na + for osmotic adjustment. Hariadi et al. (2011) previously showed in quinoa that accumulation of Na + and K + was responsible for >95 % of cell turgor in old leaves and between 80 and 100 % in young leaves. A further role in the maintenance of turgor was also attributed to Cl − accumulated in roots ( James et al. 2006 ). Our results showed that the Cl − concentration was more than enough to contribute to osmotic adjustment maintaining root turgor as previously demonstrated in seedling of Stylosanthes guianensis by Veraplakorn et al. (2013) . Thus, it appears that the better germination and growth of cv. Titicaca observed in NaCl with respect to the other salts and SW may be achieved by the accumulation of inorganic osmolytes, particularly of Na + in shoots, and of Cl − in roots. The differences in ion uptake and distribution may be ascribed to properties of the roots. Roots have a high degree of plasticity, enabling plants to cope with a wide range of soil constraints ( Ho et al. 2005 ; Panuccio et al. 2011 ). Root morphology is a compromise among costs of resource capture, transport and efficiency ( Malamy 2005 ). Some morphological modifications at the individual root level can affect the structural and physiological characteristics of the entire root system and this can change water uptake and nutrient supply by plants. Specific root length, indicating root functionality ( Ryser 2006 ), characterizes the economic aspects of a root system, defining the cost-benefit ratio. Generally, under high salinity the costs per root length is minimized because of the growth limiting conditions. SW (50 %) reduced root growth and elongation, suggesting a decrease in photosynthate supply from the shoot. At the highest NaCl concentration, the greatest SRL ratio suggests the plants maximized the effectiveness of roots in water and nutrient uptake ( Fitter 1991 ). At the lowest concentrations of NaCl, KCl, CaCl 2 and MgCl 2 , the high root tissue density and root fineness ratios indicated that the seedlings explored a larger soil volume per unit of root surface area under stress than in its absence. In short, our data suggest that root morphology modifications should not be considered as a simple growth reduction, but rather as an induced reorientation of growth to avoid stress.
The results of this study clearly indicated that salt tolerance in this variety of quinoa is largely conferred by a delicate balance between osmotic adjustment and ion accumulation, showing differences in the ion compartmentalization between root and shoot. The greater negative effect of SW compared with NaCl, MgCl 2 CaCl 2 and KCl used separately suggests an additive and/or an interactive effect of these salts which cause an accumulation of ions in excess or leading to ion toxicity.
In conclusion, the present findings allow us to speculate that quinoa cv. Titicaca is a NaCl-tolerant cultivar of quinoa. Osmotic adjustment to NaCl salinity is largely conferred by inorganic ions, especially Na + , the main osmoregulatory material in the seedlings. The high SRL contributed to a high relative NaCl salinity tolerance in Titicaca, maintaining water and nutrient uptake. Higher SW toxicity may have been caused by SO 4 2− accumulation in seedlings that affected Titicaca germination and growth more than Cl − . Even if salinity reduced the productivity in terms of biomass, there was an increase in the antioxidant compounds, important health-protecting factors in food. On the basis of salt soil classifications currently used in all countries of the world, our results suggest that saline-sodic soils may be suitable for the cultivation of quinoa.
The research in the Mediterranea University laboratory and travelling was funded by Fattoria della Piana Company and by COST (STSM FA0901).
S.S.A. participated in the experiments, M.R.P. and A.M. did the experiments, analysed the data and wrote the manuscript, S.E.J. participated in the writing of the manuscript, acquired the funds for S.S.A. through the COST action ‘Putting Halophytes to Work’, and provided quinoa seed material for the study.
None declared.
The authors thank Carmelo Mallamaci for technical assistance and for taking care of the plants.
Google Scholar
Google Preview
Author notes
| Month: | Total Views: |
|---|---|
| January 2017 | 172 |
| February 2017 | 503 |
| March 2017 | 440 |
| April 2017 | 433 |
| May 2017 | 158 |
| June 2017 | 81 |
| July 2017 | 45 |
| August 2017 | 83 |
| September 2017 | 343 |
| October 2017 | 788 |
| November 2017 | 250 |
| December 2017 | 1,389 |
| January 2018 | 1,650 |
| February 2018 | 2,833 |
| March 2018 | 3,188 |
| April 2018 | 2,452 |
| May 2018 | 2,595 |
| June 2018 | 2,152 |
| July 2018 | 1,463 |
| August 2018 | 1,863 |
| September 2018 | 2,785 |
| October 2018 | 3,043 |
| November 2018 | 2,575 |
| December 2018 | 1,911 |
| January 2019 | 2,110 |
| February 2019 | 3,153 |
| March 2019 | 3,342 |
| April 2019 | 2,855 |
| May 2019 | 2,713 |
| June 2019 | 2,126 |
| July 2019 | 1,911 |
| August 2019 | 2,421 |
| September 2019 | 2,988 |
| October 2019 | 2,133 |
| November 2019 | 1,562 |
| December 2019 | 1,137 |
| January 2020 | 1,206 |
| February 2020 | 1,753 |
| March 2020 | 1,653 |
| April 2020 | 637 |
| May 2020 | 762 |
| June 2020 | 1,124 |
| July 2020 | 728 |
| August 2020 | 973 |
| September 2020 | 1,890 |
| October 2020 | 1,523 |
| November 2020 | 1,328 |
| December 2020 | 807 |
| January 2021 | 717 |
| February 2021 | 1,118 |
| March 2021 | 1,439 |
| April 2021 | 888 |
| May 2021 | 852 |
| June 2021 | 836 |
| July 2021 | 649 |
| August 2021 | 814 |
| September 2021 | 1,270 |
| October 2021 | 1,140 |
| November 2021 | 898 |
| December 2021 | 566 |
| January 2022 | 621 |
| February 2022 | 994 |
| March 2022 | 1,033 |
| April 2022 | 621 |
| May 2022 | 725 |
| June 2022 | 540 |
| July 2022 | 306 |
| August 2022 | 574 |
| September 2022 | 1,028 |
| October 2022 | 728 |
| November 2022 | 514 |
| December 2022 | 391 |
| January 2023 | 398 |
| February 2023 | 613 |
| March 2023 | 637 |
| April 2023 | 523 |
| May 2023 | 546 |
| June 2023 | 607 |
| July 2023 | 382 |
| August 2023 | 367 |
| September 2023 | 641 |
| October 2023 | 453 |
| November 2023 | 451 |
| December 2023 | 328 |
| January 2024 | 336 |
| February 2024 | 560 |
| March 2024 | 494 |
| April 2024 | 365 |
| May 2024 | 390 |
| June 2024 | 213 |
| July 2024 | 229 |
| August 2024 | 204 |
Email alerts
Citing articles via, affiliations.
- Online ISSN 2041-2851
- Copyright © 2024 Annals of Botany Company
- About Oxford Academic
- Publish journals with us
- University press partners
- What we publish
- New features
- Open access
- Institutional account management
- Rights and permissions
- Get help with access
- Accessibility
- Advertising
- Media enquiries
- Oxford University Press
- Oxford Languages
- University of Oxford
Oxford University Press is a department of the University of Oxford. It furthers the University's objective of excellence in research, scholarship, and education by publishing worldwide
- Copyright © 2024 Oxford University Press
- Cookie settings
- Cookie policy
- Privacy policy
- Legal notice
This Feature Is Available To Subscribers Only
Sign In or Create an Account
This PDF is available to Subscribers Only
For full access to this pdf, sign in to an existing account, or purchase an annual subscription.
Open Access is an initiative that aims to make scientific research freely available to all. To date our community has made over 100 million downloads. It’s based on principles of collaboration, unobstructed discovery, and, most importantly, scientific progression. As PhD students, we found it difficult to access the research we needed, so we decided to create a new Open Access publisher that levels the playing field for scientists across the world. How? By making research easy to access, and puts the academic needs of the researchers before the business interests of publishers.
We are a community of more than 103,000 authors and editors from 3,291 institutions spanning 160 countries, including Nobel Prize winners and some of the world’s most-cited researchers. Publishing on IntechOpen allows authors to earn citations and find new collaborators, meaning more people see your work not only from your own field of study, but from other related fields too.
Brief introduction to this section that descibes Open Access especially from an IntechOpen perspective
Want to get in touch? Contact our London head office or media team here
Our team is growing all the time, so we’re always on the lookout for smart people who want to help us reshape the world of scientific publishing.
Home > Books > Abiotic Stress in Plants
Effects of Salinity on Seed Germination and Early Seedling Stage
Submitted: 02 June 2020 Reviewed: 19 August 2020 Published: 07 October 2020
DOI: 10.5772/intechopen.93647
Cite this chapter
There are two ways to cite this chapter:
From the Edited Volume
Abiotic Stress in Plants
Edited by Shah Fahad, Shah Saud, Yajun Chen, Chao Wu and Depeng Wang
To purchase hard copies of this book, please contact the representative in India: CBS Publishers & Distributors Pvt. Ltd. www.cbspd.com | [email protected]
Chapter metrics overview
2,511 Chapter Downloads
Impact of this chapter
Total Chapter Downloads on intechopen.com
Total Chapter Views on intechopen.com
Salinity is the major environmental stress source that restricts on agricultural productivity and sustainability in arid and semiarid regions by a reduction in the germination rate and a delay in the initiation of germination and subsequent seedling establishment. Salt negatively effects the crop production worldwide. Because most of the cultivated plants are salt-sensitive glycophytes. Salt stress affects the seed germination and seedling establishment through osmotic stress, ion toxicity, and oxidative stress. Salinity may adversely influence seed germination by decreasing the amounts of seed germination stimulants such as GAs, enhancing ABA amounts, and altering membrane permeability and water behavior in the seed. Rapid seed germination and subsequent seedling establishment are important factors affecting crop production under salinity conditions. Seed priming is one of the useful physiological approaches for adaptation of glycophyte species to saline conditions during germination and subsequent seedling establishment. In seed priming, seeds are exposed to an eliciting solution for a certain period that allows partial hydration without radicle protrusion. Seed priming is a simple, low cost, and powerful biotechnological tool used to overcome the salinity problem in agricultural lands.
- germination
- seed priming
- plant hormones
Author Information
Cüneyt uçarlı *.
- Department of Molecular Biology and Genetics, Istanbul University, Istanbul, Turkey
*Address all correspondence to: [email protected]
1. Introduction
Seed dormancy and germination are distinct physiological processes, and the transition from dormancy to germination is not only a critical developmental step in the life cycle of higher plants but also determines the failure or success of the subsequent seedling establishment and plant growth [ 1 ]. Seed germination begins with the water uptake of dry seed (imbibition) and ends with radicle protrusion. Seed germination is affected by adverse environmental conditions including salinity, high temperature, and drought [ 2 ].
It is estimated that about approximately 7% of world land is affected by salinity and approximately 20% of 230 million ha irrigated land is salt-affected [ 3 ]. This number could be increased in the future due to increased land salinization as a consequence of contaminated artificial irrigation, climate change, and unsuitable land management. Salinity is a major stress responsible for the inhibition of seed germination or reduction in germination percentage and a delay in germination time in crops. At present, around 30 crop plants provide 90% of plant-based human food and the majority of these crops are not salt tolerant, even salt-sensitive, called glycophytes [ 4 ]. There have been high yield losses in these crops under moderate salinity (EC 4–8 dS m −1 , approximately 40–80 mM NaCl) [ 5 ].
High salinity leads a decrease in osmotic potential of ambient soil water, resulting with a decrease in water uptake by dry seeds (imbibition). Besides, the absorption of excess Na + and Cl − ions from soils creates ionic stress and cause toxicity which contributing to disruption in biochemical processes including nucleic and protein metabolism, energy production, and respiration [ 6 ]. Salinity also damages the nutrient and hormone balances, especially gibberellin (GA)/abscisic acid (ABA), during germination. As a result, high salinity level causes a delay in germination, even inhibition of seed germination depending on salt tolerance of plants. Dynamic balance between the generation and scavenging of reactive oxygen species (ROS) such as hydroxyl radicals, superoxide, and hydrogen peroxide could be disturbed by high salinity stress. ROS damage the macromolecules including proteins, carbohydrates, nucleic acids, and lipids, or cellular structures like membranes, resulting with inhibition of seed germination [ 7 ].
Germination has been found to be under strict regulation of plant hormones, especially GA and ABA [ 8 ]. ABA promotes seed dormancy and inhibits germination of seed, whereas GAs release dormancy and stimulate germination. Plant hormones ethylene (ET), and brassinosteroids (BRs) also have positive effect on seed germination by controlling the inhibitory effects of ABA on germination and rupturing testa and endosperm [ 9 , 10 ]. The plant hormones widely took part in determining the physiological state of a seed and regulating the germination process by interacting each other [ 11 ]. Hormones are regulated by distinct transcription factors and signaling components including NO and H 2 O 2 , showing the complexity of seed germination regulation. While some plant genes control the activity of plant hormones, and the other plant genes are activated by plant hormones [ 10 ]. Signaling molecules, such as NO and H 2 O 2 , also promotes germination and reduce the dormancy by enhancing ABA catabolism and GA biosynthesis [ 12 ].
Rapid seed germination and subsequent seedling establishment are important factors determining crop production and yield under salinity stress. One of the useful physiological approaches for glycophytes to adapt saline condition is seed priming [ 7 ]. Seed priming is an easy, low cost and low risk technique. The seeds are hydrated in specific solutions including plant hormones (GA3, ET, auxins, kinetin), antioxidant compounds (ascorbic acid, glutathione, tocopherone) organic solutes (proline, glycine betaine), inorganic salts (KNO 3 , CaCl 2 , and KCl), and particular bacteria and fungi species for a certain time to allow metabolic process of germination, followed by drying the seed to inhibit occurring of radicle protrusion [ 13 ].
2. Soil salinity and salinity stress
Plants, being sessile nature, are simultaneously subjected to various adverse conditions including salinity, drought, cold, heat, excess water, and heavy metals, which limit their development and growth. Salinity is the major environmental stress source that restricts on agricultural productivity and sustainability in arid and semiarid regions [ 14 ]. Salinity is a global issue that affects about 7% of the world’s total land area, including 20% total cultivated lands and 33% of irrigated land, causing estimated yield losses of 20% worldwide [ 15 , 16 ]. Besides, it is estimated that every year 10 million ha of agricultural land destroyed by salinized soil [ 17 ]. This rate can be increased by global climate change, use of contaminated irrigation water, intensive farming and poor drainage [ 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 ]. Without proper and sustainable control, salinity-affected areas will increase to more than 50% of the world’s total arable land by 2050 [ 15 ]. This rate can be accelerated by increase in sea water level by climate change, excessive use of groundwater for irrigation, increasing use of low-quality water for irrigation and massive introduction of irrigation associated with intensive farming and poor drainage [ 57 ].
non-saline (ECe ≤ 2 dS m −1 ): salinity effects mostly negligible;
very slightly saline (ECe = 2–4 dS m −1 ): yields of very sensitive crops may be restricted;
slightly saline (ECe = 4–8 dS m −1 ): yields of many crops are restricted;
moderately saline (ECe = 8–16 dS m −1 ): only salt tolerant crops yield satisfactorily; and
strongly saline (ECe ≥ 16 dS m −1 ): only a few very salt tolerant crops yield satisfactorily.
3. Seed germination
Seed germination is a complex multi-stage developmental process and regulated by internal and external factors. Internal factors include proteins, plant hormones (gibberellins/ABA balance, ethylene, and auxin), chromatin-related factors such as methylation, acetylation, histone ubiquitination, related genes (maturating genes and hormonal and epigenetics-regulating genes), non-enzymatic processes, seed age, seed size, and structural components of seed including (endosperm and seed coat). Besides, external factors containing moisture, light, salinity, temperature, acidity, and nutrient also affect the seed germination [ 60 , 61 ].
Seed germination begins with imbibition, the uptake of water by the dry mature seed, and ends with visible protrusion of radicle through testa [ 62 ]. Successful germination requires optimum environmental conditions, including water, oxygen, and temperature to initiate this process. Germination/sprouting is regulated by plant hormones such as gibberellic acid (GA), abscisic acid (ABA), ethylene, auxins, cytokinins, and brassinosteroids [ 63 ]. Among them, ABA and GA are two important regulators, which play antagonistic roles in seed dormancy and germination [ 64 ].
The process of seed germination can be divided into three phases ( Figure 1 ) [ 65 ]. Phase I begins with imbibition of dry seeds and ends with the early plateau phase of water uptake. Phase II includes reactivation of metabolisms, significant induction of hormonal and enzyme activity using surviving structures and components in the desiccated cells, genes involved in amino acid and nucleic acid synthesis, restarting of cellular respiration with genesis of mitochondria, mobilization of reserved, RNA and protein synthesis machinery [ 66 , 67 ]. Phase III is post-germination stage involves establishment of seedling and the induction of genes for photosynthetic metabolism after radicle cells elongate and divide [ 68 ].

Major events associated with germination and subsequent post-germinative growth (based on [ 13 , 65 ]).
Gibberellins and ABA are two key phytohormones regulating seed germination and seedling growth [ 69 ]. While GA breaks dormancy and enhances the seed germination and seedling, ABA inhibits germination and enhances seed dormancy [ 10 ]. However, the ratio of the two hormones, rather than the absolute level of each hormone, plays a key role in regulating the breaking of seed dormancy and the onset of germination [ 70 ]. GA/ABA balance determines fate of the seed; germination or dormancy. Gibberellins induce the synthesis and production of α-amylase, proteases, and β-glucanases, resulting in the germination of seeds [ 71 ]. GAs also stimulate the genes involved in weakening of endosperm and expansion of embryo cell [ 10 ]. On the other hand, ABA suppresses expression of many hydrolytic enzyme genes to prevent viviparous germination and inhibits promoting effect of GA on radicle growth and embryo expansion by inhibiting water uptake and hence cell-wall loosening, which is a key step to start germination [ 72 ].
Ethylene is a gaseous hormone involved in various processes, including positive regulation of seed germination. Ethylene breaks the primary and secondary dormancy and promotes seed germination by reducing ABA levels or sensitivity [ 73 ]. Brassinosteroids (BRs) and auxin induce the secretion of ethylene which works in conjunction with GAs to induce germination [ 10 ]. Auxins reduce seed sensitivity to ABA by overexpressing microRNAs and interacting with GAs to counteract ABA suppression during germination [ 74 , 75 ].
Low temperature decreases seed dormancy and enhances germination in many species, while high temperature has the negative effect on germination and induces secondary dormancy [ 70 ]. High temperature down-regulates the genes involved in synthesis of GA synthesis and deactivation of ABA, whereas genes involved in ABA synthesis are up-regulated by high temperature. Therefore, transcriptional changes in ABA and GA metabolism and signal pathways results with inhibition of germination or a delay in germination [ 76 ]. Light has been considered both to stimulate germination and to terminate dormancy by increasing the expression of GA anabolic genes, GA3ox1 and GA3ox2, and repressing expression of GA catabolism gene GA2ox2 [ 77 ].
In addition to phytohormones, several signal molecules, including as nitric oxide (NO) and reactive oxygen species (ROS), also regulate seed dormancy and germination [ 68 ]. ROS is an important regulator during seed germination because of the interaction with lipids, DNA, and protein molecules, as well as phytohormones including ABA and GA in the cell [ 78 ]. The biochemical and cellular reactions stimulated by water uptake are accompanied by the generation of ROS [ 79 ]. Hydrogen peroxide (H 2 O 2 ) serves as a signaling hub for the regulation of seed dormancy and germination; the accurate regulation of H 2 O 2 accumulation by the cell antioxidant mechanism is important to achieve a balance between oxidative signaling that enhances germination and oxidative damage that inhibits germination or delays in germination time [ 80 ]. N compounds, including NO, promotes seed germination through increasing amylase activities, adjusting K + /Na + balance, and enhancing seed respiration and ATP production [ 81 ].
4. Effect of salinity on seed germination and early seedling stage
Salinity affects seed germination process through osmotic stress, ion-specific effects and oxidative stress, shown by decreasing germination rate and extended germination time [ 82 ]. Salinity increases external osmotic potential that reduces water uptake during imbibition [ 83 ]. Salinity may affect the germination of seeds by the toxic effects of excess sodium and chloride ions on embryo viability [ 84 , 85 ]. The toxic effects include disruption to the structure of enzymes and other macromolecules, damage to cell organelles and the plasma membrane, the disruption of respiration, photosynthesis and protein synthesis [ 85 , 86 , 87 ].
In general, seed germination progresses in three phases under normal conditions. Seed germination begins with the rapid water uptake by dry seed (imbibition) (Phase I). A plateau phase, known as phase II, follows this phase. The cellular metabolisms are reactivated, and water uptake is restricted in phase II. This is followed by phase III, a post-germination phase, which is characterized by continuous water uptake until germination is complete ( Figure 1 ). Based on these three phases, the inhibition of seed germination or delaying in germination time under salinity stress may be generally ascribed to osmotic stress in the phase I and ionic stress in the phase II. Osmotic stress and ionic stress interact together to inhibit or delay germination of seed during the phase III [ 88 ].
Salinity may adversely influence seed germination by decreasing the amounts of seed germination stimulants such as GAs, enhancing ABA amounts, and altering membrane permeability and water behavior in the seed [ 89 ]. In higher plants, salinity has been demonstrated to change expression profiles of the genes encoding GA metabolic enzymes, including copalyl diphosphate synthase (CPS), ent-kaurene synthase (KS), ent-kaurene oxidase (KO), ent-kaurenoic acid oxidase (KOA), GA 20-oxidase (GA20ox), GA 3-oxidase (GA3ox) and GA 2-oxidase (GA2ox), resulting with change in endogenous GA levels during germination [ 12 ].
The germination of seeds is characterized by transcriptional induction of hydrolytic enzymes such as α-amylase [ 90 ]. The α-amylase is excreted into the endosperm to break the stored starch to metabolizable sugars that provide ready energy and nutrients for the growing embryo and radicle. Salinity stress may have much effect on delayed germination time than on final germination percentage for most crops. A delay of water uptake and a decrease in the activity of α-amylase with an increase in the concentration of NaCl may be main reasons for delaying of the germination time [ 91 ]. The decrease in the α-amylase activity have been reported to be higher in the salt-sensitive genotypes than in the salt-tolerant genotypes. This reduction in the α-amylase activity results with a significant reduction in the translocation of sugars, essential for the developing embryo. Besides, decreasing sugar concentrations also change the osmotic potential of growing cells, resulting in a decrease in water uptake [ 88 ].
Both osmotic and ionic effects of salt stress leads to generation of excess reactive oxygen species (ROS) and oxidative damage, which disrupts proteins, lipids, and nucleic acids or the cellular structure including lipid membrane [ 83 ].
Plants can be divided into two main groups based on their response to saline stress; salt-tolerant halophytes and salt-sensitive glycophytes (non-halophytes) [ 6 ]. The halophytes are plants that are able to grow in the presence of high salt concentrations that generate a low water potential of the soil and kill 99% of other species. They are adapted to survive and complete their life cycle under saline levels of higher than 200 mM NaCl. However, seed germination was also affected under salt stress and germination percentage was reduced to less than 10% under 1.7 M NaCl [ 92 , 93 ]. In halophytes, maximum salt tolerance for seed germination has been reported to vary from 1.7 to 0.26 M NaCl depending on halophyte species and other environment conditions such as temperature, moisture, and light ( Table 1 ).
| Plant species | Maximum salt tolerance | Salt tolerance type | Reference |
|---|---|---|---|
| 1.7 M NaCl | Halophyte | [ ] | |
| 1.5 M NaCl | Halophyte | [ ] | |
| 1.5 M NaCl | Halophyte | [ ] | |
| 1.3 M NaCl | Halophyte | [ ] | |
| 1.3 M NaCl | Halophyte | [ ] | |
| 1.0 M NaCl | Halophyte | [ ] | |
| 0.85 M NaCl | Halophyte | [ ] | |
| 0.8 M NaCl | Halophyte | [ ] | |
| 0.6 M NaCl | Halophyte | [ ] | |
| 0.5 M NaCl | Halophyte | [ ] | |
| 0.4 M NaCl | Halophyte | [ ] | |
| 0.3 M NaCl | Halophyte | [ ] | |
| 0.26 M NaCl | Halophyte | [ ] | |
| Quinoa ( Willd.) | 0.3 M NaCl | Halophyte | [ ] |
| Barley ( L.). | 0.25 M NaCl | Glycophyte | [ ] |
| Maize (Zea mays) | 0.24 M NaCl | Glycophyte | [ ] |
| Chicory ( L.) | 0.21 M NaCl | Glycophyte | [ ] |
| Lentil ( Medik.) | 0.2 M NaCl | Glycophyte | [ ] |
| 0.2 M NaCl | Glycophyte | [ ] | |
| Peanut ( ) | 0.2 M NaCl | Glycophyte | [ ] |
| Rice ( ) | 0.16 M NaCl | Glycophyte | [ ] |
| Fig ( L.) | 0.17 M NaCl | Glycophyte | [ ] |
| Button grass ( ) | 0.1 M NaCl | Glycophyte | [ ] |
| Sorghum ( Moench) | 0.1 M NaCl | Glycophyte | [ ] |
| Ryegrass ( ) | 0.1 M NaCl | Glycophyte | [ ] |
| Chickpea ( L.) | 0.09 M NaCl | Glycophyte | [ ] |
| Tomato ( ) | 0.05 M NaCl | Glycophyte | [ ] |
Maximum salt tolerance of halophytes and glycophytes at the germination stage.
Maximum NaCl concentration at which seed germination percentage reduced to 10–20%.
A majority of the common crops, such as tomato, bean, rice, corn, etc., are salinity sensitive or even hypersensitive and they are described as glycophytes [ 5 ]. The glycophytes contain 99% of the world’s flora and are susceptible to even low levels of salinity (ECe < 4 dS m −1 , approximately 40 mM NaCl) [ 92 ]. Under conditions of moderate salinity (EC 4–8 dS m −1 ), all important glycophytic crops reduce average yields by 50–80% [ 118 ]. Seed germination in glycophytes is severely inhibited under salinity due to both osmotic stress and ionic toxicity stress, while halophytes are less affected by osmotic stress during germination [ 12 ].
5. Alleviation salinity stress on germination by seed priming
Most crops are highly susceptible to saline soil, even when soil has electrical conductivity (ECe) as low as 3 dS m −1 [ 119 ]. Therefore, salinity stress appears to be a major limitation factor for crop productivity. Seed germination and seedling establishments are the two critical stages in plant growth. These stages are the most sensitive to environmental conditions including salinity [ 120 ]. Plants are usually seeded within the top layer of the soil which is more saline than lower layers [ 121 ]. Salinity stress may delay or prevent germination of germination of high quality seeds, resulting with crop loss. Rapid seed germination and subsequent seedling establishment are important factors affecting crop production under salinity conditions. Therefore, to decrease the negative effects of salinity stress on seed germination, it is important to know to what extent the genotypic variation in the water uptake pattern during these phases is associated with the salt tolerance of genotypes at the germination stage.
Seed priming is one of the useful physiological approaches for adaptation of glycophyte species to saline conditions during germination and subsequent seedling establishment. Seed priming is a simple, low cost and powerful biotechnological tool used to overcome the salinity problem by promoting seed germination and seedling establishment in agricultural lands [ 122 ]. Seed are exposed to an eliciting solution for a constant period that allows partial hydration, but radicle emergence does not occur by re-drying of seed. Seed germination occurs three distinct phases: (i) imbibition, (ii) lag phase (reactivation of metabolisms) and (iii) protrusion of the radicle through the testa. The goal of seed priming is to extend the lag phase, which allows pre-germinative physiological and biochemical processes, but prevent the seed transition towards full germination [ 123 ]. Enhanced and uniformed germination of primed seeds occurs by reduction in the lag time of imbibition, activation of enzyme involved in seed germination, initiation of biochemical mechanisms of cell repair, increase in the RNA content and DNA replication, decrease in ROS and lipid peroxidation with increased activity of antioxidant enzymes including as superoxide dismutase, catalase, and glutathione reductase, and increase in osmotic adjustment and starch metabolism [ 124 , 125 ].
Several methods of seed priming have been developed in order to revive seeds under salt stress conditions. Some of these methods are hydro-priming, osmopriming, solid matrix priming, hormonal-priming, bio-priming, chemical priming, and nutripriming [ 13 ]. In recent years, many studies have been reported to exhibit the positive effects of seed priming on germination under salinity conditions in many crops ( Table 2 ).
| Plant | Treatment | Alleviating effect | Reference |
|---|---|---|---|
| Barley ( cv. Bülbül 89) | Priming with aqueous solution of 30 μM H O for 24 h at room temperature | H O increased the germination index from 16.71 to 25.07%, and from 8.19 to 14.65% under 250 mM and 300 mM NaCl, respectively | [ ] |
| Tomato ( cv. Hezuo 903) | Priming with 100 μM Epigallocatechin-3-Gallate (EGCG) at 28 ± 3°C | EGCG increased germination rate and index from 84.7 to 97.0%, and from 29.4 to 35.2%, respectively | [ ] |
| Wheat ( cv. Chamran) | Priming with 0.5 mM spermidine for 24 h, 25 mM proline for 2 days, or 1.5 mM silicon (K SiO ) for 6 h | Spermidine, proline, and K SiO enhanced the germination rate by 32, 18, and 17%, respectively, under salinity stress (20 dS m ) | [ ] |
| , | Priming with 0.2 g/L GA3 solution for 12 h at room temperature without light. | GA3 enhanced germination percentage from 16.67, 26.67, and 50 to 60, 73.3, and 86.67% in , and respectively, and resulted in 20% reduction in mean germination time under salinity stress (12 dS m ) | [ ] |
| Pakchoi ( L. cv Tiancuiqing) | Priming with sodium nitroprusside (SNP) for 2 h in dark at 25 ± 1°C | Germination potential, germination index, and vitality index were increased by 7.67%, 14.20% and 74.51% after 10 μM SNP pre-treatment under 100 mM NaCl | [ ] |
| Soaking with 10 mM Ca | Ca significantly increased the germination percentage and recovery germination percentage under 200 mM NaCl | [ ] | |
| Melon ( ) | Priming with 10–50 μM melatonin for 6 h | Melatonin increase the germination percentage from 50 to 80% under salinity stress (14 dS m ) | [ ] |
| Wheat ( cv. Khirman) | Priming with 50 mg L ascorbate, 50 mM proline, 25 μM triacontanol, or 100 μM indole acetic acid for 12 h | Priming treatments significantly enhanced germination index and final germination percentage, and reduced mean germination time under salinity stress (12 dS m ) | [ ] |
| Grain sorghum ( Moench) | Priming with 100–500 mg L nano-iron oxide (n-Fe O ) for 10 h and soaking with 10 mg L n-Fe O for 3 days | Treatments improved the speed and percent of germination under 150 mM NaCl | [ ] |
| Lentil ( cv. Ncir) | Soaking with 0.5 mM salicylic acid or 0.1 mM H O at 25°C in the dark | Salicylic acid and H O enhanced the germination percentage from 71 to 86 and 87%, respectively | [ ] |
| Priming with 80 μM salicylic acid (SA) | SA significantly increased germination rate, germination potential, and germination index of the seeds under 200 mM NaCl | [ ] | |
| Sweet sorghum ( cv. Chuntian 1) | Priming with 288 μM Gibberellin (GA3) for 32–48 h | GA3 significantly increased the water uptake, resulting with increased cumulative germination percentage and germination index under 100 mM NaCl | [ ] |
| Maize ( ) | Priming with 2 mM silicon (K SiO ) for 7 days at 25°C in the dark | Silicon significantly enhanced the germination rate and percentage, as well as vitality index under 90 mM NaCl | [ ] |
| Oat ( cv. NDO-2) | Priming with 150 ppm gibberellin (GA3) for 24 h | GA3 enhanced the germination percentage from 56.64 to 76.03% under 100 mM NaCl | [ ] |
| Cucumber ( cv. Jinyou 1) | Priming with 0.3 mM silicon (NaSi) for 36 h | Silicon enhanced the germination percentage and index, and seedling vigor index under 200 mM NaCl | [ ] |
| Priming with 200 μM melatonin | Melatonin significantly increased germination rate, potential and index under 200 mM NaCl | [ ]. | |
| Priming with 10 mM ethephon, 5 μM fusicoccin or 50 μM kinetin | Fusicoccin, kinetin, and ethephon increased the germination percentage from 10 to 40, 50, and 84%, respectively under 900 mM NaCl | [ ] | |
| cv. Jisheng 3 | Priming with 200 μM gibberellins (GA4 + 7), 200 μM fluridone (FLU), 200 μM cytokinin (CK), 100 μM sodium nitroprusside (SNP), or 100 μM thiourea (TH) in the dark or light | GA and FLU significantly increased the germination percentage from 7 to 23 and 59% in the light, respectively, while SNP, CK and TH increased the germination percentage from 9 to 54, 55, and 30%, respectively, in the dark under 200 mM NaCl | [ ] |
| Inoculation with SP1016-20 | Inoculation with enhanced the final germination percentage and mean daily germination from 21.3 to 46.7%, and from 1.6 to 4.5%, respectively, under 510 mM NaCl | [ ] |
The functions of seed priming in plant at the germination stage under salinity condition.
Hydro-priming is the simplest and one of the mostly used seed priming method. Hydro-priming depends on seed soaking in pure water without chemical substances for 6–24 h and re-drying to original moisture content prior to sowing without emergence of radicle [ 144 ]. This method is a low-cost and environmentally friendly due to no use of additional chemicals. The uncontrolled water uptake by seeds is major disadvantage of this technique. Rapid hydration may cause leakage of essential nutrients out of the seed during germination, resulting in seed damage in some species [ 145 ].
Osmo-priming, also known as osmotic conditioning, involves soaking seeds in aerated low water potential solution including sugar, polyethylene glycol (PEG), glycerol, sorbitol, or mannitol with low water potential instead of pure water, followed by air drying before sowing. Due to low water potential of osmotic solutions, water is absorbed slowly by dry seed, which allows gradual seed imbibition [ 146 ]. While osmo-priming promotes activation of early phases of germination, inhibiting radicle emergence. Osmo-priming improves seed germination and enhances general crop performance under salt conditions. Water potential of osmotic agent is critical factor since main purpose is to restrict oxidative damage caused by ROS by inhibiting excess water from entering [ 147 ]. If inorganic salts such as NaCl, KCl, KNO 3 , K 3 PO 4 , MgSO 4 , and CaCl 2 are used as an osmo-priming agent, the method is generally referred as halopriming.
In hormonal priming, seed imbibition occurs in the presence of plan hormones such as GA3, ethylene, auxins, and salicylic acid, which can gave effect on seed metabolism. Chemical priming is a promising seed priming technique to enhance germination under high salinity stress. Seeds were pre-treated with different chemical solutions used as priming agents. Chemical agents includes a wide range of both natural and synthetic compounds such as antioxidants (ascorbic acid, glutathione, tocopherol, and melatonin), sodium hydrosulfide, polyamines hydrogen peroxide, sodium nitroprusside, urea, selenium, chitosan, fungicide, etc. [ 13 ].
Biopriming involves seed imbibition together with particular bacteria or fungi. These microorganisms are able to create endophytic connections with the plant. As other priming method, this treatment increases rate and uniformity of germination under salt conditions, as well as protects seeds against the soil and seed-borne pathogens [ 147 ]. The most frequently used biopriming species are Bacillus spp., Enterobacter spp., Pseudomonas spp., and Trichoderma spp. [ 148 ].
Seed priming efficiency is influence by many factors and strongly depends on treated plant species and chosen priming technique. Physical and chemical factors including osmotica and water potential, priming agent, duration, temperature, presence or absence of light, aeration, and seed condition also influence priming success and determine germination rate and time, seedling vigor, and further plant development [ 13 , 144 ].
Conflict of interest
No conflict of interest.
- 1. Liu Y, Ye N, Liu R, Chen M, Zhang J. H 2 O 2 mediates the regulation of ABA catabolism and GA biosynthesis in Arabidopsis seed dormancy and germination. Journal of Experimental Botany. 2010; 61 :2979-2990
- 2. Wahid A, Perveena M, Gelania S, Basra SMA. Pretreatment of seed with H 2 O 2 improves salt tolerance of wheat seedlings by alleviation of oxidative damage and expression of stress proteins. Journal of Plant Physiology. 2007; 164 :283-294
- 3. Parihar P, Singh S, Singh R, Singh VP, Prasad SM. Effect of salinity stress on plants and its tolerance strategies: A review. Environmental Science and Pollution Research International. 2014; 22 :4056-4075
- 4. Zörb C, Geilfus CM, Dietz KJ. Salinity and crop yield. Plant Biology. 2019; 21 :31-38
- 5. Koyro HOW, Lieth H, Said S. Salt tolerance of Chenopodium quinoa Willd, grains of the Andes, influence of salinity on biomass production, yield, composition of reserves in the seeds, water and solute. In: Lieth H, Garcia Sucre M, Herzog B, editors. Mangroves and Halophytes, Restoration and Utilisation III. Tasks for Vegetation Sciences. Vol. 43. Dordrecht: Springer Netherlands; 2008. pp. 133-145
- 6. Mwando E, Han Y, Angessa TT, Zhou G, Hill CB, Zhang XQ , et al. Genome-wide association study of salinity tolerance during germination in barley ( Hordeum vulgare L.). Frontiers in Plant Science. 2020; 11 :118
- 7. Ibrahim EA. Seed priming to alleviate salinity stress in germinating seeds. Journal of Plant Physiology. 2016; 192 :38-46
- 8. Han C, Yang PF. Studies on the molecular mechanisms of seed germination. Proteomics. 2015; 15 :1671-1679
- 9. Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annual Review of Plant Biology. 2008; 59 :387-415
- 10. Miransari M, Smith D. Plant hormones and seed germination. Environmental and Experimental Botany. 2014; 99 :110-121
- 11. Zhang C, Luo W, Li Y, Zhang X, Bai X, Niu Z, et al. Transcriptomic analysis of seed germination under salt stress in two desert sister species ( Populus euphratica and P. pruinosa ). Frontiers in Genetics. 2019; 10 :1-16
- 12. Llanes A, Andrade A, Masciarelli O, Alemano S, Luna V. Drought and salinity alter endogenous hormonal profiles at the seed germination phase. Seed Science Research. 2016; 26 (1):1-13
- 13. Lutts S, Benincasa P, Wojtyla L, S SK, Pace R, Lechowska K, et al. Seed priming: New comprehensive approaches for an old empirical technique. In: Araujo S, Balestrazzi A, editors. New Challenges in Seed Biology–Basic and Translational Research Driving Seed Technology. Rijeka, Croazia: IntechOpen; 2016. pp. 1-40
- 14. Foti C, Khah EM, Pavli OI. Germination profiling of lentil genotypes subjected to salinity stress. Plant Biology. 2019; 21 (3):480-486
- 15. Jamil A, Riaz S, Ashraf M, Foolad MR. Gene expression profiling of plants under salt stress. CRC Critical Reviews in Plant Science. 2011; 30 (5):435-458
- 16. Ashraf M, Akram NA. Improving salinity tolerance of plants through conventional breeding and genetic engineering: An analytical comparison. Biotechnology Advances. 2009; 27 :744-752
- 17. Pimentel D, Berger B, Filiberto D, Newton M, Wolfe B, Karabinakis E, et al. Water resources: Agricultural and environmental issues. BioScience. 2004; 54 :909-918
- 18. Isayenkov SV, Maathuis FJM. Plant salinity stress: Many unanswered questions remain. Frontiers in Plant Science. 2019; 10 :80
- 19. Adnan M, Zahir S, Fahad S, Arif M, Mukhtar A, Imtiaz AK, et al. Phosphate-solubilizing bacteria nullify the antagonistic effect of soil calcification on bioavailability of phosphorus in alkaline soils. Scientific Reports. 2018; 8 :4339
- 20. Akram R, Turan V, Hammad HM, Ahmad S, Hussain S, Hasnain A, et al. Fate of organic and inorganic pollutants in paddy soils. In: Hashmi MZ, Varma A, editors. Environmental Pollution of Paddy Soils, Soil Biology. Switzerland: Springer; 2018. pp. 197-214
- 21. Akram R, Turan V, Wahid A, Ijaz M, Shahid MA, Kaleem S, et al. Paddy land pollutants and their role in climate change. In: Hashmi MZ, Varma A, editors. Environmental Pollution of Paddy Soils, Soil Biology. Switzerland: Springer; 2018. pp. 113-124
- 22. Aziz K, Daniel KYT, Fazal M, Muhammad ZA, Farooq S, Fan W, et al. Nitrogen nutrition in cotton and control strategies for greenhouse gas emissions: A review. Environmental Science and Pollution Research. 2017; 24 :23471-23487
- 23. Aziz K, Daniel KYT, Muhammad ZA, Honghai L, Shahbaz AT, Mir A, et al. Nitrogen fertility and abiotic stresses management in cotton crop: A review. Environmental Science and Pollution Research. 2017; 24 :14551-14566
- 24. Depeng W, Fahad S, Saud S, Muhammad K, Aziz K, Mohammad NK, et al. Morphological acclimation to agronomic manipulation in leaf dispersion and orientation to promote “Ideotype” breeding: Evidence from 3D visual modeling of “super” rice ( Oryza sativa L.). Plant Physiology and Biochemistry. 2019; 135 :499-510
- 25. Fahad S, Bajwa AA, Nazir U, Anjum SA, Farooq A, Zohaib A, et al. Crop production under drought and heat stress: Plant responses and management options. Frontiers in Plant Science. 2017; 8 :1147
- 26. Fahad S, Bano A. Effect of salicylic acid on physiological and biochemical characterization of maize grown in saline area. Pakistan Journal of Botany. 2012; 44 :1433-1438
- 27. Fahad S, Chen Y, Saud S, Wang K, Xiong D, Chen C, et al. Ultraviolet radiation effect on photosynthetic pigments, biochemical attributes, antioxidant enzyme activity and hormonal contents of wheat. Journal of Food, Agriculture and Environment. 2013; 11 (3&4):1635-1641
- 28. Fahad S, Hussain S, Bano A, Saud S, Hassan S, Shan D, et al. Potential role of phytohormones and plant growth-promoting rhizobacteria in abiotic stresses: Consequences for changing environment. Environmental Science and Pollution Research. 2014; 22 (7):4907-4921
- 29. Fahad S, Hussain S, Matloob A, Khan FA, Khaliq A, Saud S, et al. Phytohormones and plant responses to salinity stress: A review. Plant Growth Regulation. 2014; 75 (2):391-404
- 30. Fahad S, Hussain S, Saud S, Hassan S, Chauhan BS, Khan F, et al. Responses of rapid viscoanalyzer profile and other rice grain qualities to exogenously applied plant growth regulators under high day and high night temperatures. PLOS One. 2016; 11 (7):e0159590
- 31. Fahad S, Hussain S, Saud S, Hassan S, Ihsan Z, Shah AN, et al. Exogenously applied plant growth regulators enhance the morphophysiological growth and yield of rice under high temperature. Frontiers in Plant Science. 2016; 7 :1250
- 32. Fahad S, Hussain S, Saud S, Hassan S, Tanveer M, Ihsan MZ, et al. A combined application of biochar and phosphorus alleviates heat-induced adversities on physiological, agronomical and quality attributes of rice. Plant Physiology and Biochemistry. 2016; 103 :191-198
- 33. Fahad S, Hussain S, Saud S, Khan F, Hassan S, Amanallah J, et al. Exogenously applied plant growth regulators affect heat-stressed rice pollens. Journal of Agronomy and Crop Science. 2016; 202 :139-150
- 34. Fahad S, Hussain S, Saud S, Tanveer M, Bajwa AA, Hassan S, et al. A biochar application protects rice pollen from high-temperature stress. Plant Physiology and Biochemistry. 2015; 96 :281-287
- 35. Fahad S, Muhammad ZI, Abdul K, Ihsanullah D, Saud S, Saleh A. Consequences of high temperature under changing climate optima for rice pollen characteristics-concepts and perspectives. Archives of Agronomy and Soil Science. 2018; 64 (11):1473-1488
- 36. Fahad S, Nie L, Chen Y, Wu C, Xiong D, Saud S, et al. Crop plant hormones and environmental stress. Sustainable Agriculture Reviews. 2015; 15 :371-400
- 37. Fahad S, Adnan M, Hassan S, Saud S, Hussain S, Wu C, et al. Rice responses and tolerance to high temperature. In: Hasanuzzaman M, Fujita M, Nahar K, Biswas JK, editors. Advances in Rice Research for Abiotic Stress Tolerance. Cambridge: Woodhead Publishing; 2019. pp. 201-224
- 38. Fahad S, Rehman A, Shahzad B, Tanveer M, Saud S, Kamran M, et al. Rice responses and tolerance to metal/metalloid toxicity. In: Hasanuzzaman M, Fujita M, Nahar K, Biswas JK, editors. Advances in Rice Research for Abiotic Stress Tolerance. Cambridge: Woodhead Publishing; 2019. pp. 299-312
- 39. Habib ur R, Ashfaq A, Aftab W, Manzoor H, Fahd R, Wajid I, et al. Application of CSM-CROPGRO-cotton model for cultivars and optimum planting dates: Evaluation in changing semi-arid climate. Field Crops Research. 2019; 238 :139-152
- 40. Hafiz MH, Muhammad A, Farhat A, Hafiz FB, Saeed AQ , Muhammad M, et al. Environmental factors affecting the frequency of road traffic accidents: A case study of sub-urban area of Pakistan. Environmental Science and Pollution Research. 2019; 26 :11674-11685
- 41. Hafiz MH, Wajid F, Farhat A, Fahad S, Shafqat S, Wajid N, et al. Maize plant nitrogenuptake dynamics at limited irrigation water and nitrogen. Environmental Science and Pollution Research. 2016; 24 (3):2549-2557
- 42. Hesham FA, Fahad S. Melatonin application enhances biochar efficiency for drought tolerance in maize varieties: Modifications in physio-biochemical machinery. Agronomy Journal. 2020; 2020 :1-22
- 43. Hussain MA, Fahad S, Rahat S, Muhammad FJ, Muhammad M, Qasid A, et al. Multifunctional role of brassinosteroid and its analogues in plants. Plant Growth Regulation. 2020. DOI: 10.1007/s10725-020-00647-8 (In Press)
- 44. Kamarn M, Wenwen C, Irshad A, Xiangping M, Xudong Z, Wennan S, et al. Effect of paclobutrazol, a potential growth regulator on stalk mechanical strength, lignin accumulation and its relation with lodging resistance of maize. Plant Growth Regulation. 2017; 84 :317-332
- 45. Muhammad Z, Abdul MK, Abdul MS, Kenneth BM, Muhammad S, Shahen S, et al. Performance of Aeluropus lagopoides (mangrove grass) ecotypes, a potential turfgrass, under high saline conditions. Environmental Science and Pollution Research. 2019; 26 :13410-13421
- 46. Qamar-uz Z, Zubair A, Muhammad Y, Muhammad ZI, Abdul K, Fahad S, et al. Zinc biofortification in rice: Leveraging agriculture to moderate hidden hunger in developing countries. Archives of Agronomy and Soil Science. 2017; 64 :147-161
- 47. Sajjad H, Muhammad M, Ashfaq A, Waseem A, Hafiz MH, Mazhar A, et al. Using GIS tools to detect the land use/land cover changes during forty years in Lodhran district of Pakistan. Environmental Science and Pollution Research. 2019. DOI: 10.1007/s11356-019-06072-3 (In Press)
- 48. Saud S, Chen Y, Fahad S, Hussain S, Na L, Xin L, et al. Silicate application increases the photosynthesis and its associated metabolic activities in Kentucky bluegrass under drought stress and post-drought recovery. Environmental Science and Pollution Research. 2016; 23 (17):17647-17655
- 49. Saud S, Chen Y, Long B, Fahad S, Sadiq A. The different impact on the growth of cool season turf grass under the various conditions on salinity and drought stress. International Journal of Agricultural Science and Research. 2013; 3 :77-84
- 50. Saud S, Fahad S, Cui G, Chen Y, Anwar S. Determining nitrogen isotopes discrimination under drought stress on enzymatic activities, nitrogen isotope abundance and water contents of Kentucky bluegrass. Scientific Reports. 2020; 10 :6415
- 51. Saud S, Fahad S, Yajun C, Ihsan MZ, Hammad HM, Nasim W, et al. Effects of nitrogen supply on water stress and recovery mechanisms in Kentucky bluegrass plants. Frontiers in Plant Science. 2017; 8 :983
- 52. Saud S, Li X, Chen Y, Zhang L, Fahad S, Hussain S, et al. Silicon application increases drought tolerance of Kentucky bluegrass by improving plant water relations and morph physiological functions. The Scientific World Journal. 2014; 2014 :10. Article ID: 368694
- 53. Shah F, Lixiao N, Kehui C, Tariq S, Wei W, Chang C, et al. Rice grain yield and component responses to near 2°C of warming. Field Crops Research. 2013; 157 :98-110
- 54. Wajid N, Ashfaq A, Asad A, Muhammad T, Muhammad A, Muhammad S, et al. Radiation efficiency and nitrogen fertilizer impacts on sunflower crop in contrasting environments of Punjab. Environmental Science and Pollution Research. 2017; 25 :1822-1836
- 55. Yang Z, Zhang Z, Zhang T, Fahad S, Cui K, Nie L, et al. Effect of season-long temperature increases on rice cultivars grown in the central and southern regions of China. Frontiers in Plant Science. 2017; 8 :1908
- 56. Zahida Z, Hafiz FB, Zulfiqar AS, Ghulam MS, Fahad S, Muhammad RA, et al. Effect of water management and silicon on germination, growth, phosphorus and arsenic uptake in rice. Ecotoxicology and Environmental Safety. 2017; 144 :11-18
- 57. Machado R, Serralheiro R. Soil Salinity: Effect on vegetable crop growth. Management Practices to Prevent and Mitigate Soil Salinization. Horticulturae. 2017; 3 (2):30
- 58. Hasanuzzaman M, Nahar K Fujita M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In: Ahmad P, Azooz MM, Prasad MNV, editors. Ecophysiology and Responses of Plants under Salt Stress. New York: Springer-Verlag; 2013. pp. 25-87
- 59. USSL Staff. Diagnosis and Improvement of Saline and Alkali Soils. USDA Handbook No 60. Washington DC, USA; 1954. p. 160
- 60. An Y-Q , Lin L. Transcriptional regulatory programs underlying barley germination and regulatory functions of gibberellin and abscisic acid. BMC Plant Biology. 2011; 11 :105
- 61. Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006; 171 (3):501-523
- 62. Bewley JD. Seed germination and dormancy. The Plant Cell. 1997; 9 :1055-1066
- 63. Nonogaki H, Bassel GW, Bewley JD. Germination - still a mystery. Plant Science. 2010; 179 :574-581
- 64. Koornneef M, Bentsink L, Hilhorst H. Seed dormancy and germination. Current Opinion in Plant Biology. 2002; 5 :33-36
- 65. Weitbrecht K, Müller K, Leubner-Metzger G. First off the mark: Early seed germination. Journal of Experimental Botany. 2011; 62 (10):3289-3309
- 66. Nonogaki H. Seed dormancy and germination-emerging mechanisms and new hypotheses. Frontiers in Plant Science. 2014; 5 :233
- 67. Bewley JD, Bradford KJ, Hilhorst HWM, Nonogaki HS. Physiology of Development, Germination and Dormancy. New York: Springer; 2013
- 68. Ma Z, Marsolais F, Bykova NV, Igamberdiev AU. Nitric oxide and reactive oxygen species mediate metabolic changes in barley seed embryo during germination. Frontiers in Plant Science. 2016; 7 :138
- 69. Bentsink L, Koornneef M. Seed Dormancy and Germination. Arabidopsis Book; 2008; 6 :e0119
- 70. Gazzarrini S, Tsai AYL. Hormone cross-talk during seed germination. Essays in Biochemistry. 2015; 58 :151-164
- 71. Yamaguchi S. Gibberellin metabolism and its regulation. Annual Review of Plant Biology. 2008; 59 :225-251
- 72. Gimeno-Gilles C, Lelievre E, Viau L, Malik-Ghulam M, Ricoult C, Niebel A, et al. ABA-mediated inhibition of germination is related to the inhibition of genes encoding cell-wall biosynthetic and architecture: Modifying enzymes and structural proteins in Medicago truncatula embryo axis. Molecular Plant. 2009; 2 (1):108-119
- 73. Corbineau F, Xia Q , Bailly C, El-Maarouf-Bouteau H. Ethylene, a key factor in the regulation of seed dormancy. Frontiers in Plant Science. 2014; 5 :539
- 74. Liu PP, Montgomery T, Fahlgren N, Kasschau KD, Nonogaki H, Carrington JC. Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. The Plant Journal. 2007; 52 :133-146
- 75. Hentrich M, Bottcher C, Duchting P, Cheng Y, Zhao Y, Berkowitz O, et al. The jasmonic acid signaling pathway is linked to auxin homeostasis through the modulation of YUCCA8 and YUCCA9 gene expression. Plant Journal. 2013; 74 :626-637
- 76. Toh S, Imamura A, Watanabe A, Nakabayashi K, Okamoto M, Jikumaru Y, et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiology. 2008; 146 :1368-1385
- 77. Cho JN, Ryu JY, Jeong YM, Park J, Song JJ, Amasino RM, et al. Control of seed germination by light- induced histone arginine demethylation activity. Developmental Cell. 2012; 22 :736-748
- 78. Oracz K, Karpiński S. Phytohormones signaling pathways and ROS involvement in seed germination. Frontiers in Plant Science. 2016; 7 :864
- 79. Bailly C, El-Maarouf-Bouteau H, Corbineau F. From intracellular signaling networks to cell death: The dual role of reactive oxygen species in seed physiology. Comptes Rendus Biologies. 2008; 331 :806-814
- 80. Wojtyla L, Lechowska K, Kubala S, Garnczarska M. Different modes of hydrogen peroxide action during seed germination. Frontiers in Plant Science. 2016; 7 :66
- 81. Zheng C, Jiang D, Liub F, Dai T, Liu W, Jing Q , et al. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity. Environmental and Experimental Botany. 2009; 67 :222-227
- 82. Munns R. Comparative physiology of salt and water stress. Plant, Cell & Environment. 2002; 25 :239-250
- 83. Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008; 59 :651-681
- 84. Jahromi F, Aroca R, Porcel R, Ruiz-Lozano JM. Influence of salinity on the in vitro development of Glomus intraradices and on the in vivo physiological and molecular responses of mycorrhizal lettuce plants. Microbial Ecology. 2008; 55 :45-53
- 85. Daszkowska-Golec A. Arabidopsis seed germination under abiotic stress as a concert of action of phytohormones. OMICS. 2011; 15 :763-774
- 86. Parida AK, Das AB. Salt tolerance and salinity effects on plants: A review. Ecotoxicology and Environmental Safety. 2005; 60 (3):324-349
- 87. Panda SK, Khan MHG. Growth, oxidative damage and antioxidant responses in Greengram ( Vigna radiata L.) under short-term salinity stress and its recovery. Journal of Agronomy and Crop Science. 2009; 195 :442-454
- 88. El-Hendawy S, Elshafei A, Al-Suhaibani N, Alotabi M, Hassan W, Dewir YH, et al. Assessment of the salt tolerance of wheat genotypes during the germination stage based on germination ability parameters and associated SSR markers. Journal of Plant Interactions. 2019; 14 (1):151-163
- 89. Lee SC, Luan S. ABA signal transduction at the crossroad of biotic and abiotic stress responses. Plant, Cell & Environment. 2012; 35 :53-60
- 90. Weiss D, Ori N. Mechanisms of cross talk between gibberellin and other hormones. Plant Physiology. 2007; 144 :1240-1246
- 91. Kaneko M, Itoh H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M. The α-amylase induction in endosperm during rice seed germination is caused by gibberellin synthesized in epithelium. Plant Physiology. 2002; 128 :1264-1270
- 92. Flowers TJ, Colmer TD. Salinity tolerance in halophytes. The New Phytologist. 2008; 179 (4):945-963
- 93. Chapman VJ. Salt Marshes and Salt Deserts of the World. New York: Interscience Publishers; 1960
- 94. Wang L, Huang Z, Baskin CC, Baskin JM, Dong M. Germination of dimorphic seeds of the desert annual halophyte Suaeda aralocaspica (Chenopodiaceae), a C4 plant without kranz anatomy. Annals of Botany. 2008; 102 :757-776
- 95. Woodell SRJ. Salinity and seed germination patterns in coastal plants. Vegetation. 1985; 61 :223-229
- 96. Redondo S, Rubio-Casal AE, Castillo JM, Luque CJ, Álvarez AA, Luque T, et al. Influences of salinity and light on germination of three Sarcocornia taxa with contrasted habitats. Aquatic Botany. 2004; 78 :255-264
- 97. Huang Z, Zhang X, Zheng G, Gutterman Y. Influence of light, temperature, salinity and storage on seed germination of Haloxylon ammodendron . Journal of Arid Environments. 2003; 55 :453-464
- 98. Khan MA, Gul B, Weber DJ. Effect of salinity and temperature on the germination of Kochia scoparia. Wetlands Ecology and Management. 2001; 9 :483-489
- 99. Orlovsky NS, Japakova UN, Shulgina I, Volis S. Comparative study of seed germination and growth of Kochia prostrata and Kochia scoparia (Chenopodiaceae) under salinity. Journal of Arid Environments. 2011; 75 :532-537
- 100. El-Keblawy A, Al-Shamsi N. Effects of salinity, temperature and light on seed germination of Haloxylon salicornicum , a common perennial shrub of the Arabian deserts. Seed Science and Technology. 2008; 36 :679-688
- 101. Fos M, Alfonso L, Ferrer-Gallego PP, Laguna E. Effect of salinity, temperature and hypersaline conditions on the seed germination in Limonium mansanetianum an endemic and threatened Mediterranean species. Plant Biosystems - An International Journal Dealing with all Aspects of Plant Biology. 2020; 0 (0):1-7
- 102. Zia S, Khan MA. Effect of light, salinity and temperature on the germination of Limonium stocksii. Canadian Journal of Botany. 2004; 82 :151-157
- 103. Yildiz M, Cenkci S, Kargioglu M. Effects of salinity, temperature, and light on seed germination in two Turkish endemic halophytes, Limonium iconicum and L. lilacinum (Plumbaginaceae). Seed Science and Technology. 2008; 36 :646-656
- 104. Li J, Yin LY, Jongsma MA, Wang CY. Effect of light, hydro-priming and abiotic stress on seed germination, and shoot and root growth pyrethrum ( Tanacetum cinerariifolium ). Industrial Crops and Products. 2011; 34 :1543-1549
- 105. Causin HF, Bordón DAE, Burrieza H. Salinity tolerance mechanisms during germination and early seedling growth in Chenopodium quinoa Wild. genotypes with different sensitivity to saline stress. Environmental and Experimental Botany. 2020; 172 :103995
- 106. El Goumi Y, Fakiri M, Lamsaouri O, Benchekroun M. Salt stress effect on seed germination and some physiological traits in three Moroccan barley ( Hordeum vulgare L.) cultivars. Journal of Materials and Environmental Science. 2014; 5 (2):625-632
- 107. Khodarahmpour Z, Ifar M, Motamedi M. Effects of NaCl salinity on maize ( Zea mays L.) at germination and early seedling stage. African Journal of Biotechnology. 2012; 11 (2):298-304
- 108. Vahabinia F, Pirdashti H, Bakhshandeh E. Environmental factors’ effect on seed germination and seedling growth of chicory ( Cichorium intybus L.) as an important medicinal plant. Acta Physiologiae Plantarum. 2019; 41 (2):1-13
- 109. Wu H, Guo J, Wang C, Li K, Zhang X, Yang Z, et al. An effective screening method and a reliable screening trait for salt tolerance of Brassica napus at the germination stage. Frontiers in Plant Science. 2019; 10 :530
- 110. Chakraborty K, Bishi SK, Goswami N, Singh AL, Bhaduri D, Zala PV. Salinity-induced changes in seed germination and the expression profile of antioxidant enzymes in peanut as early and late responses in emerging radicles. Acta Physiologiae Plantarum. 2019; 41 (8):1-16
- 111. Hakim MA, Juraimi AS, Begum M, Hanafi MM, Ismail MR, Selamat A. Effect of salt stress on germination and early seedling growth of rice ( Oryza sativa L.). African Journal of Biotechnology. 2010; 9 (13):1911-1918
- 112. Pakyürek M, Dumanoğlu H. Effects of saline stress on in vitro seed germination and seedling growth of some Turkish fig cultivars ( Ficus carica L.). Applied Ecology and Environmental Research. 2019; 17 (6):13485-13492
- 113. Asaduzzaman M, Koetz E, Rahman A. Factors affecting seed germination and emergence of button grass ( Dactyloctenium radulans ) (R.Br.) P.Beauv. Weed Biology and Management. 2019; 19 (3):85-92
- 114. Zhu G, An L, Jiao X, Chen X, Zhou G, McLaughlin N. Effects of gibberellic acid on water uptake and germination of sweet sorghum seeds under salinity stress. Chilean Journal of Agricultural Research. 2019; 79 (3):415-424
- 115. Rahman A, Asaduzzaman M. Statistical modelling of seed germination and seedlings root response of annual ryegrass ( Lolium rigidum ) to different stress. Agricultural Research. 2019; 8 (2):262-269
- 116. Lavrenko SO, Lavrenko NM, Lykhovyd PV. Effect of degree of salinity on seed germination and initial growth of chickpea ( Cicer arietinum ). Biosystems Diversity. 2019; 27 (2):101-105
- 117. Moles TM, Guglielminet L, Reyes TH. Differential effects of sodium chloride on germination and post-germination stages of two tomato genotypes. Scientia Horticulturae. 2019; 257 :108730
- 118. Panta S, Flowers T, Lane P, Doyle R, Haros G, Shabala S. Halophyte agriculture: Success stories. Environmental and Experimental Botany. 2014; 107 :71-83
- 119. Francois LE, Maas EV. Crop response and management on salt-affected soils. In: Pessarakli M, editor. Handbook of Plant and Crop Stress. New York: Marcel Dekker; 1994. pp. 149-181
- 120. Ali E, Hussain N, Shamsi IH, Jabeen Z, Siddiqui MH, Jiang L. Role of jasmonic acid in improving tolerance of rapeseed ( Brassica napus L.) to Cd toxicity. Journal of Zhejiang University: Science B (Biomedicine & Biotechnology). 2018; 19 (2):130-146
- 121. Esechie HA, Al-Saidi A, Al-Khanjari S. Effect of sodium chloride salinity on seedling emergence in chickpea. Journal of Agronomy and Crop Science. 2002; 188 :155-160
- 122. Maiti R, Pramanik K. Vegetable seed priming: A low cost, simple and powerful techniques for farmers’ livelihood. International Journal of Bio-resource and Stress Management. 2013; 4 :475-481
- 123. Hussain T, Koyro HW, Huchzermeyer B, Khan MA. Eco-physiological adaptations of Panicum antidotale to hyperosmotic salinity: Water and ion relations and anti-oxidant feedback. Flora. 2015; 212 :30-37
- 124. Lemmens E, Deleu LJ, De Brier N, De Man WL, De Proft M, Prinsen E, et al. The impact of hydro-priming and Osmo-priming on seedling characteristics, plant hormone concentrations, activity of selected hydrolytic enzymes, and Cell Wall and Phytate hydrolysis in sprouted wheat ( Triticum aestivum L.). ACS Omega. 2019; 4 (26):22089-22100
- 125. del Carmen Martínez-Ballesta M, Egea-Gilabert C, Conesa E, Ochoa J, Vicente MJ, Franco JA, et al. The importance of ion homeostasis and nutrient status in seed development and germination. Agronomy. 2020; 10 (4):504
- 126. Kilic S, Kahraman A. The mitigation effects of exogenous hydrogen peroxide when alleviating seed germination and seedling growth inhibition on salinity-induced stress in barley. Polish Journal of Environmental Studies. 2016; 25 (3):1053-1059
- 127. Ahammed GJ, Li Y, Li X, Han WY, Chen S. Epigallocatechin-3-Gallate alleviates salinity-retarded seed germination and oxidative stress in tomato. Journal of Plant Growth Regulation. 2018; 37 (4):1349-1356
- 128. Feghhenabi F, Hadi H, Khodaverdiloo H, van Genuchten MT. Seed priming alleviated salinity stress during germination and emergence of wheat (Triticum aestivum L.). Agricultural Water Management. 2020; 231 :106022
- 129. Tsegay BA, Andargie M. Seed priming with gibberellic acid (GA 3) alleviates salinity induced inhibition of germination and seedling growth of Zea mays L., Pisum sativum Var. abyssinicum A. Braun and Lathyrus sativus L. Journal of Crop Science and Biotechnology. 2018; 21 (3):261-267
- 130. Ren Y, Wang W, He J, Zhang L, Wei Y, Yang M. Nitric oxide alleviates salt stress in seed germination and early seedling growth of pakchoi ( Brassica chinensis L.) by enhancing physiological and biochemical parameters. Ecotoxicology and Environmental Safety. 2020; 187 :109785
- 131. Zhang DW, Vu TS, Huang J, Chi CY, Xing Y, Fu DD, et al. Effects of calcium on germination and seedling growth in melilotus officinalis L. (Fabaceae) under salt stress. Pakistan Journal of Botany. 2019; 51 :1-9
- 132. Luis Castañares J, Alberto Bouzo C. Effect of exogenous melatonin on seed germination and seedling growth in melon ( Cucumis melo L.) under salt stress. Horticultural Plant Journal. 2019; 5 (2):79-87
- 133. Mahboob W, Khan A. Seed priming modulates germination potential, osmoprotectants accumulation and ionic uptake in wheat seedlings under salt stress screening for abiotic stress view project screening of salt tolerant wheat genotypes view project. International Journal of Agricultural and Biology. 2019; 22 :594-600
- 134. Maswada HF, Djanaguiraman M, Prasad PVV. Seed treatment with nano-iron (III) oxide enhances germination, seeding growth and salinity tolerance of sorghum. Journal of Agronomy and Crop Science. 2018; 204 (6):577-587
- 135. Bouallègue A, Souissi F, Nouairi I, Souibgui M, Abbes Z, Mhadhbi H. Physiological and biochemicals changes modulated by seeds’ priming of lentil ( Lens culinaris L.) under salt stress at germination stage. Acta Scientiarum Polonorum Hortorum Cultus. 2019; 18 (5):27-38
- 136. Liu J, Li L, Yuan F, Chen M. Exogenous salicylic acid improves the germination of Limonium bicolor seeds under salt stress. Plant Signaling & Behavior. 2019; 14 :10
- 137. Khan WUD, Aziz T, Waraich EA, Khalid M. Silicon application improves germination and vegetative growth in maize grown under salt stress. Pakistan Journal of Agricultural Sciences. 2015; 52 (4):937-944
- 138. Chauhan A, Abuamarah BA, Kumar A, Verma JS, Ghramh HA, Ali K, et al. Influence of gibberellic acid and different salt concentrations on germination percentage and physiological parameters of oat cultivars. Saudi Journal of Biological Sciences. 2019; 26 (6):1298-1304
- 139. Gou T, Chen X, Han R, Liu J, Zhu Y, Gong H. Silicon can improve seed germination and ameliorate oxidative damage of bud seedlings in cucumber under salt stress. Acta Physiologiae Plantarum. 2020; 42 (1):1-11
- 140. Li J, Zhao C, Zhang M, Yuan F, Chen M. Exogenous melatonin improves seed germination in Limonium bicolor under salt stress. Plant Signaling & Behavior. 2019; 14 (11):1659705
- 141. Khan MA, Gul B, Weber DJ. Action of plant growth regulators and salinity on the seed germination of Ceratoides lanata . Canadian Journal of Botany. 2004; 82 :37-42
- 142. Wu YP, Chen F, Hu XW, Baskin CC, Baskin JM. Alleviation of salinity stress on germination of Leymus chinensis seeds by plant growth regulators and nitrogenous compounds under contrasting light/dark conditions. Grass and Forage Science. 2016; 71 (3):497-506
- 143. Figueira C, Ferreira MJ, Silva H, Cunha A. Improved germination efficiency of Salicornia ramosissima seeds inoculated with Bacillus aryabhattai SP1016-20. The Annals of Applied Biology. 2019; 174 (3):319-328
- 144. Waqas M, Korres NE, Khan MD, Nizami A, Deeba F, Ali I, et al. Advances in the concept and methods of seed priming. In: Hasanuzzaman M, Fotopoulos V, editors. Priming and Pretreatment of Seeds and Seedlings. Singapore: Springer; 2019. pp. 11-41
- 145. Nawaz J, Hussain M, Jabbar A, Nadeem GA, Sajid M, Subtain M, et al. Seed priming a technique. International Journal of Agriculture and Crop Sciences. 2013; 6 (20):1373-1381
- 146. Di Girolamo G, Barbanti L. Treatment conditions and biochemical processes influencing seed priming effectiveness. Italian Journal of Agronomy. 2012; 7 :8-18
- 147. Abdelhamid MT, El-masry RR, Darwish DS, Abdalla MMF, Oba S, Ragab R, et al. Mechanisms of seed priming involved in salt stress amelioration. In: Hasanuzzaman M, Fotopoulos V, editors. Priming and Pretreatment of Seeds and Seedlings. Singapore: Springer; 2019. pp. 219-251
- 148. Niranjan Raj S, Shetty NP, Shetty HS. Seed bio-priming with Pseudomonas fluorescens isolates enhances growth of pearl millet plants and induces resistance against downy mildew. International Journal of Pest Management. 2004; 50 :41-48
© 2020 The Author(s). Licensee IntechOpen. This chapter is distributed under the terms of the Creative Commons Attribution 3.0 License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Continue reading from the same book
Edited by Shah Fahad
Published: 21 July 2021
By Haifa Abdulaziz S. Alhaithloul, Abdelghafar M. Abu...
1208 downloads
By Nawab Ali and Mohammad Akmal
756 downloads
By Tinashe Zenda, Songtao Liu and Huijun Duan
937 downloads
IntechOpen Author/Editor? To get your discount, log in .
Discounts available on purchase of multiple copies. View rates
Local taxes (VAT) are calculated in later steps, if applicable.
Support: [email protected]
- Science & Math
- Sociology & Philosophy
- Law & Politics
Lab Explained: Effect of Salinity on Seed Germination
- Lab Explained: Effect of Salinity…
Research Question
How does salinity affect the seed germination of Solanum lycopersicum?
Introduction
Relevance to Real world
Covering 14% of Australia and home to over two million people, the Murray Darling Basin is Australia’s largest and most iconic river system ( figure 1 ) (McCormick, 2015). As over one-third of Australia’s food supply is produced by the Basin (Discover Murray, 2016), increased salinity levels affect both Australia’s agriculture and the overall economy.
Deep beneath the Murray Darling Basin, lies salty groundwater which flows through the river, out Murray’s mouth, and into the ocean (Government of South Australia, 2019). Before the development of agriculture, local farming regions were covered by native vegetation. The native vegetation thrived from the rainfall, absorbing the water for survival. Therefore, less water is soaked up by the soil and moved into the salty groundwater.
Since there has been an increase in demand for agricultural supplies, the native vegetation has been cleared; replaced with irrigated crops. Resultantly, more water is absorbed by the soil, increasing the amount of water moving into the salty groundwater, transferring into the river. Key species including the Eucalyptus camaldulensis (river redgums) have specific salinity thresholds that are notwithstanding these rising levels; causing habitat loss for many species (Government of South Australia, 2019).
Furthermore, loss of habitat creates a struggle for survival of both flora and fauna as they are more vulnerable to environmental pressures.
Process of seed germination
The initial phase of plant growth is seed germination. Seed germination is recognised as the original sprouting from the seeds to the development into plants (Robb, 2017). Before germination occurs, environmental conditions must trigger the conversion of ‘food’ being released from the seed endosperm to energy for the seed embryo; conditions include water, temperature, sunlight and air.
Relative oxygen species (ROS) are chemically reactive species that contain oxygen (such as peroxides). When ROS are released, O 2 oxidises into different substances creating oxidative stress. ROS play a large role during seed germination (Arbor Assays, 2017). Maintaining the correct ROS levels between a small range will ensure germination of the seeds.
Varying to low, seeds will never leave dormancy and varying too high and the seeds will suffer extreme oxidative damage during seed and will be non-viable. During the early stages, seedlings are most vulnerable and heavily rely on water intake. Once a seed is exposed to favourable conditions, water and oxygen are absorbed through the seed coat.
The embryo cell begins to swell and the radicle emerges from the seed coats (figure 2) (University of Illinois Extension, 2019).
Effect of Salinity on Seed Germination
Plant stress is any unfavourable factors that alter a plant’s equilibrium, growth or development (Lichtenthaler, 1996) . During the development of seeds, the breakdown of ROS creates oxidative stress. In the presence and in increasing concentrations, salinity triggers a high accumulation of ROS levels, causing damage (AbdElgawad, et al., 2016). Dormancy is the prolonged development of seed embryos due to unsuitable conditions (R. B. & S. B., 1977) and until the appropriate environmental conditions are met, seeds will not germinate.
As mentioned above, water intake is vital to the development of seeds and exposure to salt stress increase the requirement of water. Osmosis is the diffusion of water from an area of high concentration water molecules to another in a lower concentration through a partially permeable membrane (BBC Bitesize, 2019).
In plants, water is attracted to the region of the cell membrane with the highest concentration of salt. If the highest concentration is outside the cell membrane, water escapes the cell to bond with the sodium chloride (figure 3) (Crowe, 2019). Osmotic stress is the abrupt change in the solute concentration around a cell, creating a rapid change in the movement of water across its cell membrane.
In the presence of increased salt concentrations, osmotic stress occurs as the water moves across the membrane towards the salt. Furthermore, increased sodium chloride concentrations accumulates the soluble solutes around the seeds, increasing the osmotic pressure. Additionally causing excessive uptake of ions, resulting in toxicity in the plant (Jones, 1986).
Applications to Similar Experiments
Similar studies, where Solanum Lycopersicum seeds were treated with increasing concentrations of sodium chloride, have shown that the germination rate decreased with increasing salinity and the germination period took longer (Singer, 1994; El-Habbasha et al ., 1996; Cuartero and Fernández-Muñoz, 1999). Correlating with these studies are the works of Meza et al . (2007), observing the same trend: germination decreased with increased salt concentrations.
While the following investigation will produce different values for the percentage of seeds germinating, based on the theory, the central relationship shared between the independent and dependent variables will be inverse. Different plants have different salinity thresholds. For this experiment, it is important to consider plants that are salt-tolerant (halophytes) to enable real-world applications.
Solanum Lycopersicum (tomato seeds) have an average tolerance. Therefore, they are suitable for determining how salinity affects seed germination.
To determine the effect of increasing concentrations of sodium chloride on the seed germination of Solanum Lycopersicum (tomato plant).
It is predicted that if the concentration of sodium chloride is increased, then the percentage of seed germination will decrease.
Hypothesis Justification
Before seeds can germinate, certain conditions must be suitable for the seed embryo to survive; temperature, water, sunlight and air (White, 2016). During dormancy, tomato seeds store ‘food’ in their endosperms. Adequate amounts of the correct water quality, and the ‘food’ will release from the endosperm and convert into energy for the development of the embryo.
During this experiment, the increasing salinity concentrations should create less acceptable water quality for the ‘food’ to be released and slowing the development of the embryo.
The hypothesis is validated by a study by Miquel et al., who during a similar experiment documented that salinity can affect the germination of seeds by producing osmotic potential, preventing water uptake (Kaymakanova, 2014). In this study, the results showed a decrease in the percentage of seeds that germinated as the salinity concentration increased.
Experimental Design
Table 1. Experimental design
| Variable Type: | Variable: | Description: | How it was measured/ controlled: |
| Independent | Concentration of Sodium Chloride | Concentration of Salt for trials (g): 0% (normal water)2%4%6% | Measured using a milligram scale based on measurements of parts per million/milligrams (mg) before the experiment began. |
| Dependent | Percentage of seed germination | Percentage of seed germination is the percentage of seeds that clearly show the emerged radicle. | The percentage of germination is measured by the number of radicles that have emerged within that petri dish. |
| Controlled | Amount of sprays for the seeds each day | The cotton wool buds lining the petri dishes must be kept damp at all times. | To ensure the cotton wool is always damp, each day the experimentalist will lightly spray each petri dish 6 times, targeting all areas. To ensure that the seeds do not drown, each time, the petri dish is investigated for loose drops of water. |
| Time of Experimentation | For one week, the experiment occurs twice each day. The seeds are sprayed at 7am and 5 pm. | Every day an alarm is kept to remind the experimentalist when to spray the trials, therefore keeping consistency. | |
| Source of water | The source and quality of water is controlled so that the trials will equally show the results. | The concentrations of salt are changed for the experiment to be 0%, 2%, 4% and 6%. To make these concentrations, see the calculations in . | |
| Time duration for experiments | During the experiments, it takes the same amount of time every day. | Spray bottles allow the whole surface area of the cotton wools to be covered at equal amounts. If I was to drop a small amount of water into the petri dishes, the water wouldn’t reach some of the seeds. | |
| Spray bottles, petri dishes, cotton wool and the type of tomato seed | The spray bottles, petri dishes, cotton wool and the types of tomato seed were all bought from the same supplier. | To ensure reliability, both the spray bottles and tomato seeds were all bought from Bunnings and then the petri dishes and the cotton wool were supplied by the school. | |
| Uncontrolled | Oxygen supply | Availability of oxygen in the air. | The oxygen supply is uncontrollable as the experiment is placed inside on a cupboard. |
| Ambient Temperature | The temperature of the room throughout the trials. | The ambient temperature does vary, however, there is nothing that can be changed to keep the temperature as a constant. |
- 2L x tap water
- 60g x Sodium Chloride
- 4 x 500ml Spray bottles
- 1 x Thistle Funnel
- 1 x Stirring rod
- 20 x Petri dishes
- 140 x Cotton wool
- 1 x Permanent marker
- 400 x Tomato seeds
- Table 2 – Example labels for the clear plastic petri dishes.
| Trial 1 – 0% | Trial 1 – 2% | Trial 1 – 4% | Trial 1 – 6% |
| Trial 2 – 0% | Trial 2 – 2% | Trial 2 – 4% | Trial 2 – 6% |
| Trial 3 – 0% | Trial 3 – 2% | Trial 3 – 4% | Trial 3 – 6% |
| Trial 4 – 0% | Trial 4 – 2% | Trial 4 – 4% | Trial 4 – 6% |
| Trial 5 – 0% | Trial 5 – 2% | Trial 5 – 4% | Trial 5 – 6% |
- Table 3 – Example labels for the spray bottles.
| 0% Concentration | 2% Concentration | 4% Concentration | 6% Concentration |
- Calculate the correct amount of salt needed for the concentrations ( appendix 1 ).
- With the scales, weigh the correct amount of salt needed for the spray bottles (10g, 20g and 30g) and place into the bottom of the respective empty spray bottles using the funnel.
- Using the stirring rod, combine the salt and tap water for the spray bottles: 2%, 4% and 6%. Stop stirring once the salt has completely dissolved.
- Repeat step 6 for the following 19 trials.
- Count 20 seeds for the petri dish and gently place on top of the cotton wool ( figure 5 ).
- Seeds need to be placed at an approximate even distance away from each other.
- Repeat step 7 for the following 19 trials.
- Spray the seeds in the petri dish with the respective spray bottle until it is completely wet (ten sprays).
- There should be no standing water at the bottom of the dish.
- Repeat step 8 for the following 19 trials.
- Repeat step 9 for the following 19 trials.
- Twice a day, spray the correct dishes with the respective spray bottle (ten sprays).
- Repeat step 9 and 10 for the following 19 trials.
- After seven days of trialing, count and record the number of seeds which have germinated in each dish.
Preliminary Results
Table 4 – Number of Seeds Germinating at increasing sodium chloride concentrations
| Sodium Chloride Concentration (%) | |||||
| 0 | 2 | 4 | 6 | ||
| Number of Seeds Germinated | 1 | 12 | 5 | 0 | 0 |
| 2 | 15 | 3 | 0 | 0 | |
| 3 | 13 | 4 | 0 | 0 | |
| 4 | 11 | 0 | 0 | 0 | |
| 5 | 14 | 2 | 0 | 0 |
Table 5 – Percentage of Seeds Germinating at increasing sodium chloride concentrations (calculation method is shown in Appendix 1 )
| Sodium Chloride Concentration (%) | |||||
| 0 | 2 | 4 | 6 | ||
| Percentage of Seeds Germinated | 1 | 60 | 25 | 0 | 0 |
| 2 | 75 | 15 | 0 | 0 | |
| 3 | 65 | 2 | 0 | 0 | |
| 4 | 55 | 0 | 0 | 0 | |
| 5 | 70 | 10 | 0 | 0 |
Graph 1 – Preliminary Results of Seed Germination
The concentrations of salinity tested for this experiment is 0%, 2%, 4% and 6%. As predicted, the most percentage of germination is from the concentration with no salinity. However, after 2%, there is no germination from the seeds. Solanum lycopersicum is asalt-tolerant plant, therefore there should be an increase in the germination percentages.
In comparison to an investigation with similar purpose (Demir, Mavi, Ozcoban, & Okcu, 2003), the results showed that 4% and 6% still had small percentages of seeds germinating. Therefore, the method needs to be altered to increase the seeds germinating. Observations within the preliminary trials revealed that seeds were becoming dehydrated as there was not enough water supplied, the cotton wool the seeds were placed on was thinly spread and the location was in a windy environment.
Improving these factors could potentially show the desired results.
Table 6 – Method Refinements
| Method Refinements | Justification |
| Location | In the preliminary trials, the petri dishes were placed on a table outside, exposed to light, wind and varying temperatures. As the location is a controlled variable, I decided it was best if I placed them on a table inside a bedroom, at an ambient temperature. |
| Number of sprays | Observations from the preliminary trials include dryness of the cotton wool and crystallisation of the sodium chloride. Both factors are due to low amounts of water. Therefore, by increasing the number of sprays from six to ten, the trials are always damp. |
| Amount of cotton wool | Cotton wool, during the experiment, acts as soil in the real world context. Cotton wool absorbs the water, but maintains dampness for long periods of time, depending on the amount of sprays and the amount of cotton wool. Therefore, the more cotton wool there is, the longer the seeds will be in damp conditions. Therefore, the amount of cotton wool used has been increased from five pieces to eight. |
Safety Considerations / Risk Assessment
+ A risk assignment has been attached, see appendix 2 .
Table 7 – The table below exhibits the safety considerations for this experiment.
| Object | Hazards | Control Measures |
| Tomato Seeds | Allergies to seeds. Avoid eating the seeds as they may be treated with toxic fungicides. | Keep dry |
| Water | Spillage. Chemical contamination | If water is spilled, clean immediately or use a wet floor sign to indicate the spillage. Water in a laboratory should not be drunk, due to the possibility of chemical contamination. |
| Spray Bottles | May be used to spray others. | Use responsibly. Avoid the eyes |
Table 8 – Number of Seeds Germinating at increasing sodium chloride concentrations
| Sodium Chloride concentration (%) | |||||
| 0 | 2 | 4 | 6 | ||
| Trials | 1 | 17 | 12 | 8 | 3 |
| 2 | 15 | 13 | 11 | 5 | |
| 3 | 18 | 10 | 10 | 4 | |
| 4 | 19 | 9 | 9 | 3 | |
| 5 | 16 | 15 | 6 | 2 |
Processed Data
+ The following results tables has been calculated using the method in ( appendix 1)
Table 9 – Percentage of Seeds Germinating at increasing sodium chloride concentrations
| Sodium Chloride concentration (%) | |||||
| 0 | 2 | 4 | 6 | ||
| Trials | Trial 1 | 85 | 60 | 40 | 15 |
| Trial 2 | 75 | 65 | 55 | 25 | |
| Trial 3 | 90 | 50 | 50 | 20 | |
| Trial 4 | 95 | 45 | 45 | 15 | |
| Trial 5 | 80 | 75 | 30 | 10 |
Table 10 – Number of Seeds Germinating at increasing sodium chloride concentrations
| Sodium Chloride concentration (%) | ||||
| 0 | 2 | 4 | 6 | |
| Average Percentage of seeds germinated | 85 | 59 | 44 | 17 |
Graph 2 – Percentage of seeds germinated
Graph 2 reveals an inverse relationship between salinity and seed germination: as salinity increases, seed germination decreases. These results display a linear decrease and is proved to be considerably accurate as the R 2 value is 0.989.
Observations
- Over the course of the trialing period, most of the seeds began to germinate after the fourth day.
- During preliminary trials, with only 6 sprays, the cotton wool became hard and crystallised. However with 10 sprays, the cotton wool maintained the perfect dampness with no crystallization. Salt did develop around the seeds.
- Observing the seeds over the trialing period, showed that as the salinity increased, the less seeds were germinating.
Before seeds can germinate, the surrounding environment must display favourable conditions, causing the release of the energy stored within the embryo in the form of food. Seeds rely on the water intake to receive the proper nutrients to survive. In Graph 2 , the control trials (no sodium chloride) reached an average 85% seeds germinated. As the percentage of salinity increased, there is a noticeable decrease within the trials: 59%, 44% and finally 17% respectively.
The accuracy of these results are supported by the R 2 value which is 0.989. The closer the value is to 1, the more accuracy and close fitting to a trend line the data is. Thus it is fair to say that overall, the trials show a linear decrease. These results correlate with several different studies, supporting the hypothesis that there is a clear inverse relationship shared between both the independent and dependent variable (the concentration of sodium chloride and the percentage of seed germination respectively).
For the survival of any seed during the early stages, naturally there is a small percentage of sodium chloride. While a key component of the process of seed germination is relative oxygen species (ROS), when exposed to the salinity, oxygen species react to form oxidative stress on the seed (Arbor Assays, 2017). The levels of ROS are very delicate, too low leads to dormancy and too high causes damage and therefore it is important to understand the variables that can alter the equilibrium.
In relation to this investigation, approximately none of the seeds reached ROS levels too low to remain in dormancy, but rather they were too high the trials became damaged. Larger concentrations of salinity create an accumulation of high ROS levels, thus it is reasonable to say the seeds were damaged because of the intensity of the salinity (AbdElgawad, et al., 2016). Concentrations 0-2% showed the highest and most successful percentage of seed germination, in comparison to 4-6% which showed the lowest.
There are only a few requirements for the germination of seeds: proper oxygen, temperature, water, and nutrients. If seeds do not receive these vital components, they will experience dormancy. These components trigger the process of ‘food’ being released from the endosperm into energy for the seed embryo. Water and oxygen are absorbed through the seed coat (figure 2) and the radicle will begin to emerge. In this investigation, different concentration of salinity was tested on the seeds.
Water within the plant cell is attracted to the salt ions, and will quickly move across the cell membrane to be with the highest concentration of sodium chloride. This process is known as osmosis. However, under increasing sodium chloride concentrations, osmosis begins to be another form of stress on the plant. During the experiment, it is appropriate to conclude that within the larger sodium chloride concentrations, osmotic stress was interfering with the germination. As a result of the relationship between the variables, the larger the concentration, the less percentage of seed germination.
It can be expected that more water is needed to fully hydrate the seeds to maintain the ROS levels (Zhang, Irving, Tian, & Zhoua, 2012). Consequently, the more water required increases the osmotic pressure. Furthermore, increased sodium chloride concentrations accumulate the soluble solutes around the seeds, increasing the osmotic pressure (Jones, 1986).
Due to the osmotic pressure created by sodium chloride, water can no longer enter the seed coat; instead, salt enters and causes toxicity to plants. This leads to underdeveloped growth and reduction in yield (Cocoponics, 2011). Based on the observations, the seeds with the higher concentrations all appeared dehydrated, in comparison to the 0-2% trials which were damp. The dehydration and resultantly little germination of the seeds is supported by the concept of osmotic pressure.
Overall within the experiment, the comparison percentage germination between 0% concentration and 6% concentration is 68%. The results show that the best concentration for the most percentage of seed germination occurs under the concentration of no salinity (0%). However, in relation to the Murray Darling Basin, there will always be some form of salinity flowing through the basin to escape at the Murry mouth, due to the salty groundwater below the surface.
While the ocean has a salinity percentage of 3.5% (Science Daily, 2017), the Murray Darling Basin is considered a combination of many fresh water rivers. Therefore, the average salinity percentage must be lower than 3.5%. Concentrations of 4% and 6% would have devastating effects on the ecosystem where as 2% will not. From the above results, it can be concluded that if salinity percentage of the Murray Darling Basin remains below 2%, the ecosystem will flourish.
Concluding the results from several different reports and studies (Singer, 1994; El-Habbasha et al ., 1996; Cuartero and Fernández-Muñoz, 1999) and the results from this experiment, the hypothesis has been supported, outlining the clear linear inverse relationship between the concentration of salinity and the percentage of seed germination.
During this investigation, the experiment produced the results that were hypothesised. However, the results would not have been as valid if preliminary trials were not completed. Preliminary trials aim to insure accuracy and effectiveness within the method. In comparison to the primary data, the preliminary trials did not perform as well, due to not enough water, in a dry sunny location and too many cotton wool balls.
Resultantly, these issues were fixed in the method refinements. Despite the actions to increase reliability and accuracy, there are a few sources that can risk and effect the overall results due to errors. The table below (table 11) outlines the sources of potential error.
Table 11 – Sources, effect and solution for potential errors
| Source of Error | Error | Effect |
| Number of Trials | Only 5 trials were conducted for each concentration. As five is a small number, the results are very limited and provide a large amount of error. | Having a smaller number of trials limits the overall results and therefore the conclusion of the experiment (whether the hypothesis is supported or rejected). A smaller number of trials decrease the reliability of this experiment. |
| Number of Concentrations and the percentage of each | During the experiment the concentrations used were 0%, 2%, 4% and 6%. These concentrations could potentially limit the results. | During the experiment, the results found that even at 6% salinity, there were a few seeds still germinating. This limits the results as there could have been germination at 8%. |
| Trialing period | Trialing period for the seed germination only ran for seven days. The cut off for trials could limit the results. | The trialing period ran for only seven days, despite there being enough time during the investigation to increase the number of days. Overall, different percentages or trends may have emerged and therefore effecting whether the hypothesis is supported or rejected. |
| Type of plant species | The experiment was only limited to Solanum lycopersicum (tomato seeds). | Solanum lycopersicum (tomato seeds) are considered salt-tolerant plants. When determining the effect of salinity on germination, both halophytes and glycophytes. Therefore, the results only provide the answer to how do salt-tolerant plants react to salinity, instead of including all types. |
| Not washing the seeds before | None of the seeds were washed before the experiment occurred. | An observation on the germination of the seeds was that it was only within the last few days did the seeds begin to germinate. A potential error is not washing the seeds before the experiment. This alters the seeds natural defences against environmental stressors, ultimately allowing an increase in the rate of germination (Rhoades, 2018). |
| Parallax Error | Displacement of measurement positions can occur when viewing measuring equipment at an oblique angle. Parallax error can cause altered perceptions and reading measurements. | Contributes to minor errors within measuring amount of materials for different concentrations and measuring the radius of inhibition. This further affects accuracy of results. |
Improvements to experiment
For better and improved results for future experiments, improvements can be used to increase the accuracy. An adjustment as simple as increasing the number of trials from 5 per concentration to 10 per concentration creates a more reliable data plot as the results are focused on a widespread of data. More trials allows room for outliers and can fully represent the data being displayed.
The more trials completed, allows for the experimentalist to give a properly educated conclusion after the experiment. As this investigation is specifically focused on the vegetation surrounding the Murray Darling Basin, future experiments should expand the types of seeds being studied to the rather prominent native halophyte seeds that are crucial to the environment.
Furthermore, increasing the range between the concentrations, and the different types of seeds, allows for experimentalists to determine which seeds have a higher/ lower salt tolerance and will survive in the changing environment. Additionally, this will create a widespread of data, allowing for all trends and outliers.
Another improvement to further this study can be to extend the trialing period focus on growth rates as well as germination. If future experiments can determine the growth rates of surrounding local plants to the Murray Darling Basin and the seed germination while exposed to environmental stressors, farmers can determine better seeds to plant in certain paddocks based on those factors; increasing both the agricultural and economical value of Australia.
This investigation supported the hypothesis that if the concentration of sodium chloride is increased, then the percentage of seed germination will decrease. After the first few days, the seeds began to germinate; the most being 0% followed in a linear decrease by 2%, 4% and finally 6%.
The importance of these results is that it can give an idea as to the appropriate levels of salinity the Murray Darling Basin must be maintained at in order to achieve a thriving ecosystem. In the future, further studies can extend to the growth rates of plants and environmental factors cause harm. Further studies can be used to support the decisions made affecting the Murray Darling Basin to minimise the impact on the environment.
Appendix 1 – Calculations
AbdElgawad, H., Zinta, G., Hegab, M. M., Pandey, R., Asard, H., & Abuelsoud, W. (2016, March 8). High Salinity Induces Different Oxidative Stress and Antioxidant Responses in Maize Seedlings Organs . Retrieved from US National Library of Medicine – National Institutes of Health: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4781871/
Arbor Assays. (2017, October 5). Effects of Oxidative Stress on Germination . Retrieved from Arbor Assays: http://www.arborassays.com/effects-oxidative-stress-germination/
BBC Bitesize. (2019). Transport in cells – AQA . Retrieved July 17, 2019, from BBC Bitesize: https://www.bbc.com/bitesize/guides/zs63tv4/revision/4
Cocoponics. (2011, October 3). How salt affects Seed Germination . Retrieved from Cocoponics Community: http://www.cocoponics.co/germination-2/salt-affects-seed-germination
Crowe, B. (2019). What Does a High Concentration of Salt Do to a Cell Membrane? Retrieved July 17, 2019, from Seattle PI: https://education.seattlepi.com/high-concentration-salt-cell-membrane-4414.html
Cuartero, J., & Fernández-Muñoz, R. (1998, November 30). Tomato and salinity. Scientia Horticulturae, 78 (1-4), 83-125.
Demir, I., Mavi, K., Ozcoban, M., & Okcu, G. (2003). Effect of salt stress on germination and seedling growth in serially harvested aubergine (Solanum melongena L.) seeds during development. Israel Journal of Plant Sciences, 51 (2), 125-131.
Discover Murray. (2016). Murray Darling Basin . Retrieved from Discover Murray: http://www.murrayriver.com.au/about-the-murray/murray-darling-basin/
El-Habbasha, K., Shaheen, A., & Rizk, F. (1998). Germination of some tomato cultivars as affected by salinity stress condition. Egyptian Documentation and Information Centre for Agriculture, 23 (2), 179-190.
Government of South Australia. (2019, March 3). Salinity . Retrieved from Natural Resources SA Murray Darling Basin: https://www.naturalresources.sa.gov.au/samurraydarlingbasin/water/river-murray/issues-for-river-health/salinity
Jones, R. (1986, June). High salt tolerance potential in Lycopersicon species during germination. Euphytica, 35 (2), 575–582. Retrieved from Springer Link: https://link.springer.com/article/10.1007/BF00021866
Kaymakanova, M. (2014). Effect of Salinity on Germination and Seed Physiology in Bean (Phaseolus Vulgaris L.). Agricultural University Plovdiv. Bulgaria: Taylor and Francis Group, LLC.
Kranner, I., Minibayeva, F. V., Beckett, R. P., & Seal, C. E. (2010, September 10). What is stress? Concepts, definitions and applications in seed science . Retrieved from New Phytologist: https://nph.onlinelibrary.wiley.com/doi/10.1111/j.1469-8137.2010.03461.x
Lichtenthaler. (1996). Vegetation stress: An introduction to the stress concept in plants. PLANT PHYSIOLOGY , 4-14. Retrieved from Web of Science: http://cel.webofknowledge.com/InboundService.do?customersID=atyponcel&smartRedirect=yes&mode=FullRecord&IsProductCode=Yes&product=CEL&Init=Yes&Func=Frame&action=retrieve&SrcApp=literatum&SrcAuth=atyponcel&SID=C3UyoChxpOizTuv7f5a&UT=WOS%3AA1996UL69800002
Mavi, K. (2008, October). Effect of salt and osmotic stresses on the germination of pepper seeds of different maturation stages . Retrieved from SciELO: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S1516-89132008000500004
McCormick, B. (2015). Parliament of Australia . Retrieved July 16, 2019, from Murray-Darling Basin management: https://www.aph.gov.au/About_Parliament/Parliamentary_Departments/Parliamentary_Library/pubs/BriefingBook44p/MurryDarlingBasin
Meza, N., Arizaleta, M., & Bautista, D. (2007). Efecto de la salinidad en la germinación y emergencia de semillas de parchita. Passiflora edulis f. flavicarpa, 24 (1), 69-80.
R. B., T., & S. B., H. (1977, June). Annual Reviews. Retrieved July 16, 2019, from Dormancy in Seeds: https://www.annualreviews.org/doi/pdf/10.1146/annurev.pp.28.060177.001555
Rhoades, H. (2018, August 5). How To Soak Seeds Before Planting And The Reasons For Soaking Seeds . Retrieved from Gardening How To: https://www.gardeningknowhow.com/garden-how-to/propagation/seeds/soaking-seeds.htm
Robb, A. (2017). Study.com . Retrieved July 16, 2019, from What is Seed Germination? – Definition, Process, Steps & Factors: https://study.com/academy/lesson/what-is-seed-germination-definition-process-steps-factors.html
Science Daily. (2017, August). Sea water . Retrieved from Science Daily: https://www.sciencedaily.com/terms/seawater.htm
Singer, S. M. (1997). Germination responses of some tomato genotypes as affected by salinity and temperature stress. Egyptian Documentation and Information Centre for Agriculture, 21 (1), 47-64.
University of Illinois Extension. (2019). Background Information . Retrieved from The Great Plant Escape: https://web.extension.illinois.edu/gpe/tg/c3-background.html
White, N. (2016, May 6). Conditions Necessary for Germination . Retrieved July 17, 2019, from Tisiawikispace2016: https://sites.google.com/site/tisiawikispace2016/mysciencewikipage/conditionsnecessaryforgermination
Zhang, H., Irving, L., Tian, Y., & Zhoua, D. (2012). Influence of salinity and temperature on seed germination rate and the hydrotime model parameters for the halophyte, Chloris virgata, and the glycophyte, Digitaria sanguinalis. South African Journal of Botany, 78 , 203-210.
Related Posts
- Lab Report: The Impacts of Salinity on Modern Agriculture
- Lab Explained: The Effect of Ocean Water and Distilled Water on Iron
- Phet Projectile Motion Lab: Lab Answers
- Common Ion Effect
- Soil Salinity
Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Post comment
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Open access
- Published: 30 June 2020
A Salt Tolerance Evaluation Method for Sunflower ( Helianthus annuus L.) at the Seed Germination Stage
- Wenhui Li 1 ,
- Huizhen Zhang 1 ,
- Youling Zeng 1 ,
- Lijun Xiang 2 ,
- Zhonghua Lei 2 ,
- Qixiu Huang 2 ,
- Tianye Li 1 ,
- Fei Shen 1 &
- Quan Cheng 1
Scientific Reports volume 10 , Article number: 10626 ( 2020 ) Cite this article
12k Accesses
51 Citations
Metrics details
- Plant breeding
- Plant stress responses
Salinity is a major abiotic stress that affects plant growth and development and leads to crop yield loss. Many crop species are more sensitive to salinity stress at the seed germination stage than at other developmental stages. Some studies have shown that sunflower is tolerant to salinity to a certain degree. However, no systematic screening data for sunflower germplasms are available for salinity stress. In this study, 552 sunflower germplasms with different genetic backgrounds were evaluated for salt tolerance. Among them, 30 and 53 sunflower germplasms were identified as highly salt-tolerant and salt-tolerant germplasms, respectively, while 80 and 23 were grouped as salt-sensitive and highly salt-sensitive materials, respectively. Of all the traits tested, the germination index and the germination vigor index were the two most reliable traits, showing the highest correlation with salt tolerance during the seed germination stage of sunflower. Thus, a highly efficient and reliable method for evaluating salinity tolerance of sunflower seed germination was established. These results provided a good foundation for studying salt-tolerance mechanisms and breeding highly salt-tolerant sunflower cultivars.
Similar content being viewed by others
Improving the genetic potential of okra ( Abelmoschus esculentus L.) germplasm to tolerate salinity stress

Development of a robust hydroponic method for screening of sunflower ( Helianthus annuus L.) accessions for tolerance to heat and osmotic stress
Investigation of salt tolerance in cotton germplasm by analyzing agro-physiological traits and ERF genes expression
Introduction.
Salinity is a major abiotic stress that affects plant growth and development, thus resulting in crop yield loss. High salt stress disrupts homeostasis of water potentials and ion distributions, leading to molecular damage, reduced growth and even cell death 1 . Worldwide, more than 800 million hectares of land are affected by salt, equivalent to 6% of the total land area 2 , affecting more than 20% of today’s agriculture 3 , 4 , 5 , 6 , 7 . A more efficient way to use land with saline soil is to screen existing germplasms and develop new crop varieties with high tolerance to salinity stress 8 . Sunflower ( Helianthus annuus L.) is the fifth most widely grown edible oil crop in the world, and its planting area exceeds 22.9 million hectares across 60 countries with a total value of over $40 billion annually 9 . The development and breeding of salt-tolerant sunflower varieties are very necessary and have enormous economic potential 10 , 11 , 12 , 13 . Thus far, crossbreeding is still a commonly used breeding method; however, the traditional breeding methods focus on screening germplasms with desired traits, such as those with high tolerance to salinity.
Sunflower is a crop with moderate salt- tolerance 14 . However, salinity stress is still a major constraint in sunflower breeding owing to inadequate rainfall failing to leach salt from the root zone and high evapotranspiration often exceeding rainfall 15 . Generally, the ability of a crop to survive and grow under saline conditions depends on its salt tolerance, which can vary among different crops and growth stages 16 . Seed germination is the first stage of crop growth and development during the plant life cycle. Thus, high germination ability of crops in saline soil is necessary for later growth and development. It has been reported that salt stress can lead to a significant reduction in germination rate, as it reduces the ability of plants to uptake water from the soil, resulting in the growth inhibition and yield loss. Accordingly, to accelerate salt tolerance breeding of sunflower, an effective method to evaluate and obtain salt-tolerant germplasms at the germination stage or other growth stages in sunflower is urgently needed. In the current study, we establish a high-quality reference for the screening and evaluation of salt-tolerant sunflower germplasms at the seed germination stage.
Evaluation methods for the screening of salt-tolerant germplasms have been developed in various plant species at the seed germination stage. However, the methods are inconsistent among different crops 2 , 17 . In oilseed rape, the fresh weight of shoot is an effective screening feature for salt tolerance at the germination stage 17 , and among the traits examined in a recent study, the germination index of sweet sorghum ( Sorghum bicolor (L.) Moench.) had the highest correlation with salt tolerance 2 . Salt tolerance is a complex quantitative trait, and the measurement of a single trait poorly reflects the tolerance of plants to stress. Membership function analysis is used to integrate more traits in order to screen and evaluate plant tolerance germplasms in various plant species 18 , 19 . The drought tolerance of wheat resources can be divided into five distinct grades according to mean and standard deviation 19 , 20 . Wu et al . applied multiple regression to establish a quantitative evaluation model for salt tolerance of rapeseed inbred lines at the germination stage 17 . For sunflower, there is currently no reliable method for evaluating and/or screening germplasms with salinity tolerance. A recent study showed that seed shape is a potential predictor of salt tolerance in sunflower 21 . Two additional studies have investigated the salt tolerance index of mature sunflower plants, but we considered it was also unreliable to provide an effective evaluation with insufficient samples 22 , 23 . In addition, there seems to be little research on salt tolerance at the seed germination stage in sunflower.
In this study, the optimal concentration for salt tolerance screening of sunflower germplasms was first determined. Subsequently, 552 sunflower germplasms (inbred lines) were phenotyped for a variety of traits at the seed germination stage, and a quantitative evaluation model developed from multiple regression analysis was established for salt tolerance. Besides, the germination index and the germination vigor index were found to be the two most reliable traits for salt tolerance of sunflower at the germination stage based on correlation analysis. These results improve the current basis for sunflower breeding and the exploration of salt tolerance mechanisms.
Materials and methods
Plant materials.
The seeds of 729 sunflower germplasms (maintained by the Institute of Economic Crops, Xinjiang Academy of Agricultural Sciences, China) were collected from different regions of China and other countries. These germplasms had different genetic backgrounds and were labeled with numbers that described each line. The first two numbers corresponded to harvest year of resource seeds, while the third represented the various types of lines; in the third numbers, 1 or 2 indicated restorer lines, and 6 indicated sterile lines or maintainer lines. A pre-experiment was first conducted to ensure the viability of all seeds based on high germination rates (>99%). Only such high-quality seeds were used in subsequent experiments to evaluate salt tolerance at the germination stage.
Determination of salt stress concentration
The ZSADT variety of oil sunflower is widely cultivated as a major parental line for sunflower breeding in Xinjiang, China, and it has some excellent agronomic traits, such as early maturity, dwarf phenotype, lodging resistance and large floral disc size. It was used to determine the optimal salt concentration for the salt-tolerant screening of sunflower germplasms and a method of salt tolerance evaluation was developed at the seed germination stage. NaCl concentrations in the test included 25, 50, 75, 100, 150, 200, 250, 300, and 400 mM NaCl. All seeds were shelled, sown in 9-cm-diameter Petri dishes and cultured in an incubator at 28 °C/18 °C (day/night) with 16 h light/8 h dark. Seeds treated with distilled water (0 mM NaCl) served as controls. Four biological replicates were designed per treatment in one experiment. When the radicle length reached half of the seed length, the seed was considered to be germinated. The combination of the germination rate and growth inhibition phenotype under salt stress was applied to determine the appropriate salt concentration.
Determination of physiological parameters under salt stress during seed germination
Eleven sunflower seeds from each germplasm line were sown and treated with 300 mM NaCl (as treatment) and distilled water (as control), respectively. Four biological replicates were conducted for each treatment.
The number of germinated seeds was recorded every day for one week. On the seventh day after sowing, the root length (RL) and fresh weight (FW) of the seedlings were measured. The dry weight (DW) was determined after the seedlings were dried in a 150 °C-oven until they reached a constant weight.
To evaluate the salt tolerance of sunflower germplasms at the seed germination stage, germination rate (GR), germination index (GI), germination energy (GE), germination vigor index (GVI), and water content (WC) were calculated with the following formula, respectively:
In each of these formula, T is the number of days after sowing, G t is the total number of germinated seeds on the T th day, G 1 and G 7 are the total numbers of seed germinated on the 1 st and 7 th days after sowing, N is the total number of seeds, and AFW is the average FW of seedlings.
To describe the differences in salt tolerance among sunflower germplasms, the salt tolerance index (STI) of each physiological parameter was also measured.
Here, STI i is the STI of trait i , V i n and V i c represent the values of trait i in the salt-stressed treatment and control, respectively. Each trait of each germplasm has its own STI.
Salt tolerance evaluation
The salt tolerance levels of sunflower germplasm lines were evaluated with the fuzzy comprehensive evaluation method using the membership function value (MFV) 21 . The salt tolerance MFV was calculated using the following equation:
Here, X i represents the membership function value of the i trait in a germplasm, X is the STI value of the i trait in the germplasm, X max and X min are the maximum and minimum values of the STI of the i trait observed in all germplasms, respectively. Therefore, each trait has its own MFV, ranging from 0 to 1.
According to a previously reported method 20 , the salt tolerance levels of sunflower germplasms were divided into five grades based on mean value ( \(\bar{X}\) ) and standard deviation (SD) of MFV: \((1){X}_{i}\ge \bar{X}+1.64SD\) , highly salt-tolerant (HST); \((2)\bar{X}+1.64SD > {X}_{i}\ge \bar{X}+1SD\) , salt-tolerant (ST); \((3)\bar{X}+1SD > {X}_{i}\ge \bar{X}-1SD\) , moderately salt-tolerant (MST); \((4)\bar{X}-1SD > {X}_{i}\ge \bar{X}-1.64SD\) , salt-sensitive (SS); \((5)\bar{X}-1.64SD > {X}_{i}\) , highly-saltsensitive (HSS).
Statistical analysis
SPSS 25 (IBM Corp., Armonk, NY, USA) was employed to perform multiple regression analysis on the mean MFV (the dependent Y variable) and STI value (the independent STI i variable). The following mathematical evaluation model for salt tolerance was established: \(Y={\beta }_{GR}ST{I}_{GR}+{\beta }_{GI}ST{I}_{GI}+{\beta }_{GE}ST{I}_{GE}+{\beta }_{RL}ST{I}_{RL}+\) \({\beta }_{FW}ST{I}_{FW}+{\beta }_{MC}ST{I}_{MC}+\mu \) , where Y represents the mean MFV, β is a nonnormalized coefficient, and μ is a constant representing the random error term.
To determine a suitable salt concentration for screening the salt tolerance of sunflower germplasms, ZSADT, a parental line, was used and the GR and germinated growth phenotype of ZSADT were recorded and photographed after 7 days of salt-stressed treatment (Fig. 1 ).
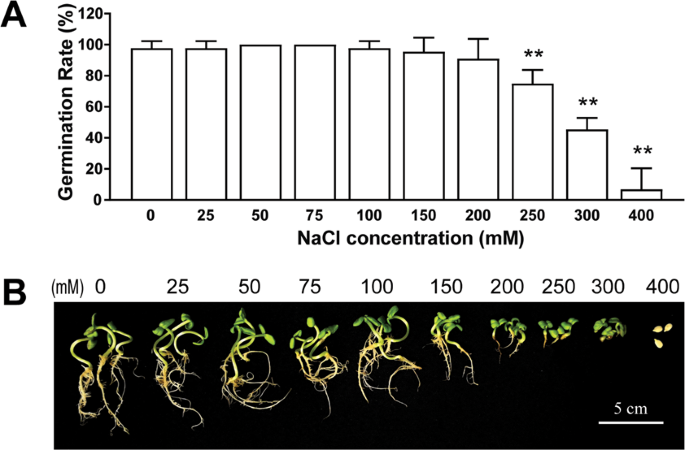
The optimum NaCl concentration for salt tolerance evaluation was determined according to the two indexes of germination rate and germination phenotype with the parental breeding line ZSADT. ( A ) Germination rate and ( B ) germination phenotype of ZSADT seeds treated with different NaCl concentrations for seven days. **Indicated significant difference ( p < 0.01) between treatment and control.
GR and seedling growth were significantly different among the different concentration treatments after 7 days (Fig. 1 , p < 0.01). For low salinity treatments, up to 200 mM NaCl, the germination rate was not affected. NaCl concentrations above 200 mM significantly inhibited sunflower seed germination in a dose-dependent manner. At a NaCl concentration of 300 mM, more than half of seeds did not germinate, while the 400 mM NaCl treatment inhibited almost all seed germination, with only 6.8% final germinating (Fig. 1A ). The phenotypic analysis under salt stress revealed that sunflower seedling growth and development after germination were more sensitive to salinity relative to seed germination. Although no significant growth inhibition were observed, when seedlings were exposed to NaCl concentrations up to 100 mM, 150 mM NaCl treatment significantly inhibited both leaf and root growth by day 7. Although the majority of seeds germinated when exposed to 200–300 mM NaCl, the growth of seedlings was almost completely inhibited (Fig. 1B ). To ensure that biomass would permit measurements of indicators such as root length, fresh weight and water content as well, 300 mM NaCl was selected for evaluation of salt tolerance among sunflower germplasms at the germination stage.
Effects of NaCl stress on plant traits of sunflower germplasm lines at the germination stage
Seeds from a total of 729 sunflower germplasm lines were harvested and made available. To ensure the accuracy of tests on salinity stress response, Pre-germination experiments were carried out to obtain the germplasm seeds with high vigor that the germination rate exceeded 99% for the screening of the salt tolerance, and finally, only 552 germplasm lines were candidates.
The STI value can be used to evaluate the effect of NaCl on the salt tolerance parameters of the sunflower germplasms. Larger STI values represent a smaller impact, while smaller values indicate greater impacts. The STI values under 300 mM NaCl for GR, GI, GE, RL, GVI, FW and WC of the 552 sunflower germplasm lines were shown in the supplementary material (Table S1 ). Under the 300 mM NaCl treatment, the GRs of four germplasm lines, 152021, 152094, 156084 and 156096, were significantly inhibited, but those of 10 other germplasms, including 151082, 152552, 152452 and 152400, were almost unaffected. Consistent with the GR results, the GI values of four germplasms, 152021, 152094, 156084 and 156096, were also significantly inhibited. GE was the most affected, with 328 germplasms having a GE of 0. The average STI value for GE among the 552 germplasms was only 0.062. The STI values of RL and GVI differed significantly among all germplasms. Because germination without cotyledon growth occurred, FW and WC of 27 germplasms couldn’t be measured. For 9 and 25 germplasms, FW and WC, respectively, were unaffected or slightly increased. Four germplasms, including 152021, did not germinate, and all parameters could not be measured, as they were apparently most affected by NaCl (Table S1 ).
To comprehensively evaluate the salt tolerance of 552 germplasm lines, the MFV for each parameter of each germplasm and mean MFV were calculated (Table S2 ). The distribution of mean MFV is shown in Fig. 2A . The mean MFV ranged from 0.152 to 0.715 with an average of 0.287 ± 0.143. Germplasm line 505 had the highest mean MFV; four germplasm lines, 156084, 152021, 152094 and 156096, had the lowest mean MFV (0.000).
Classification of 552 sunflower germplasm lines based on mean membership function values (mean MFV). ( A ) Distribution of mean MFVs. ( B ) Classification of 552 sunflower germplasms according to salt tolerance based on mean MFVs. HST, highly salt-tolerant; ST, salt-tolerant; MST, moderately salt-tolerant; SS, salt-sensitive; HSS: highly salt-sensitive.
Based on the salinity response and salt tolerance, 552 tested sunflower germplasms were divided into five grades: (1) 30 germplasm lines were highly salt-tolerant (HST, \({X}_{i}\ge \bar{X}+1.64SD\) , \({\rm{mean}}\,{\rm{MFV}}\,\ge \,0.5216\) ); (2) 53 were salt-tolerant (ST, \(\bar{X}+1.64SD > {X}_{i}\ge \bar{X}+1SD\) , 0.5216> mean MFV ≥0.4302); (3) 366 were moderately salt-tolerant (MST, \(\bar{X}+1SD > {X}_{i}\ge \bar{X}-1SD\) , \(0.4302 > {\rm{mean}}\,{\rm{MFV}}\ge 0.1446\) ); (4) 80 were salt-sensitive (SS, \(\bar{X}-1SD > {X}_{i}\ge \bar{X}-1.64SD\) , \(0.1446 > {\rm{mean}}\,{\rm{MFV}}\,\ge \,0.0532\) ); and (5) 23 were highly salt-sensitive (HSS, \(\bar{X}-1.64SD\, > \,{X}_{i}\) , 0.0532> mean MFV) (Fig. 2B , Table S2 ). The five most salt-tolerant and five most salt-sensitive germplasm lines were listed in Table 1 . Based on this study, the majority of sunflower germplasm lines were moderately salt-tolerant; only a small proportion of germplasm lines were highly tolerant or highly sensitive to salinity.
Correlation analysis of parameter STI
The correlation coefficients between different parameters were analyzed (Table 2 ). Among all the parameters assessed, the correlation between GR and GI was the highest ( r = 0.897), followed by FW and WC ( r = 0.812). The correlation between RL and GE was the lowest ( r = 0.192), followed by GE and WC ( r = 0.214). The correlation between GE or RL and other parameters was also low.
Mean MFV reflects the salt tolerance of germplasms. The larger the mean MFV of a germplasm is, the stronger its salt tolerance is. In this study, the STI of GR, GI, GE, RL, GVI, FW and WC together determined the mean MFV. Therefore, for each germplasm, the mean MFV value is determined by the STI of each parameter. To find the most reliable parameters reflecting salt tolerance, a linear model was fitted between each STI and mean MFV (Fig. 3 ). The coefficient of determination between the mean MFV and GI was the highest ( R 2 = 0.817), while those for GR, FW and GVI were slightly lower, ( R 2 = 0.784, R 2 = 0.719 and R 2 = 0.708, respectively. The coefficient of determination for RL was the lowest ( R 2 = 0.261). The normalized beta coefficients between mean MFV and GI as well as mean MFV and GVI were also higher. Overall, our results suggested that GI and GVI can be used as reliable traits to evaluate the salt tolerance of sunflower germplasm lines at the germination stage.
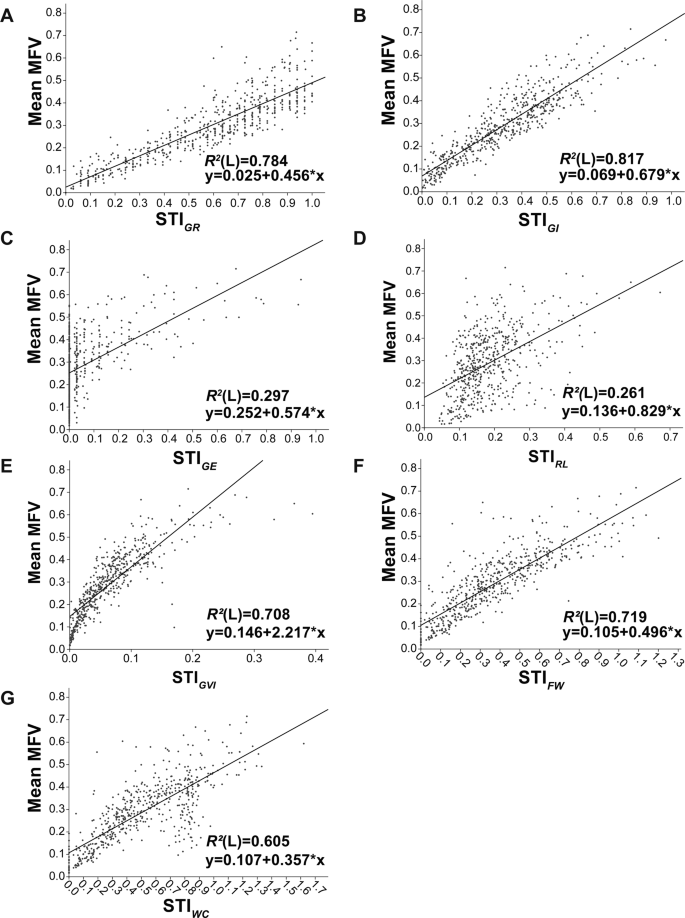
A linear correlation analysis between the STI values of each parameter and mean MFV. ( A ) Relationship between the STI of germination rate (STI GR ) and mean MFV; ( B ) Relationship between the STI of germination index (STI GI ) and mean MFV; ( C ) Relationship between the STI of the germination energy (STI GE ) and mean MFV; ( D ) Relationship between STI of root length (STI RL ) and mean MFV; ( E ) Relationship between STI of germination vitality index (STI GVI ) and mean MFV; ( F ) Relationship between STI of fresh weight (STI FW ) and mean MFV; ( G ) Relationship between the STI of water content (STI WC ) and mean MFV; R 2 (L) is the coefficient of determination.
Establishment of a model for evaluating salt tolerance in sunflower
Based on the mean MFV of 552 sunflower germplasm lines and STI values for seven parameters, a quantitative model for evaluating salt tolerance in sunflower was established; a linear equation was developed using multiple regression. As shown in Table 3 , the unstandardized coefficients of the STI for GR, GI, GE, RL, GVI, FW and WC were 0.143, 0.146, 0.152, 0.213, 0.369, 0.119 and 0.088, respectively. The random error term was −3.33 × 10 −16 . Therefore, \(Y=0.143\times {{\rm{STI}}}_{GR}+0.146\times {{\rm{STI}}}_{GI}+0.152\times {{\rm{STI}}}_{GE}+0.213\times {{\rm{STI}}}_{RL}+0.369\times {{\rm{STI}}}_{GVI}+0.119\times {{\rm{STI}}}_{FW}+0.088\times {{\rm{STI}}}_{WC}\)
where Y represented the salt tolerance of sunflower germplasm lines. According to the classification criteria of this study, the salt tolerance of sunflower germplasms could be divided into five grades. When \(Y\ge 0.5216\) indicated highly salt-tolerant (HST), \(0.5216 > Y\ge 0.4302\,\) indicated salt-tolerant (ST), \(0.4302 > Y\ge 0.1446\) indicated moderately salt-tolerant (MST), \(0.1446 > Y\ge 0.0532\) indicated salt-sensitive (SS), and Y < 0.0532 indicated highly salt-sensitive (HSS).
To test whether the quantitative evaluation model was useful for predicting the salt tolerance of the sunflower germplasms, the Y values of the 552 germplasms were calculated (Table S3 ) and datawere used from three random germplasms of each of the five salt-tolerance grades, as a total of 15, listed in Table 4 . The absolute value of the difference between Y and the mean MFV was also calculated (Table S3 and Table 4 ). The average difference between Y and mean MFV was only 0.000462, while the maximum and minimum were 0.002949 and 3.33 × 10 −16 , respectively. The values of mean MFV and Y were very close. Overall, our model was reliable and salt tolerance can be predicted by calculating the Y value of any sunflower germplasm using the STI values of growth parameters, such as GR, GI, GE, RL, GVI, FW and WC during the germination stage.
Throughout their long-term evolution, plants have established complex mechanisms for responding to different environmental stresses, including salinity. However, different germplasms within the same species show different responses to the same stress owing to germplasms being grown under different environmental conditions and/or being bred for different specific purposes. The ability of a plant to resist salt stress varies widely among species and varieties 24 . Generally, sunflower has moderate salt tolerance, but little has been reported about its resistance to salt stress 25 . Specifically, different germplasms have shown significant differences in their growth parameters under NaCl stress. The different responses of germplasms or genotypes to salt stress have previously been reported in other plant species 26 , 27 , 28 . Seed germination is the first stage of plant growth, and crops are more susceptible to stress during the germination stage. Salt stress is one of the most serious stresses faced by crops, and salinity can cause significant decreases in seed germination rate. When plant is planted in saline-alkali soil, the germination of salt-sensitive germplasm is significantly inhibited, resulting in crop yield loss and even plant death 29 , 30 . One potential mechanism is high NaCl stress causing dysfunction in seed metabolism, further resulting in inhibition of seed germination 31 . Studies have shown that the size and shape of seeds 32 , KNO 3 treatment 33 , antioxidant levels 34 and gene expression 35 can affect the salt tolerance of sunflower during germination. However, using a single feature to identify salt tolerance may be inadequate. The physiological and biochemical indexes related to salt tolerance can be used as criteria for the screening of salt tolerance. However, when more sunflower germplasms are used for screening, the process is cumbersome and time-consuming. In this study, a simple and efficient method of identifying salt tolerance through the effect of salt treatment on sunflower phenotypic characteristics was developed.
Salinity not only affects seed germination but also seedling growth and development. Thus, germination rate alone cannot accurately evaluate the salt tolerance of sunflower seed germination, other related traits should also be included involving in evaluating plant responses to salinity. In this study, we used multiple parameters to evaluate the salt tolerance of sunflower germplasms by mean MFV at the germination stage. Among the 552 sunflower germplasms, 83 showed salt tolerance, 366 showed moderate salt tolerance, and 103 showed salt sensitivity (Fig. 2 , Table S2 ). The maximum mean MFV was 0.715, indicating that 300 mM NaCl has a greater impact on sunflower seed germination across germplasms. Compared with salt-tolerant germplasms, some germplasms showed lower mean MFV, indicating that these germplasms had lower salt tolerance at the germination stage. Salt stress causes osmotic damage and ion stress to plants, resulting in accumulation of reactive oxygen species 36 . The salt-tolerant germplasms screened in this study may have superior active oxygen scavenging capacity, synthesize more osmotic adjustment substances and/or have accumulated more inorganic ions to obtain higher salt tolerance 37 , 38 .
Salt stress damages plants, leading to a variety of physiological and biochemical changes. However, not all parameters are useful in salt tolerance screening. In hybrid breeding, the lack of an accurate and reliable salt tolerance evaluation parameter is one of the factors limiting the success rate of conventional breeding for salt tolerance 39 . To more efficiently determine the salt tolerance of sunflower germplasms during germination, it is necessary to identify some reliable traits as indicators of salt tolerance. In this study, GI and GVI are reliable traits for evaluating the salt tolerance of sunflower germplasms.
Moreover, when defining the salt tolerance of one or several sunflower germplasms, it is difficult to judge the salt tolerance of the germplasms without a large number of other germplasms for comparison. To evaluate the salt tolerance of sunflower germplasms easily and reliably, a mathematical formula was established by multiple regression analysis, and the salt tolerance of sunflower germplasms was evaluated by calculating the Y value. According to the calculated Y value, the salt tolerance of the germplasms can be divided into five grades. The larger the Y value is, the higher the salt tolerance is. This study is the first to establish a mathematical evaluation model for salt tolerance of sunflower germplasms at the seed germination stage, and this regression formula can be applied to screen the salt tolerance of other sunflower germplasm lines for mechanical exploration and breeding of salt tolerance of sunflower.
GI and GVI are two reliable traits for evaluating the salt tolerance of sunflower germplasms under 300 mM NaCl treatment. A mathematical model was also developed to evaluate the salt tolerance at the germination stage. Based on the model, 552 sunflower germplasms were classified into five grades: 30 HST, 53 ST, 366 MST, 80 SS and 23 HSS. These results have important theoretical and practical values for the evaluation salt tolerance of sunflower germplasms and breeding new cultivars with high salt-tolerance as well as exploration of the mechanisms underlying salt tolerance.
Zhu, J. K. Plant salt tolerance. Trends Plant Sci. 6 , 66–71 (2001).
Article CAS Google Scholar
Ding, T. L. et al . Evaluation of salt-tolerant germplasm and screening of the salt-tolerance traits of sweet sorghum in the germination stage. Funct. Plant Biol. 45 , 1073–1081 (2018).
Mickelbart, M. V., Hasegawa, P. M. & Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 16 , 237–251 (2015).
Sumner, M. E. Sodic Soils:Distribution, Properties, Management and Environmental Consequences. 1 edn, (Oxford University Press, 1998).
Vinocur, B. & Altman, A. Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. Curr. Opin. Biotechnol. 16 , 123–132 (2005).
Song, J. & Wang, B. S. Using euhalophytes to understand salt tolerance and to develop saline agriculture: Suaeda salsa as a promising model. Ann. Bot. 115 , 541–553 (2015).
Yang, Z. & Wang, B. S. Present status of saline soil resources and countermeasures for improvement and utilization in china. Shandong Agricultural Sciences. 47 (4), 125–130 (2015).
MathSciNet Google Scholar
Ashraf, M. Y., Awan, A. R. & Mahmood, K. Rehabilitation of saline ecosystems through cultivation of salt tolerant plants. Pak. J. Bot. 44 , 69–75 (2012).
CAS Google Scholar
Hu, J. et al . Genetics, Genomics and Breeding of Sunflower. (Taylor & Francis, 2010).
Rauf, S. et al . Progress in modification of sunflower oil to expand its industrial value. J. Sci. Food Agric. 97 , 1997–2006 (2017).
Rauf, S., Shahzad, M., Teixeira da Silva, J. A. & Noorka, I. R. Biomass partitioning and genetic analyses of salinity tolerance in sunflower ( Helianthus annuus L.). J. Crop Sci. Biotech. 15 , 205–217 (2012).
Article Google Scholar
Guo, S. S., Ge, Y. & Jom, K. N. A review of phytochemistry, metabolite changes, and medicinal uses of the common sunflower seed and sprouts ( Helianthus annuus L.). Chem. Cent. J. 11 , 95 (2017).
Dimitrijevic, A. et al . Oleic acid variation and marker-assisted detection of Pervenets mutation in high- and low-oleic sunflower cross. Crop Breed. Appl. Biot. 17 , 235–241 (2017).
Shi, D. C. & Sheng, Y. M. Effect of various salt-alkaline mixed stress conditions on sunflower seedlings and analysis of their stress factors. Environ. Exp. Bot. 54 , 8–21 (2005).
Nolan, B. T. et al . Factors influencing ground-water recharge in the eastern United States. J. Hydrol. 332 , 187–205 (2007).
Article ADS Google Scholar
Akbari, G., Sanavy, S. A. & Yousefzadeh, S. Effect of auxin and salt stress (NaCl) on seed germination of wheat cultivars ( Triticum aestivum L.). Pak. J. Biol. Sci. 10 , 2557–2561 (2007).
Wu, H. et al . An effective screening method and a reliable screening trait for salt tolerance of Brassica napus at the germination stage. Front. Plant Sci. 10 , 530 (2019).
Liu, N. et al . Evaluation of mercury resistance and accumulation characteristics in wheat using a modified membership function. Ecol. Indic. 78 , 292–300 (2017).
Liu, Y. X. et al . Evaluation of agronomic traits and drought tolerance of winter wheat accessions from the usda-ars national small grains collection. Agronomy-Basel 7 , 51 (2017).
Chen, X. J., Min, D. H., Yasir, T. A. & Hu, Y. G. Evaluation of 14 morphological, yield-related and physiological traits as indicators of drought tolerance in Chinese winter bread wheat revealed by analysis of the membership function value of drought tolerance (MFVD). Field Crops Res. 137 , 195–201 (2012).
Wang, J., Zhao, Y. G., Pang, H. C., Zhang, L. & Li, Y. Y. Grain shape as a predictor of salt tolerance in sunflower. Agron. J. 108 , 2280–2288 (2016).
Zhang, J. L., Zhang, G. B. & Wang, D. Comparison and physiological index selection of salt tolerance on sunflower. Chinese Journal of Oil Crop Sciences 28 , 176–179 (2006).
Yu, H. F. et al . Analysis of yield related traits of sunflower salt tolerance. Acta Agriculturae Boreali-Sinica 28 , 192–198 (2013).
Google Scholar
Ashraf, M. & Wu, L. Breeding for salinity tolerance in plants. Crit. Rev. Plant Sci. 13 , 17–42 (1994).
Liu, X. N. & Baird, W. V. Differential expression of genes regulated in response to drought or salinity stress in sunflower. Crop Sci. 43 , 678–687 (2003).
Noreen, Z. & Ashraf, M. & Mahmood-Ul-Hassan. Inter-accessional variation for salt tolerance in pea ( Pisum sativum L.) at germination and screening stage. Pak. J. Bot. 39 , 2075–2085 (2007).
Maliro, M. F. A., McNeil, D., Redden, B., Kollmorgen, J. F. & Pittock, C. Sampling strategies and screening of chickpea ( Cicer arietinum L.) germplasm for salt tolerance. Genet. Resour. Crop Evol. 55 , 53–63 (2008).
Oyiga, B. C. et al . Identification and characterization of salt tolerance of wheat germplasm using a multivariable screening approach. J. Agron Crop. Sci. 202 , 472–485 (2016).
Khajeh-Hosseini, M., Powell, A. A. & Bingham, I. J. The interaction between salinity stress and seed vigour during germination of soyabean seeds. Seed Science and Technology 31 , 715–725 (2003).
Hakim, M. A. et al . Effect of salt stress on germination and early seedling growth of rice ( Oryza sativa L.). Afr. J. Biotechnol. 9 , 1911–1918 (2010).
Ayaz, F. A., Kadioglu, A. & Turgut, R. Water stress effects on the content of low molecular weight carbohydrates and phenolic acids in Ctenanthe setosa (Rosc.) Eichler. Can. J. Plant Sci. 80 , 373–378 (2000).
Kaya, M. D., Okçu, G., Atak, M., Çıkılı, Y. & Kolsarıcı, Ö. Seed treatments to overcome salt and drought stress during germination in sunflower ( Helianthus annuus L.). Eur. J. Agron. 24 , 291–295 (2006).
Zhang, J. & Kirkham, M. B. Antioxidant responses to drought in sunflower and sorghum seedlings. New phytol. 132 , 361–373 (1996).
Saadia, M., Jamil, A., Ashraf, M. & Akram, N. A. Comparative study of SOS2 and a novel PMP3-1 gene expression in two sunflower ( Helianthus annuus L.) lines differing in salt tolerance. Appl. Biochem. Biotechnol. 170 , 980–987 (2013).
Parida, A. K. & Das, A. B. Salt tolerance and salinity effects on plants: a review. Ecotoxicol. Environ. Saf. 60 , 324–349 (2005).
Munns, R. & Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59 , 651–68 (2008).
Ashraf, M. & Foolad, M. R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59 , 206–216 (2007).
Zeng, L., Shannon, M. C. & Grieve, C. M. Evaluation of salt tolerance in rice genotypes by multiple agronomic parameters. Euphytica 127 , 235–245 (2002).
Download references
Acknowledgements
This work was financially supported by the Key R & D projects in Xinjiang Uygur Autonomous Region (No. 2018B01006-2, cooperated with Xinjiang Academy of Agricultural Sciences), the “One Belt and One Road” biotechnology collaborative innovation project (No. BRI-ASTIP- MISACC-02-2017) and the Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. 2020D01C020).
Author information
Authors and affiliations.
Xinjiang Key Laboratory of Biological Resources and Genetic Engineering, College of Life Science and Technology, Xinjiang University, Urumqi, 830046, China
Wenhui Li, Huizhen Zhang, Youling Zeng, Tianye Li, Fei Shen & Quan Cheng
Institute of Economic Crops, Xinjiang Academy of Agricultural Sciences, Urumqi, China
Lijun Xiang, Zhonghua Lei & Qixiu Huang
You can also search for this author in PubMed Google Scholar
Contributions
W.L., Y.Z. and H.Z. designed the experiments and analyzed the data. L.X., Z.L. and Q.H. provided the sunflower germplasms used as experimental materials. W.L. and Y.Z. wrote the manuscript. W.L., H.Z., T.L., F.S. and Q.C. performed the experiments. All authors reviewed the manuscript.
Corresponding authors
Correspondence to Youling Zeng or Lijun Xiang .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary table s1, supplementary table s2, supplementary table s3, rights and permissions.
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Li, W., Zhang, H., Zeng, Y. et al. A Salt Tolerance Evaluation Method for Sunflower ( Helianthus annuus L.) at the Seed Germination Stage. Sci Rep 10 , 10626 (2020). https://doi.org/10.1038/s41598-020-67210-3
Download citation
Received : 21 January 2020
Accepted : 01 June 2020
Published : 30 June 2020
DOI : https://doi.org/10.1038/s41598-020-67210-3
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
A method for screening salt stress tolerance in indian mustard (brassica juncea) (l.) czern & coss at seedling stage.
- Garima Aggarwal
- Premnath Edhigalla
- Sanjeet Singh Sandal
Scientific Reports (2024)
A plausible screening approach for moisture stress tolerance in finger millet (Eleusine coracana L.) germplasm accessions using membership function value at the seedling stage
- Mayank Pratap Singh Bangari
- Asha Sastya
- Yellodu Adi Reddy Nanja Reddy
Genetic Resources and Crop Evolution (2024)
Priming of Citrullus lanatus var. Colocynthoides seeds in seaweed extract improved seed germination, plant growth and performance under salinity conditions
- Asmaa M. Radwan
- Entesar A. Ahmed
- Mohamed A. Balah
Scientific Reports (2023)
Genome-wide association study of salt tolerance at the germination stage in hemp
- Jiquan Chen
- Jianguang Su
Euphytica (2023)
Evaluation of wild chrysanthemums for waterlogging tolerance at the seedling stage
- Jiangshuo Su
- Yingnan Yang
By submitting a comment you agree to abide by our Terms and Community Guidelines . If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.

Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
The Effects of Salinity on Mung Bean (Vigna radiata) Seed Germination
- Jimmy Huang
- William Lee
- Claire Weng
Vigna radiata , also known as “the green gram” or “mung bean,” is a plant species in the legume family. Mung beans typically germinate within 2-5 days, but factors such as temperature, salinity level, pH of water, and more affect germination and growth rate (Overhiser 2019). Knowing this, we aimed to determine how the salinity concentration present in the growth medium would affect the germination rate of mung beans. In this experiment, members performed the same experiment with the same measurements. Each group member exposed 25 mung bean seeds to varying salinity treatment groups: 0, 40, 80 and 120 mM/ L. The amount of seeds germinated were recorded each day. Our study found a general decreasing germination rate with increasing salinity concentrations, which can be associated with ethylene production and osmotic stress. Average germination rate after two days was determined to be 73% in the 0 mM/L, 67% in the 40 mM/L, 53% in the 80 mM/L, and 43% in the 120 mM/L; all values are significantly different from one another receptor for 0 and 40 mM/ L groups. This affirms our initial hypothesis that an increase in salinity would cause a decrease in germination. Although only mung beans were tested, results can be applied to many other dried legumes and seeds, allowing us to test more advanced hypotheses in the future. For future studies, this could be replicated with other types of beans or include greater sample sizes.
Developed By
- Français (Canada)
Information
- For Readers
- For Authors
- For Librarians

Effect of salinity on seed germination and seedling growth of bullet cultivar of chilli ( Capsicum annuum L.)
- Biochemistry & Physiology - Short Communication
- Published: 06 July 2023
- Volume 46 , pages 513–525, ( 2023 )
Cite this article

- Anup Kumar Sarkar ORCID: orcid.org/0000-0002-6418-4895 1 , 2 na1 ,
- Satyajit Oraon ORCID: orcid.org/0000-0003-3609-6422 3 na1 ,
- Subrata Mondal ORCID: orcid.org/0000-0003-4414-5669 3 &
- Sanjoy Sadhukhan ORCID: orcid.org/0000-0002-2619-8700 2
458 Accesses
3 Citations
7 Altmetric
Explore all metrics
Salinity stress is one of the most severe factors, limiting seed germination and post-germination events. Salinity produces negative effects on seed germination and seedling growth of most of the glycophytes either by inhibiting water absorption or by causing the toxicity of salt ions. Globally, sustainable salinization of arable land is rising at an alarming rate, reducing the output from formerly fertile soil. Though chilli is glycophyte, several varieties of the species show a different level of salt tolerance. The response of the “Bullet variety” of chilli ( Capsicum annuum L.) to distinct levels of sodium chloride (0, 25, 50, 100, 150, 200 mM) was explored in this communication, with an emphasis on seed germination and early seedling growth. In this study, several metrics evaluating germination and seedling are tested at varying salt levels. Such metrics reveal that the Bullet variety can tolerate a 200-mM concentration of NaCl during germination and the early seedling developmental stage. Major deviations of such metrics were observed between the 100-mM and 150-mM concentrations of NaCl.
This is a preview of subscription content, log in via an institution to check access.
Access this article
Subscribe and save.
- Get 10 units per month
- Download Article/Chapter or eBook
- 1 Unit = 1 Article or 1 Chapter
- Cancel anytime
Price includes VAT (Russian Federation)
Instant access to the full article PDF.
Rent this article via DeepDyve
Institutional subscriptions
Data Availability
Data supporting this study are included within the article and/or supporting materials.
Aamir N, Muhammad A, Muhammad AP, Irfan A (2011) Effect of halopriming on germination and seedling vigor of tomato. Afr J Agric Res 6:3551–3559. https://doi.org/10.5897/AJAR11.064
Article Google Scholar
Abdul-Baki AA, Anderson JD (1972) Physiological and biochemical deterioration of seeds. In: Kozlowski TT (ed) Seed Biology Germination Control, Metabolism and Pathology, vol 2. Academic Press, New York, pp 283–316
Chapter Google Scholar
Ai TN, Tran TNB, Lam NH, Nguyen MH, Phan CH (2021) Assessment of salinity tolerance of 4 chili pepper genotypes in Vietnam. J Southwest Jiaotong Univ 56:94–110. https://doi.org/10.35741/issn.0258-2724.56.2.9
Airaki M, Leterrier M, Mateos RM, Valderrama R, Chaki M, Barroso JB, Del Rio LA, Palma JM, Corpas FJ (2012) Metabolism of reactive oxygen species and reactive nitrogen species in pepper ( Capsicum annuum L.) plants under low temperature stress. Plant Cell & Environ 35:281–295. https://doi.org/10.1111/j.1365-3040.2011.02310.x
Article CAS Google Scholar
Akhtar MF-U-Z, Hussain A, Ahmad F, Kharal MA, Khalid I, Latif M, Jamshaid MU (2017) Screening of pepper ( Capsicum annuum L.) genotypes against salinity stress. J Environ Agric Sci 11:51–58
Google Scholar
Almansouri M, Kinet J-M, Lutts S (2001) Effect of salt and osmotic stresses on germination in durum wheat ( Triticum durum Desf.). Plant Soil 231:243–254. https://doi.org/10.1023/A:1010378409663
Al-Mudaris M (1998) Notes on various parameters recording the speed of seed germination. Der Tropenlandwirt-J Agric Tropics and Subtropics 99:147–154
Anshori M, Purwoko B, Dewi I, Ardie S, Suwarno W (2019) Selection index based on multivariate analysis for selecting doubled-haploid rice lines in lowland saline prone area. SABRAO J Breed Genet 51:161–174
Arán D, García-Duro J, Reyes O, Casal M (2013) Fire and invasive species: modifications in the germination potential of Acacia melanoxylon, Conyza canadensis and Eucalyptus globulus . For Ecol Manage 302:7–13. https://doi.org/10.1016/j.foreco.2013.02.030
Ashraf M, Wu L (1994) Breeding for salinity tolerance in plants. Crit Rev Plant Sci 13:17–42. https://doi.org/10.1080/07352689409701906
Bahuguna S, Bahuguna A, Singh N (2014) Allelopathic belongings of dried walnut leaf on seed germination and seedling growth of mustard ( Brassica campestris ) in agri-silvi system of Uttarakhand Himalaya. India Am J Food Technol 9:172–179. https://doi.org/10.3923/ajft.2014.172.179
Balasankar D, Praneetha S, Arumugam T, Jeyakumar P, Manivannan N, Arulmozhiselvan K (2017) Genotypic response of chilli ( Capsicum annuum L.) on germination and seedling characters to different salinity levels. Int J Curr Microbiol Appl Sci 6:2197–2205. https://doi.org/10.20546/ijcmas.2017.604.257
Bam R, Kumaga F, Ofori K, Asiedu E (2006) Germination, vigour and dehydrogenase activity of naturally aged rice ( Oryza sativa L.) seeds soaked in potassium and phosphorus salts. Asian J Plant Sci 5:948–955. https://doi.org/10.3923/ajps.2006.948.955
Bandyopadhyay P (2014) Root distribution pattern of pulses in response to water availability. In: Ghosh P, Kumar N, Venkatesh M, Hazra K, Nadarajan N (eds) Resource Conservation Technology in Pulses, 1st edn. Scientific Publishers (INDIA), Jodhpur, Rajasthan, India, pp 483–493
Beltrán-Morales A, Córdoba-Matson MV, García-Hernández JL, Troyo-Diéguez E, Azadi H, Ruiz-Espinoza FH, Valdez-Cepeda RD, Murillo-Amador B (2015) Salinity effects on germination and seedlings biomass of Pachycereus pecten-aboriginum : an endangered species. J Prof Assoc Cactus Development 17:107–122. https://doi.org/10.56890/jpacd.v17i.65
Bojorquez-Quintal E, Ileana E-M, Fatima M-L, Manuel M-E (2012) Plants’ challenges in a salinized World: the case of capsicum. Afr J Biotech 11:13614–13626. https://doi.org/10.5897/AJB12.2145
Bojović B, Đelić G, Topuzović M, Stanković M (2010) Effects of NaCl on seed germination in some species from families Brassicaceae and Solanaceae. Kragujevac J Sci 32:83–87
Chakma P, Hossain MM, Rabbani MG (2019) Effects of salinity stress on seed germination and seedling growth of tomato: Salinity stress on seed germination and seedling growth. J Bangladesh Agric Univ 17:490–499. https://doi.org/10.3329/jbau.v17i4.44617
Chartzoulakis K, Klapaki G (2000) Response of two greenhouse pepper hybrids to NaCl salinity during different growth stages. Sci Hortic 86:247–260. https://doi.org/10.1016/S0304-4238(00)00151-5
Chookhampaeng S, Pattanagul W, Theerakulpisut P (2007) Screening some tomato commercial cultivars from Thailand for salinity tolerance. Asian J Plant Sci 6:788–794. https://doi.org/10.3923/ajps.2007.788.794
Cunhua S, Xinna X, Baiwei L, Dan W (2012) The effect of NaCl stress on the germination of seed and growth of wild species and cultivated varieties of tomato. Afr J Biotech 11:6687–6693. https://doi.org/10.5897/AJB11.3454
Cutforth H, Angadi S, McConkey B, Miller P, Ulrich D, Gulden R, Volkmar K, Entz M, Brandt S (2013) Comparing rooting characteristics and soil water withdrawal patterns of wheat with alternative oilseed and pulse crops grown in the semiarid Canadian prairie. Can J Soil Sci 93:147–160. https://doi.org/10.4141/cjss2012-081
Czabator FJ (1962) Germination value: an index combining speed and completeness of pine seed germination. Forest Sci 8:386–396. https://doi.org/10.1093/forestscience/8.4.386
de Souza EM, da Silva MLM, Alves EU, do Nascimento MdGR, de Oliveira Pires RdSSeRM, (2021) Salt stress effects in the physiological quality of Cajanus cajan (L.) millspaugh seeds at different temperatures. Int J Dev Res 11:21933. https://doi.org/10.37118/ijdr.21933.05.2021
Debez A, Ben Hamed K, Grignon C, Abdelly C (2004) Salinity effects on germination, growth, and seed production of the halophyte Cakile maritima . Plant Soil 262:179–189. https://doi.org/10.1023/B:PLSO.0000037034.47247.67
Demir I, Okcu G (2004) Aerated hydration treatment for improved germination and seedling growth in aubergine ( Solarium melongena ) and pepper ( Capsicum annuum ). Annals Appl Biol 144:121–123. https://doi.org/10.1111/j.1744-7348.2004.tb00324.x
Demir I, Ermis S, Mavi K, Matthews S (2008) Mean germination time of pepper seed lots ( Capsicum annuum L.) predicts size and uniformity of seedlings in germination tests and transplant modules. Seed Sci Technol 36:21–30. https://doi.org/10.15258/sst.2008.36.1.02
Farid M, Nasaruddin N, Musa Y, Anshori MF, Ridwan I, Hendra J, Subroto G (2020) Genetic parameters and multivariate analysis to determine secondary traits in selecting wheat mutant adaptive on tropical lowlands. Plant Breeding and Biotechnol 8:368–377. https://doi.org/10.9787/PBB.2020.8.4.368
Flynn R, Phillips R, Ulery AL, Kochevar R, Liess L, Villa M (2002) Chile seed germination as affected by temperature and salinity. College of Agriculture and Home Economics, Cooperative Extension Service …,
Foolad M (2004) Recent Advances in genetics of salt tolerance in tomato. Plant Cell, Tissue Organ Cult 76:101–119. https://doi.org/10.1023/B:TICU.0000007308.47608.88
Foti C, Khah E, Pavli O (2019) Germination profiling of lentil genotypes subjected to salinity stress. Plant Biol 21:480–486. https://doi.org/10.1111/plb.12714
Article CAS PubMed Google Scholar
Gotame TP (2006) Morphological and physiological responses of tomato cv. NCL 1 subjected to excess water stress. Natural Resources Management Nepal Biological Society, Biratnagar & Ecological Society, Kathmandu, Nepal pp 445–450
Grozeva S, Kalapchieva S, Tringovska I (2023) In vitro screening for salinity tolerance in garden pea ( Pisum sativum L.). Horticulturae 9:338. https://doi.org/10.3390/horticulturae9030338
Guedes RS, Alves EU, Viana JS, Gonçalves EP, Lima CRd, Santos SdRNd (2013) Germination and Vigor of Apeiba tibourbou seeds submitted to water stress and to different temperatures. Ciência Florestal 23:45–53. https://doi.org/10.5902/198050988438
Hasan M, Kulsum M, Ullah M, Hossain MM, Mahmud ME (2014) Genetic diversity of some chili ( Capsicum annuum L.) genotypes. Int J Agric Res, Innov Technol 4:32–35. https://doi.org/10.3329/ijarit.v4i1.21088
Howlader MHK, Islam MN, Biswas S, Uddin ME, Shila A, Haque MZ, Mahmud N (2018) Salt tolerance of chili genotypes during germination and seedling growth. Malaysian J Halal Res 1:1–7. https://doi.org/10.26480/mjhr.02.2018.01.07
Htwe NN, Maziah M, Ling HC, Zaman FQ, Zain AM (2011) Responses of some selected Malaysian rice genotypes to callus induction under in vitro salt stress. Afr J Biotech 10:350–362
CAS Google Scholar
Hussain M, Kumar A (2022) Germination and seedling growth of sesame cultivars under salinity stresses. Curr Agric Res J 10:366–374. https://doi.org/10.12944/CARJ.10.3.20
Iroka CF, Chukwuma MO, Izundu AI, Okereke NC (2016) Evaluation of salt tolerance of some varieties of Capsicum species during germination using morphometric features. Evaluation 1:29–33
Kader M (2005) A comparison of seed germination calculation formulae and the associated interpretation of resulting data. J Proc Royal Soc New South Wales 138:65–75
Kalhori N, Ying T, Nulit R, Sahebi M, Abiri R, Atabaki N (2018) Effect of four different salts on seed germination and morphological characteristics of Oryza sativa L. cv. MR219. Int J Adv Res Botany 4:29–45
Kandil A, Sharief A, Abd EL-Fatah FM, (2019) Influence of antioxidants and salinity stress on seedling parameters of some rice cultivars. J Plant Stress Physiol 5:15–21. https://doi.org/10.25081/jpsp.2019.v5.5444
Kaveh H, Nemati H, Farsi M, Jartoodeh SV (2011) How Salinity Affect Germination and Emergence of Tomato Lines. J Biol Environ Sci 5:159–163
Khajeh-Hosseini M, Lomholt A, Matthews S (2009) Mean germination time in the laboratory estimates the relative vigour and field performance of commercial seed lots of maize ( Zea mays L.). Seed Sci Technol 37:446–456. https://doi.org/10.15258/sst.2009.37.2.17
Khayat PN, Jamaati-e-Somarin S, Zabihi-e-Mahmoodabad R, Yari A, Khayatnezhad M, Gholamin R (2010) Screening of salt tolerance canola cultivars ( Brassica napus L.) using physiological markers. World Appl Sci J 10:817–820
Khodarahmpour Z, Ifar M, Motamedi M (2012) Effects of NaCl salinity on maize ( Zea mays L.) at germination and early seedling stage. African J Biotechnol 11:298–304. https://doi.org/10.5897/AJB11.2624
Kim Y-H, Hwang S-J, Waqas M, Khan AL, Lee J-H, Lee J-D, Nguyen HT, Lee I-J (2015) Comparative analysis of endogenous hormones level in two soybean ( Glycine max L.) lines differing in waterlogging tolerance. Front Plant Sci 6:714. https://doi.org/10.3389/fpls.2015.00714
Article PubMed PubMed Central Google Scholar
Kpinkoun JK, Zanklan SA, Montcho D, Kinsou E, Komlan FA, Mensah AC, Wouyou AD, Gandonou CB (2018) Response of chili pepper ( Capsicum spp.) cultivars cultivated in benin to salt stress at germination stage. Int J Plant and Soil Sci 23:1–11. https://doi.org/10.9734/IJPSS/2018/42118
Li Y (2008) Effect of salt stress on seed germination and seedling growth of three salinity plants. Pak J Biol Sci 11:1268–1272. https://doi.org/10.3923/pjbs.2008.1268.1272
Loganayaki K, Tamizhmathi S, Brinda D, Gayathri S, Mary MC, Mohanlal V (2020) In vitro evaluation of tomato ( Lycopersicon esculentum Mill.), chilli ( Capsicum annum L), cucumber ( Cucumissativus L.) and Bhendi ( Abelmoschus esculentus L.) for salinity stress. Int J Chem Stud 8:2364–2367. https://doi.org/10.22271/chemi.2020.v8.i2aj.9104
Lynch JP, Chimungu JG, Brown KM (2014) Root anatomical phenes associated with water acquisition from drying soil: targets for crop improvement. J Exp Bot 65:6155–6166. https://doi.org/10.1093/jxb/eru162
Maggio A, Raimondi G, Martino A, De Pascale S (2007) Salt stress response in tomato beyond the salinity tolerance threshold. Environ Exp Bot 59:276–282. https://doi.org/10.35741/issn.0258-2724.56.2.9
Matthews S, Khajeh Hosseini M (2006) Mean germination time as an indicator of emergence performance in soil of seed lots of maize ( Zea mays ). Seed Science and Technology 34:339–347. https://doi.org/10.15258/sst.2006.34.2.09
Mirosavljević M, Čanak P, Ćirić M, Nastasić A, Đukić D, Rajković M (2013) Maize germination parameters and early seedlings growth under different levels of salt stress. Ratarstvo i Povrtarstvo/field and Veg Crops Res 50:49–53. https://doi.org/10.5937/ratpov50-3042
Moles TM, Guglielminetti L, Reyes TH (2019) Differential effects of sodium chloride on germination and post-germination stages of two tomato genotypes. Scientia Horticult 257:108730. https://doi.org/10.1016/j.scienta.2019.108730
Morris B, Charles S, Son S (2003) Legumes Encyclopedia of Food and Culture. Charles Scribner and Sons, New York
Munns R (2002) Comparative physiology of salt and water stress. Plant, Cell Environ 25:239–250. https://doi.org/10.1046/j.0016-8025.2001.00808.x
Mustafa M, Syahri YF, Rauf M (2019) Selection of Chilli pepper ( Capsicum frutescens L.) for salinity tolerance in seed germination. Agrotech J 4:83–90. https://doi.org/10.31327/atj.v4i2.1110
Nasrin S, Mannan MA (2019) Impact of salinity on seed germination and seedling growth of tomato. J Biosci Agric Res 21:1737–1748. https://doi.org/10.18801/jbar.210119.212
Nonogaki H, Bassel GW, Bewley JD (2010) Germination—Still a mystery. Plant Sci 179:574–581. https://doi.org/10.1016/j.plantsci.2010.02.010
Oraon S, Mondal S (2022) Allelopathic impacts of an agroforestry tree species ( Streblus asper lour.) on seed germination and seedling growth of chickpea. Legume Res-an Int J 45:1295–1300. https://doi.org/10.18805/LR-4679
Ouji A, El-Bok S, Mouelhi M, Younes MB, Kharrat M (2015) Effect of salinity stress on germination of five Tunisian lentil ( Lens culinaris L.) genotypes. Euro Sci J 11:63–75
Özdemir B, Tanyolaç ZÖ, Ulukapı K, Onus AN (2016) Evaluation of salinity tolerance level of some pepper ( Capsicum annuum L.) cultivars. Int J Agric Innov Res 5:247–251
Pandey M, Penna S (2017) Time course of physiological, biochemical, and gene expression changes under short-term salt stress in Brassicajuncea L. The Crop J 5:219–230. https://doi.org/10.1016/j.cj.2016.08.002
Panuccio M, Jacobsen S, Akhtar S, Muscolo A (2014) Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB PLANTS 6:plu047. https://doi.org/10.1093/aobpla/plu047
Article CAS PubMed PubMed Central Google Scholar
Pavli OI, Foti C, Skoufogianni G, Karastergiou G, Panagou A, Khah EM (2021) Effect of salinity on seed germination and seedling development of soybean genotypes. Int J Environ Sci Nat Resour 27:556210. https://doi.org/10.19080/IJESNR.2021.27.556210
Pradeep P (2018) Seed quality parameters (Germination percentage and seedling vigor index) of rabi sorghum seeds influenced by rice weevil infestation. MOJ Toxicol 4:391–396. https://doi.org/10.15406/mojt.2018.04.00135
Rajabi Dehnavi A, Zahedi M, Ludwiczak A, Cardenas Perez S, Piernik A (2020) Effect of salinity on seed germination and seedling development of sorghum ( Sorghum bicolor (L.) Moench) genotypes. Agronomy 10:859. https://doi.org/10.3390/agronomy10060859
Ranal MA, Santana DGd (2006) How and why to measure the germination process? Brazilian J Botany 29:1–11. https://doi.org/10.1590/S0100-84042006000100002
Ranal MA, Santana DGd, Ferreira WR, Mendes-Rodrigues C (2009) Calculating germination measurements and organizing spreadsheets. Brazilian J Botany 32:849–855. https://doi.org/10.1590/S0100-84042009000400022
Ropokis A, Ntatsi G, Kittas C, Katsoulas N, Savvas D (2018) Impact of cultivar and grafting on nutrient and water uptake by sweet pepper ( Capsicum annuum L.) grown hydroponically under mediterranean climatic conditions. Front Plant Sci 9:1244. https://doi.org/10.3389/fpls.2018.01244
Saboora A, Kiarostami K, Behroozbayati F, Hajihashemi S (2006) Salinity (NaCl) tolerance of wheat genotypes at germination and early seedling growth. Pak J Biol Sci 9:2009–2021
Sagar A, Tajkia J, Haque M, Fakir M, Hossain A (2018) Screening of Sorghum genotypes for salt-tolerance based on seed germination and seedling stage. Fundam Appl Agric 4:735–743. https://doi.org/10.5455/faa.18483
Saha R, Ahmed F, Mokarroma N, Rohman M, Golder P (2016) Physiological and biochemical changes in waterlog tolerant sesame genotypes. SAARC J Agric 14:31–45. https://doi.org/10.3329/sja.v14i2.31243
Sardoei AS, Mohammadi GA (2014) Study of salinity effect on germination of tomato ( Lycopersicon esculentum L.) genotypes. Eur J Exper Biol 4:283–287
Sarkar AK, Sadhukhan S (2022) Bioremediation of Salt-Affected Soil Through Plant-Based Strategies. In: Malik JA (ed) Advances in Bioremediation and Phytoremediation for Sustainable Soil Management. Springer, Cham, pp 81–100
Sarwar M, Ahmad S, Chattha M, Chattha M, Alam M, Anjum S, Shafeeq T, Naseem M, Mannan A (2019) Assesment of growth and productivity of cucumber ( Cucumis sativus L.) genotypes under salt stress regime. Appl Ecol Environ Res 17:10793–10806. https://doi.org/10.15666/aeer/1705_1079310806
Sholi NJ (2012) Effect of salt stress on seed germination, plant growth, photosynthesis and ion accumulation of four tomato cultivars. Am J Plant Physiol 7:269–275. https://doi.org/10.3923/ajpp.2012.269.275
Singh J, Sastry E, Singh V (2012) Effect of salinity on tomato ( Lycopersicon esculentum Mill.) during seed germination stage. Physiol Mol Biol Plants 18:45–50. https://doi.org/10.1007/s12298-011-0097-z
Article PubMed Google Scholar
Soltani E, Ghaderi-Far F, Baskin CC, Baskin JM (2015) Problems with using mean germination time to calculate rate of seed germination. Aust J Bot 63:631–635. https://doi.org/10.1071/BT15133
Stefanello R, Viana B, Goergen P, Neves L, Nunes U (2019) Germination of chia seeds submitted to saline stress. Braz J Biol 80:285–289. https://doi.org/10.1590/1519-6984.192140
Tarchoun N, Saadaoui W, Mezghani N, Pavli OI, Falleh H, Petropoulos SA (2022) The effects of salt stress on germination seedling growth and biochemical responses of tunisian squash. Plants Basel. https://doi.org/10.3390/plants11060800
Tebini M, Luu D, Mguis K, Ben Ahmed H, Meddich A, Zribi F, Chalh A (2022) Physiological exploration of intra-specific variability in salinity tolerance of amaranth. Russ J Plant Physiol 69:59. https://doi.org/10.1134/S1021443722030153
Tehseen S, Ayyub CM, Amjad M (2016) Assessment of salinity tolerance in bell pepper ( Capsicum annuum L.) genotypes on the basis of germination, emergence and growth attributes. Pakistan J Botany 48:1783–1791
Tsegay BA, Gebreslassie B (2014) The effect of salinity (NaCl) on germination and early seedling growth of Lathyrus sativus and Pisum sativum var. abyssinicum. African J Plant Sci 8:225–231. https://doi.org/10.5897/AJPS2014.1176
Wojtyla Ł, Lechowska K, Kubala S, Garnczarska M (2016) Molecular processes induced in primed seeds—increasing the potential to stabilize crop yields under drought conditions. J Plant Physiol 203:116–126. https://doi.org/10.1016/j.jplph.2016.04.008
Zerkout A, Omar H, Ibrahim MH, Mustafa M (2018) Influence of lead on in vitro seed germination and early radicle development of acacia auriculiformis cunn ex benth species. Annual Res Rev Biol 28:1–12. https://doi.org/10.9734/ARRB/2018/43393
Zhang D, Wang Z, Wang N, Gao Y, Liu Y, Wu Y, Bai Y, Zhang Z, Lin X, Dong Y (2014) Tissue culture-induced heritable genomic variation in rice, and their phenotypic implications. PLoS ONE 9:e96879. https://doi.org/10.1371/journal.pone.0096879
Download references
Acknowledgements
Seeds used in the experiment were obtained from the Department of Agronomy, Visva-Bharati, Santiniketan, West Bengal, India
Author information
Anup Kumar Sarkar and Satyajit Oraon have contributed equally.
Authors and Affiliations
Department of Botany, DukhulalNibaran Chandra College, Murshidabad, West Bengal, India
Anup Kumar Sarkar
Plant Molecular Biology Laboratory, Department of Botany, Raiganj University, Raiganj, West Bengal, India
Anup Kumar Sarkar & Sanjoy Sadhukhan
Department of Botany, Visva-Bharati, Santiniketan, West Bengal, India
Satyajit Oraon & Subrata Mondal
You can also search for this author in PubMed Google Scholar
Contributions
All the authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication. All the authors had the idea for the article; AKS and SO performed the research work and data analysis; AKS and SO drafted; figures were drawn by SO and SS. SM and SS critically revised the work.
Corresponding author
Correspondence to Sanjoy Sadhukhan .
Additional information
Publisher's note.
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 (TIF 3618 kb)
Supplementary file2 (doc 32 kb), rights and permissions.
Reprints and permissions
About this article
Sarkar, A.K., Oraon, S., Mondal, S. et al. Effect of salinity on seed germination and seedling growth of bullet cultivar of chilli ( Capsicum annuum L.). Braz. J. Bot 46 , 513–525 (2023). https://doi.org/10.1007/s40415-023-00894-9
Download citation
Received : 09 February 2023
Revised : 17 June 2023
Accepted : 19 June 2023
Published : 06 July 2023
Issue Date : September 2023
DOI : https://doi.org/10.1007/s40415-023-00894-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
- Linear regression
- Find a journal
- Publish with us
- Track your research
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
Effect of saline water on seed germination and early seedling growth of the halophyte quinoa
M. r. panuccio.
1 Department of Agriculture, Mediterranea University, località Feo di Vito, 89126 Reggio Calabria, Italy
S. E. Jacobsen
2 Department of Plant and Environmental Sciences, Faculty of Science, University of Copenhagen, Højbakkegård Allé 13, DK-2630 Tåstrup, Denmark
S. S. Akhtar
3 Sino-Danish Center for Education and Research (SDC), Beijing, China
Guest Editor: Tim J. Flowers
The introduction of new crops with improved salinity stress tolerance could preserve water quality and protect soil resources from further degradation, providing extra sources of food for salinized areas. In this context, we tested the salinity tolerance of a variety of quinoa. Quinoa, a rich source of minerals, proteins and antioxidants, is considered a major alternative crop to meet food shortages in this century. Our study indicated that salinity tolerance of quinoa is largely conferred by a delicate balance between osmotic adjustment and ion accumulation. Salinity reduced productivity in terms of biomass, but increased the levels of antioxidant compounds, which are important health-protecting factors in food, thus providing economic benefit.
Salinization is increasing on a global scale, decreasing average yields for most major crop plants. Investigations into salt resistance have, unfortunately, mainly been focused on conventional crops, with few studies screening the potential of available halophytes as new crops. This study has been carried out to investigate the mechanisms used by quinoa, a facultative halophytic species, in order to cope with high salt levels at various stages of its development. Quinoa is regarded as one of the crops that might sustain food security in this century, grown primarily for its edible seeds with their high protein content and unique amino acid composition. Although the species has been described as a facultative halophyte, and its tolerance to salt stress has been investigated, its physiological and molecular responses to seawater (SW) and other salts have not been studied. We evaluated the effects of SW and different salts on seed germination, seedling emergence and the antioxidative pathway of quinoa. Seeds were germinated in Petri dishes and seedlings grown in pots with SW solutions (25, 50, 75 and 100 %) and NaCl, CaCl 2 , KCl and MgCl 2 individually, at the concentrations in which they are present in SW. Our results demonstrated that all salts, at lower concentrations, increased the germination rate but not the germination percentages, compared with control (pure water). Conversely, seedlings were differently affected by treatments in respect to salt type and concentration. Growth parameters affected were root and shoot length, root morphology, fresh and dry weight, and water content. An efficient antioxidant mechanism was present in quinoa, activated by salts during germination and early seedling growth, as shown by the activities of antioxidant enzymes. Total antioxidant capacity was always higher under salt stress than in water. Moreover, osmotic and ionic stress factors had different degrees of influence on germination and development.
Introduction
Soil salinity and sodicity cause severe problems in agriculture worldwide, and salt tolerance in crops is an extremely important trait and a major focus of research. Detrimental effects of high salinity on crops are multifaceted and affect plants in several ways: drought stress, ion toxicity, nutritional disorders, oxidative stress, alteration of metabolic processes, membrane disorganization and reduction of cell division and expansion ( Hasegawa et al. 2000 ; Munns 2002 ; Muscolo et al. 2007 , 2013 ; Zhu 2007 ; Sidari et al. 2008 ). As a result, plant growth, development and survival are reduced ( Muscolo et al. 2011 ; Schleiff and Muscolo 2011 ). Two major stresses affecting plants under salinity are osmotic and ionic stresses. Osmotic stress, occurring immediately in the root medium on exposure to salts, can result in inhibition of water uptake, cell expansion and lateral bud development ( Munns and Tester 2008 ). Ionic stress develops when toxic ions (e.g. Na + ) accumulate in cells causing increase in leaf mortality, chlorosis, necrosis and decrease in the activity of cellular metabolism including photosynthesis ( Yeo and Flowers 1986 ; Glenn and Brown 1999 ). In fact, excess Na + and Cl − have the potential to affect plant enzymes, resulting in reduced energy production and other physiological processes ( Larcher 1980 ; Morais et al. 2012 a , b ). Ionic stress results in premature senescence of older leaves and in toxicity symptoms (chlorosis, necrosis) in mature leaves due to high Na + and Cl − which affect plants by disrupting protein synthesis and by interfering with enzyme activity ( Munns and Termaat 1986 ; Hasegawa et al. 2000 ; Munns 2002 ).
In order to counteract the detrimental effects of salinity on agricultural production, extensive research on plant screening for salt tolerance has been conducted, with the aim of providing more tolerant cultivars. However, these studies have mainly focused on conventional crops, screening criteria and investigating how plants tolerate salts ( Shannon and Noble 1990 ; Chen et al. 2005 ; Sevengor et al. 2011 ). Unfortunately, there are few investigations on screening of available halophytes and their responses to saline conditions ( Flowers et al. 2010 ). The seed crop quinoa is a facultative halophyte native to the Andean region of Bolivia and Peru, and a member of the Amaranthaceae: quinoa is traditionally cultivated across a range of extreme environments. Due to its huge genetic variability, the species can be grown under unfavourable soil and climatic conditions ( Ruiz-Carrasco et al. 2011 ), showing a diverse tolerance to a wide range of abiotic stresses such as frost, salinity and drought, as well as an ability to grow on marginal soils ( Jacobsen et al. 2005 , 2007 , 2009 ; Maughan et al. 2009 ; Sun et al. 2014 ). Some varieties can grow in salt concentrations similar to those found in seawater (SW, 40 dS m −1 ) and even higher ( Jacobsen et al. 2001 ; Adolf et al. 2012 , 2013 ; Shabala et al. 2012 , 2013 ), well above the threshold for any known crop species.
Quinoa is considered a major alternative crop to meet food shortages in this century ( Jensen et al. 2000 ; Jacobsen et al. 2003 ; Sanchez et al. 2003 ; Trognitz 2003 ; Ruiz et al. 2014 ), for its gluten-free seeds and also as its grains provide a rich source of a wide range of minerals (Ca, P, Mg, Fe and Zn), vitamins (B 1 , B 9 , C and E), linolenate, natural antioxidants and high-quality protein, containing ample amounts of essential amino acids such as lysine and methionine ( Abugoch et al. 2008 ; Koyro and Eisa 2008 ). Quinoa's tolerance to high salinity at the primary stages of seed germination is based upon alterations in the levels of primary metabolites and enzyme activity ( González and Prado 1992 ; Adolf et al. 2013 ). Most of the studies on the effect of salinity on seed germination of halophytes have, however, been conducted using NaCl solutions. Such investigations may not provide information on germination under field conditions, because soils contain different salts, which may collectively influence germination in different ways from their individual effects ( Ungar 1996 ). Sea salt mimics the composition of saline soil solutions and can be used to study the synergistic effect of different salts on seed germination ( Liu et al. 2006 ). Therefore, the work presented here was carried out to examine the effects of SW and its component salts on seed germination, seedling emergence and the antioxidative pathway of quinoa cv. Titicaca, as well as the relative importance of two components (ionic and osmotic) of salinity stress.
Quinoa cultivars have been shown to differ in salt tolerance ( Bonales-Alatorre et al. 2013 ). In general, varieties originating from salt-affected areas are adapted to saline conditions and hence are less affected by salinity ( Adolf et al. 2012 ; Shabala et al. 2013 ) than those from non-saline areas. In this study, we used the Danish-bred quinoa cv. Titicaca ( Jacobsen et al. 2010 ; Adolf et al. 2012 ) to verify the salinity tolerance of a variety well adapted to European climatic conditions. Quinoa production may be a viable option for farmers interested in a high-value crop with regional production and local markets in Mediterranean countries where saline water and soil salinity are major risks for the future of agricultural development. Here fresh water resources are limited, while food requirements and pressure from climate change are still growing. The use of saline water resources may constitute a remedy for the current water scarcity. For these reasons, quinoa offers the possibility of an alternative, promising, cash crop to be cultivated in arid and semiarid environments that are prohibitive for other species and so may be able to utilize saline soils in a sustainable and productive way.
Plant material
Mature seeds of the Danish-bred quinoa ( Chenopodium quinoa cv. Titicaca) (provided by Department of Plant Environmental Science, University of Copenhagen) were stored at 5 °C until the start of experiments. Two different experiments were carried out in a growth chamber (Green line WRS 96-85, KW, Scientific Equipment, Italy) (temperature of 25 ± 1 °C in the dark with a relative humidity of 70 %) to characterize the responses of quinoa to salt stress. Seed germination and biochemical responses were studied in the first experiment, while morphological, physiological and biochemical responses of seedlings were studied in the second experiment.
Experiment 1: seed germination
Germination conditions and experimental design.
Seeds were surface-sterilized for 20 min in 20 % (v/v) sodium hypochlorite, rinsed and soaked for 1 h in distilled water. The sterilization procedure is required to eliminate saponine from seeds and to avoid contamination by microorganisms during the germination process. The entire sterilization procedure, including soaking, took 1 h and did not affect the germination process ( Ruiz-Carrasco et al. 2011 ; Burrieza et al. 2012 ). For the germination tests, five 50-seed replicates were used with either Mediterranean SW collected from the Tirreno sea (Calabria Southern Italy) with a salinity of 38 % ( Cotruvo 2005 ) or solutions of NaCl, CaCl 2 , KCl or MgCl 2 at the concentration in which they were in the SW and at various dilutions. In the experiment, five different concentrations of NaCl (0, 100, 200, 300 and 400 mM); KCl (0, 2.54, 5.08, 7.62 and 10.2 mM); CaCl 2 (0, 2.54, 5.08, 7.62 and 10.2 mM) and MgCl 2 (0, 13.4, 26.7, 40.1 and 53.5 mM) were used to test whether the various ions differently affected germination indexes and to verify possible antagonistic or synergic ion effects on seed germination. Seeds were placed on filter paper in 9 cm diameter Petri dishes containing 3 mL of each solution. The Petri dishes were hermetically sealed with Parafilm to prevent evaporation and kept in the growth chamber at a temperature of 25 ± 1 °C in the dark with a relative humidity of 70 %. Seeds were considered germinated when the radicle had extended at least 2 mm.
Germination indexes
The number of seeds germinated was recorded daily for up to 7 days. From these germination counts, several germination attributes were calculated to characterize the salt tolerance, including germination percentage (%) at 1 and 7 days, coefficient of velocity of germination (CVG) ( Kader and Jutzi 2004 ), germination rate index (GRI) ( Kader 2005 ) and mean germination time (MGT) ( Kader 2005 ), as follows:
where N is the number of seeds germinated on day i , and T i is the number of days from sowing. The CVG gives an indication of the rapidity of germination: it increases when the number of germinated seeds increases and the time required for germination decreases. The GRI reflects the percentage of germination on each day of the germination period. Higher GRI values indicate higher and faster germination. The lower the MGT, the faster a population of seeds has germinated.
Determination of ionic and osmotic effect
According to Munns et al. (1995) , the decrease in germination under saline conditions is the consequence of the combined effect of osmotic (OE) and ionic (IE) factors; consequently, the total effect (TE) of salinity on germination can be defined as
To resolve this equation, the osmotic components (OE) were determined by germinating seeds in distilled water (zero osmolality) and in solutions of polyethylene glycol (PEG 8000) with an osmolality equivalent to the concentrations of the various salts that reduced germination by 50 % (LD 50max ). Consequently, OE corresponds to the difference between the germination values obtained in pure water (GH 2 O) and those obtained in the isotonic solutions (GOs).
A cryoscopic osmometer (OSMOMAT 030 GONOTEC) was used in order to determine the osmolality of each PEG and saline solution. The TE of salinity was obtained by means of the difference between the germination values under non-saline conditions with water (GH 2 O) and germination obtained with the LD 50max saline concentrations. This germination is termed GLD 50max , thus TE was determined through:
Based on the values of TE and OE, the ionic effect (IE) was calculated as
Determination of enzyme activities
Seeds (0.5 g) that had been soaked for 3 days in the test solutions were ground using a chilled mortar and pestle and homogenized in 0.1 M phosphate buffer solution (pH 7.0) containing 100 mg soluble polyvinylpolypyrrolidone and 0.1 mM ethylenediamine tetra acetic acid (EDTA). The homogenate was filtered through two layers of muslin cloth and centrifuged at 15 000 g for 15 min at 4 °C. The resulting supernatant was used to evaluate the activity of catalase (CAT, EC 1.11.1.6), peroxidase (POX, EC 1.11.1.7), ascorbate peroxidase (APX, EC 1.11.1.11) and superoxide dismutase (SOD EC 1.15.1.1). All enzyme activities were measured at 25 °C by a UV–visible light spectrophotometer (UV-1800 CE, Shimadzu, Japan).
Catalase activity was determined by monitoring the disappearance of H 2 O 2 at 240 nm, calculated using its extinction coefficient ( ε ) = 0.036 mM −1 cm −1 . The reaction mixture contained 1 mL of potassium phosphate buffer (50 mM, pH 7.0), 40 μL of enzyme extract and 5 μL of H 2 O 2 ( Beaumont et al. 1990 ).
Ascorbate peroxidase activity was assayed according to Nakano and Asada (1981) . The reaction mixture (1.5 mL) contained 50 mM phosphate buffer (pH 6.0), 0.1 μM EDTA, 0.5 mM ascorbate, 1.0 mM H 2 O 2 and 50 μL enzyme extract. The reaction was started by the addition of H 2 O 2 and ascorbate oxidation measured at 290 nm for 1 min. Enzyme activity was quantified using the molar extinction coefficient for ascorbate (2.8 mM −1 cm −1 ).
Peroxidase activity was measured on the basis of determination of guaiacol oxidation at 436 nm for 90 s ( Panda et al. 2003 ). The reaction mixture contained 1 mL of potassium phosphate buffer (0.1 M, pH 7.0), 20 μL of guaiacol, 40 μL of enzyme extract and 15 μL of H 2 O 2 . Peroxidase activity was quantified by the amount of tetraguaiacol formed using its extinction coefficient ( ε ) = 25.5 mM −1 cm −1 .
Superoxide dismutase activity was estimated by recording the decrease in the absorbance of superoxide nitro-blue tetrazolium complex by the enzyme ( Gupta et al. 1993 ). The reaction mixture (3 mL) contained 0.1 mL of 200 mM methionine, 01 mL of 2.25 mM nitro-blue tetrazolium, 0.1 mL of 3 mM EDTA, 1.5 mL of 100 mM potassium phosphate buffer, 1 mL of distilled water and 0.05 mL of enzyme extract. The assay was performed in duplicate for each sample. Two tubes without enzyme extract were used as a background control. The reaction was started by adding 0.1 mL of riboflavin (60 μM) and placing the tubes below a light source of two 15 W florescent lamps for 15 min. The reaction was stopped by switching off the light and covering the tubes with black cloth. Tubes without enzyme developed maximum colour. A non-irradiated complete reaction mixture which did not develop colour served as the blank. Absorbance was recorded at 560 nm and one unit of enzyme activity was taken as the quantity of enzyme which reduced the absorbance of samples to 50 % in comparison with tubes lacking enzymes.
For CAT, APX, SOD and POX activities, the results were expressed as enzyme units (U) per mg fresh weight. One unit of enzyme was defined as the amount of enzyme necessary to decompose 1 μmol of substrate per min at 25 °C.
Determination of total antioxidant capacity
Seeds (treated with different salt solutions for 3 days) were homogenized in a chilled mortar with distilled water at a ratio of 1 : 4 (seeds/water; w/v) and centrifuged at 14 000 g for 30 min. All steps were performed at 4 °C. The supernatants were filtered through two layers of muslin cloth and were used to determine the total antioxidant capacity by the spectrophotometric method of Prieto et al. (1999) . Aqueous extracts of the seeds were mixed in Eppendorf tubes with 1 mL of reagent solution (0.6 M H 2 SO 4 , 28 mM sodium phosphate, 4 mM ammonium molybdate mixture). The tubes were incubated for 90 min at 95 °C, then cooled to room temperature, and the absorbance read at 695 nm against a blank (mixture without seed extract). The assay was conducted in triplicate and the total antioxidant activity expressed as the absorbance of the sample at 695 nm. The higher the absorbance value, the higher the antioxidant activity ( Prasad et al. 2009 ).
Determination of total phenolic content
Total phenolic content was determined with the Folin–Ciocalteu reagent according to a modified procedure described by Singleton and Rossi (1965) . Briefly, 0.50 mL of the aqueous extract of the seeds was reacted with 2.5 mL of Folin–Ciocalteu reagent (1 : 10 diluted with distilled water) for 4 min, and then 2 mL of saturated sodium carbonate solution (∼75 g/L) was added to the reaction mixture. The absorbance readings were taken at 760 nm after incubation at room temperature for 2 h. Tannic acid was used as a reference standard, and the results were expressed as milligram tannic acid equivalent (mg TAET/g fresh weight).
Experiment 2: morphological, physiological and biochemical responses of seedlings
Plantlet growth in pots.
Seeds were germinated in Petri dishes. After 3 days from the beginning of germination, germinated seeds were grown for 21 days in plastic pots (10 cm diameter × 7 cm height), in a growth chamber (Green line WRS 96-85, KW apparecchi scientifici, Italy), under white light (80 W m −2 , Osram HQI halogen vapor W lamp, PAR 1055 μmol m −2 s−1) in a 16/8-h photoperiod, 70 % relative humidity and at 21 °C. All pots were filled with Perlite that had been equilibrated, before transplanting the germinated seeds, with one of the different salts or SW solutions at the desired concentration. All reagents used were of the highest analytical grade and were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All pots were watered with a one-fourth strength Murashige and Skoog medium (MS /4 ) containing macro and micronutrients at pH 5.8: the pots were weighed daily, and watered when their weight decreased by 30 % (corresponding to water that was lost by evapotranspiration). The control pots were watered with MS /4 alone. Leaf and root length were evaluated 21 days after the beginning of the stress, using six plants for each treatment.
Measurement of enzyme activities
After 21 days in pots under different salinity treatments, plantlet material was ground with a mortar and pestle in 100 mM HEPES–NaOH (pH 7.5), 5 mM MgCl 2 and 1 mM dithiothreitol . The ratio of plant material to buffer was 1 : 3. The extract was filtered through two layers of muslin and clarified by centrifugation at 15 000 g for 15 min. The supernatant was used for CAT, APX, POX, SOD analyses and total antioxidant capacity as described above. All steps were performed at 4 °C.
Cations (Na + , K + , Ca 2+ Mg 2+ and NH 4 + ) and anions (Cl − and SO 4 2− ) were determined in the water extracts of treated seedlings by ion chromatography (DIONEX ICS-1100).
Measurement of root morphology
Seedlings were harvested and root weight was recorded. Roots were scanned using an Epson Expression/STD 1600 scanner and personal computer with Intel Pentium III/500 CPU, 128 MB RAM, optimized for root analyses by Regent Instrument, Inc., and their length was analysed using the WinRHIZO image analysis system (Regent Instruments, Quebec, Canada). When scanning, each root sample was placed in a rectangular glass dish (300 × 200 mm) with ∼4–5 mm of water to untangle the roots and minimize root overlap. Three replicated roots were analysed for each treatment.
Statistical analysis
All data were analysed by one-way analysis of variance (ANOVA) with the salt concentration as the grouping factor. Separate ANOVAs were performed for each of four salt types and concentrations: NaCl (0, 100, 200, 300, 400 mM); KCl (0, 2.54, 5.08, 7.62, 10.16 mM); CaCl 2 (0, 2.54, 5.08, 7.62, 10.16 mM) and MgCl 2 (0, 13.36, 26.72, 40.09, 53.46 mM). The response variables for these ANOVAs were: seed germination, seedling growth, enzyme activities, ion contents and root morphology. Since salt concentration had five levels, on all significant ANOVAs we performed Tukey's multiple comparison tests to compare all pairs of means. The germination percentage data were previously subjected to arcsine transformation but are reported in tables as untransformed values. All data collected were statistically analysed using SYSTAT 8.0 software (SPSS Inc.).
Experiment 1: Germination under saline conditions
In water, all (100 %) seeds germinated (Table 1 ). At the lower concentrations, individual salts (NaCl, CaCl 2 , KCl and MgCl 2 ) did not have any significant effects on the germination percentage of quinoa seeds. Conversely, dilute SW significantly lowered germination (Table 1 ). With increasing salt concentration, the germination percentage decreased, irrespective of the treatment, except for MgCl 2 . The strongest reduction of germination was observed in the presence of 75 and 100 % SW in comparison to the other salts. The inhibition of different salt solutions on seed germination was in the order of SW > NaCl > KCl > CaCl 2 > MgCl 2 (Table 1 ). There were no significant differences among the treatments in germination rapidity (CVG), except in the SW (Table 1 : with increasing SW concentration, the CVG decreased, with a reduction of 53 % at 75 % SW). The GRI, reflecting the percentage of germination on each day of the germination period, decreased under NaCl and SW. The strongest decrease was observed in SW. No significant differences were observed among NaCl, CaCl 2 , KCl and MgCl 2 and the control, in terms of MGT (MGT, Table 1 ). Conversely, with increasing SW percentage, the MGT increased, reaching values 10 times greater than the control and of the other treatments. The strong significant inverse relationship between SW concentrations and germination indexes confirmed the detrimental effects of the SW on seed germination (Table 1 ).
Table 1.
Germination indices: total germination; CVG, GRI and MGT determined for quinoa seeds after 7 days of germination in the presence of NaCl, CaCl 2 , KCl, MgCl 2 and SW at different concentrations. Data are expressed as percentage in respect to control. Data are the means of five replicates. *** P < 0.001; ** P < 0.01: * P < 0.05.
| Total germination (%) | CVG (%) | GRI (%) | MGT (days) | ||
|---|---|---|---|---|---|
| Control | 100 | 26.8 | 26.7 | 3.7 | |
| NaCl | 100 mM | 100 | 26.2 | 26.4 | 3.8 |
| NaCl | 200 mM | 100 | 27.0 | 28.4 | 3.7 |
| NaCl | 300 mM | 95 | 26.0 | 24.7* | 3.8 |
| NaCl | 400 mM | 80** | 26.7 | 22.2* | 3.7 |
| KCl | 2.54 mM | 96 | 26.8 | 26.7 | 3.8 |
| KCl | 5.08 mM | 95 | 26.8 | 26.7 | 3.8 |
| KCl | 7.62 mM | 93* | 26.5 | 25.3 | 3.8 |
| KCl | 10.16 mM | 86* | 26.9 | 23.9* | 3.7 |
| MgCl | 13.36 mM | 100 | 26.1 | 26.1 | 3.7 |
| MgCl | 26.73 mM | 100 | 26.9 | 28.3 | 3.7 |
| MgCl | 40.00 mM | 100 | 26.3 | 26.6 | 3.7 |
| MgCl | 53.46 mM | 100 | 27.0 | 29.1* | 3.6 |
| CaCl | 2.54 mM | 98 | 26.6 | 26.8 | 3.8 |
| CaCl | 5.08 mM | 95 | 26.4 | 25.4 | 3.8 |
| CaCl | 7.62 mM | 93* | 26.4 | 24.9 | 3.8 |
| CaCl | 10.16 mM | 93* | 27.0 | 26.8 | 3.7 |
| SW | 25 % | 85* | 25.8* | 21.4** | 3.9 |
| SW | 50 % | 65*** | 19.6** | 6.6*** | 5.1* |
| SW | 75 % | 10*** | 14.3** | 0.28*** | 35*** |
| SW | 100 % | 0 | nd | nd | nd |
Separation of ionic and osmotic components
Calculating the relative importance of the osmotic and ionic component stresses showed that the two stressful factors made a different contribution to the deterioration of germination depending on the salts used. In the presence of MgCl 2 , the two stressful factors (ionic and osmotic) had a proportional effect on the reduction of seed germination as shown by the value of the IE/OE ratio (1.0, Table 2 ). Regarding NaCl, the osmotic effect prevailed (IE/OE ratio = 0.53). In CaCl 2 and KCl, at LD 50 concentrations, seed germination decreased, mainly due to osmotic factors, as suggested by the IE/OE ratios that were always <1.0 and by IE values that were under 50 (Table 2 ). Seawater (the most toxic) affected seed germination mainly through its IE as evidenced by the IE/OE ratio >1.0 (Table 2 ).
Table 2.
Influence of osmotic and ionic factors on seed germination of Titicaca quinoa seeds in the presence of NaCl, KCl, MgCl 2 , CaCl 2 and SW at LD 50max concentration. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). The values correspond to the average of five replicates.
| Treatments | OE | TE (OE + IE) (%) | IE | IE/OE | IE/TE |
|---|---|---|---|---|---|
| NaCl | 36 | 55 | 19 | 0.53 | 35 |
| KCl | 29 | 52 | 23 | 0.79 | 44 |
| MgCl | 27 | 54 | 27 | 1.0 | 50 |
| CaCl | 33 | 58 | 25 | 0.76 | 43 |
| SW | 20 | 60 | 40 | 2.0 | 67 |
Enzyme activities, phenols and antioxidants
With increasing salt concentrations, POX activity decreased, with respect to the control in the presence of NaCl, CaCl 2 and SW. Conversely, an increase in POX activity was observed with MgCl 2 and particularly KCl (Fig. 1 A). Ascorbate peroxidase, CAT and SOD activities were always lower in control seeds compared with treated seeds; the highest concentrations of KCl and SW increased APX activity five and four times, respectively, compared with control. In NaCl and MgCl 2 , APX activity was higher at the lower, than at the higher, concentrations, and it was unaffected by CaCl 2 treatment (Fig. 1 B). Catalase activity increased with increasing concentration of CaCl 2 and SW. In contrast, in the presence of KCl and MgCl 2 , CAT activity decreased when the concentration increased (Fig. 1 C). Superoxide dismutase activity decreased as the concentrations of NaCl and CaCl 2 increased. Conversely, in the presence of increasing concentrations of KCl, MgCl 2 and SW, SOD activity increased, but to different extents. The highest values of SOD were observed in the presence of SW and KCl (Fig. 1 D).

Effect of different salts on POX, APX, CAT, SOD of quinoa seeds 3 days after sowing. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
The amount of total phenols and the total antioxidant capacity of seeds varied with the salt used. Total phenols increased in seeds treated with NaCl and SW, but the greatest increase was observed in the presence of SW (Table 3 ). Increasing the concentrations of KCl and MgCl 2 decreased total phenols; no significant differences were instead observed with increasing the concentration of CaCl 2 with respect to control and the other treatments. Total antioxidant capacity increased in all treated seeds compared with control. The highest antioxidant capacity was detected in the presence of SW (Table 3 ).
Table 3.
Total antioxidant activity and total phenol content in quinoa seeds 3 days after sowing with different salt treatments: A= control; B= 100 mM NaCl, 2.54 mM KCl, 2.54 mM CaCl 2 , 13.38 mM MgCl 2 , 25 % SW; C= 200 mM NaCl, 5.08 mM KCl, 5.08 mM CaCl 2 , 26.76 mM MgCl 2 , 50 % SW; D = 300 mM NaCl, 7.62 mM KCl, 7.62 mM CaCl 2 , 40.1 mM MgCl 2 , 75 % SW; E = 400 mM NaCl, 10.16 mM KCl, 10.16 mM CaCl 2 , 53.52 mM MgCl 2 , 100 % SW. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
| NaCl | KCl | CaCl | MgCl | SW | |
|---|---|---|---|---|---|
| Total antioxidant activity (µmol α-tocopherol/g FW) | |||||
| A | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.65 ± 0.02 | 0.65 ± 0.02 |
| B | 3.15 ± 0.02 | 3.06 ± 0.04 | 1.91 ± 0.10 | 1.95 ± 0.10 | 2.24 ± 0.08 |
| C | 2.98 ± 0.02 | 2.91 ± 0.09 | 2.62 ± 0.03 | 2.69 ± 0.02 | 4.13 ± 0.15 |
| D | 3.06 ± 0.04 | 2.89 ± 0.1 | 2.50 ± 0.02 | 2.52 ± 0.08 | 3.24 ± 0.03 |
| E | 2.44 ± 0.05 | 2.94 ± 0.05 | 2.51 ± 0.03 | 2.75 ± 0.10 | 3.17 ± 0.02 |
| Total phenols (mg TAET/g DW) | |||||
| A | 209 ± 10 | 209 ± 10 | 209 ± 10 | 209 ± 10 | 209 ± 10 |
| B | 285 ± 10 | 200 ± 15 | 198 ± 10 | 167 ± 8 | 555 ± 25 |
| C | 307 ± 8 | 180 ± 10 | 223 ± 20 | 169 ± 5 | 521 ± 10 |
| D | 370 ± 12 | 181 ± 12 | 223 ± 18 | 171 ± 10 | 625 ± 20 |
| E | 347 ± 9 | 180 ± 13 | 224 ± 22 | 163 ± 6 | 568 ± 10 |
Ion contents
In seeds 3 days after sowing, the total quantity of ions increased with increasing concentration of NaCl. A similar response was observed in the presence of SW, the only exception being at the higher concentrations (mainly ungerminated seeds) (Fig. 2 ). In the presence of KCl and CaCl 2 , the total ionic concentration gradually decreased with increasing concentrations of salts due to the increased number of non-germinated seeds (Fig. 2 ). On increasing MgCl 2 concentrations, the reduction in total ion concentration compared with control is likely due to the greater seed dry weight observed (+20 %). The ratio of cations/anions was unchanged in CaCl 2 and MgCl 2 and in NaCl up to a concentration of 400 mM. Increasing the concentration of KCl caused an increase in cations and a concomitant decrease in anion percentage (Fig. 2 ). Seawater, at the lowest concentrations (25 and 50 %), increased the total ions, lowering the amount of cations (33 %) with respect to the anions. Conversely, at the highest concentrations (75 and 100 %), SW decreased the number of germinated seeds and consequently the quantity of total ions but did not affect the cation–anion ratio (Fig. 2 ).

Total ion content, cation and anion percentages in seeds of quinoa after 3 days of different salt treatments. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
The ratio of Na + to cations and of Cl − to anions changed significantly depending on the salts used (Table 4 ). The ratio of Na + /cations increased significantly in comparison to the control with increasing the concentration of NaCl and SW. No differences were observed in the presence of MgCl 2 , while with CaCl 2 a slight decline was observed with respect to the control. The greatest significant decrease in Na + /cations ratio (ranging from 30 to 22 %) was observed in seeds under KCl treatment. For the Cl − /anions ratio, the lowest values were observed in the presence of KCl and the highest with NaCl. Increasing the concentration of SW and NaCl, increased the Na + /Cl − ratio with respect to the control, while this ratio decreased in the presence of other salts when their concentrations increased (Table 4 ). The greatest decrease in K + /Cl − ratio was observed in the presence of NaCl with a reduction ranging from 49 to 87 %. Mg 2+ /Cl − and NH 4 + /Cl − ratios decreased with respect to the control, mainly with increasing salt concentrations (Table 4 ). The Ca 2+ /Cl − ratio decreased in each treatment except for CaCl 2 and KCl. The PO 4 3− /Cl − ratio was significantly reduced compared with control in the presence of SW, NaCl and MgCl 2 (Table 4 ). The highest SO 4 2− /Cl − ratios were observed in the presence of SW and the lowest under NaCl treatment.
Table 4.
Cation and anion content against chloride, in seeds of quinoa treated with different salts, expressed as percentages. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
| Treatments | Na /cations | Cl /anions | Na /Cl | PO /Cl | SO /Cl | K /Cl | NH /Cl | Mg /Cl | Ca /Cl | |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 5.0 | 13 | 29 | 635 | 23 | 349 | 76 | 69 | 28 | |
| NaCl | 100 mM | 41 | 49 | 63 | 102 | 1.8 | 51 | 23 | 15 | 3.2 |
| NaCl | 400 mM | 72 | 64 | 76 | 54 | 0 | 13 | 5.8 | 10 | 1.9 |
| MgCl | 13.38 mM | 5.0 | 33 | 16 | 202 | 6.5 | 185 | 34 | 53 | 19 |
| MgCl | 53.52 mM | 5.0 | 63 | 6.1 | 58 | 0 | 53 | 9.6 | 44 | 6.8 |
| CaCl | 2.54 mM | 6.0 | 16 | 30 | 530 | 15 | 345 | 45 | 55 | 14 |
| CaCl | 10.16 mM | 4.3 | 37 | 11 | 170 | 0.82 | 130 | 15 | 43 | 43 |
| KCl | 2.54 mM | 3.5 | 13 | 24 | 500 | 100 | 485 | 7.4 | 62 | 29 |
| KCl | 10.16 mM | 3.2 | 45 | 18 | 120 | 2.6 | 273 | 7.3 | 29 | 45 |
| SW | 25 % | 37 | 35 | 51 | 58 | 128 | 51 | 13 | 14 | 9.3 |
| SW | 100 % | 55 | 57 | 63 | 41 | 19 | 36 | 0 | 13 | 3.2 |
Growth parameters
Seawater and NaCl, at the highest concentrations, affected the dry weights of the whole seedlings, as shown by the highest fresh weight/dry weight (FW/DW) ratio (Table 5 ), and additionally they reduced the root mass ratio (RMR). These findings suggest that the reduction of root mass may be the cause of the decrease in the total dry matter of the seedlings (Table 5 ). Investigating the root morphology showed that the total root length in all treatments was the most affected root parameter, as shown by F -ratios (Table 6 ). The plants irrigated with SW (50 %) had root lengths, surface areas and root volumes significantly lower than control (Table 6 ).
Table 5.
Total FW/DW ratio, LMR (leaf mass ratio = leaf dry weight/plant dry weight) and RMR (root mass ratio = root dry weight/plant dry weight) of quinoa seedlings after 21 days under different salt treatments. Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
| FW/DW (g plant ) | LMR (g plant ) | RMR (g plant ) | ||
|---|---|---|---|---|
| Control | 9.7 ± 0.2 | 0.81 ± 0.02 | 0.19 ± 0.01 | |
| SW | 50 % | 11.8 ± 0.2 | 0.89 ± 0.01 | 0.11 ± 0.02 |
| KCl | 5.08 mM | 8.3 ± 0.7 | 0.83 ± 0.02 | 0.17 ± 0.01 |
| KCl | 10.16 mM | 8.5 ± 0.4 | 0.84 ± 0.02 | 0.16 ± 0.01 |
| CaCl | 5.08 mM | 9.0 ± 0.5 | 0.82 ± 0.03 | 0.18 ± 0.02 |
| CaCl | 10.16 mM | 8.8 ± 0.3 | 0.83 ± 0.01 | 0.17 ± 0.01 |
| NaCl | 200 mM | 9.8 ± 0.2 | 0.82 ± 0.02 | 0.18 ± 0.02 |
| NaCl | 400 mM | 10.8 ± 0.2 | 0.90 ± 0.02 | 0.10 ± 0.01 |
| MgCl | 26.76 mM | 9.2 ± 0.3 | 0.78 ± 0.02 | 0.22 ± 0.03 |
| MgCl | 53.52 mM | 9.5 ± 0.5 | 0.82 ± 0.01 | 0.18 ± 0.02 |
Table 6.
Analysis of variance of the effect of different salt treatments on root morphology parameters of quinoa seedlings 21 days old. *** P < 0.001; ** P < 0.01; * P < 0.05.
| Treatment | Total root length | Surface area | Volume | |
|---|---|---|---|---|
| SW | 2309.20*** | 200.82*** | 132.25*** | -ratio |
| 0.99 | 0.99 | 0.98 | ||
| KCl | 56.11*** | 2.98 | 8.22* | -ratio |
| 0.97 | 0.71 | 0.86 | ||
| CaCl | 49.18*** | 27.73** | 8.33* | -ratio |
| 0.97 | 0.95 | 0.86 | ||
| MgCl | 95.77*** | 21.25** | 11.27** | -ratio |
| 0.98 | 0.94 | 0.89 | ||
| NaCl | 42.67*** | 3.94 | 7.00* | -ratio |
| 0.97 | 0.75 | 0.84 |
Root parameters
Root length to mass ratio (SRL) and root fineness (RF), under SW, were not different from control while the ratio of root mass to volume (RTD) was lower. In seedlings irrigated with 400 mM NaCl, a higher SRL value indicated longer roots per unit root mass, while RTD and RF ratios were significantly reduced (Table 7 ), suggesting a decrease in root length and dry weight of seedlings treated with NaCl (200 mM) or MgCl 2 (26, 76 mM). Root morphology parameters were significantly changed by CaCl 2 and KCl compared with control but to different extents, depending on salt type (Table 7 ). NaCl, MgCl 2 and CaCl 2 , at lower concentrations, significantly increased RTD and RF ratios. No differences were observed when CaCl 2 and NaCl concentrations increased (Table 7 ). KCl, at all concentrations, significantly increased RTD and RF ratios, inducing a root system with thinner roots in comparison with control.
Table 7.
Specific root length (SRL = root length/root DW), root tissue density (RTD = root DW/root volume), root fineness (RF = root length/root volume) of quinoa seedlings after 21 days of different salt treatments. *** P < 0.001; ** P < 0.01; * P < 0.05.
| SRL (cm/mg DW) | RTD (mg DW/cm ) | RF (cm/cm ) | ||
|---|---|---|---|---|
| Control | 18.7 | 28 | 590 | |
| SW | 50 % | 21 | 23.1* | 613 |
| SW | 100 % | – | – | – |
| KCl | 5.08 mM | 19.3 | 35** | 675* |
| KCl | 10.16 mM | 21 | 31.3* | 690** |
| CaCl | 5.08 mM | 18.6 | 31.2* | 687** |
| CaCl | 10.16 mM | 20.0 | 27.5 | 520.9* |
| MgCl | 26.76 mM | 16.8 | 33.4* | 636* |
| MgCl | 53.52 mM | 20 | 29.0 | 601 |
| NaCl | 200 mM | 18.35 | 36.6* | 659* |
| NaCl | 400 mM | 24* | 19.6** | 522* |
In 21-day-old seedlings, total percentage of ions increased in the presence of NaCl, SW and KCl at all concentrations (Fig. 3 ) and at the highest concentrations of CaCl 2 and MgCl 2 . Total cations (Fig. 3 ) decreased in the presence of NaCl at all concentrations and at the highest concentrations of SW, MgCl 2 and CaCl 2 , with a concomitant increase in anion percentages (Fig. 3 ). No significant differences, in comparison to control, were observed in the presence of KCl.

Total ion content, cation and anion percentages in quinoa seedlings after 21 days of different salt treatments. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
Different salts caused a different distribution of cations and anions between root and shoot (Fig. 4 ). More cations were accumulated in shoots than in roots, decreasing in shoots when NaCl and MgCl 2 concentrations increased, while roots accumulated more anions than cations. The highest accumulation of anions was observed with CaCl 2 and KCl but with a different trend. In CaCl 2 , the anions increased in a concentration-dependent manner; in contrast increasing KCl concentrations lowered the anion percentage (Fig. 4 ). NaCl and MgCl 2 increased the cation concentration in roots as their external concentrations increased (Fig. 4 ).

Cation and anion percentages in root and shoot of quinoa seedlings after 21 days of different salt treatments.
The ratios of Na + /total cations and of Cl − /anions changed significantly depending on the salts used (Table 8 ). The Na + /cations ratio increased in comparison to the control with increasing the concentration of NaCl and SW. In contrast, Na + /cations ratio decreased with increasing the concentration of KCl, MgCl 2 and CaCl 2 . Cl − /anions ratios increased in the different salts at all concentrations, the highest value being observed with NaCl treatment. Increasing the concentration of SW and NaCl increased the Na + /Cl − ratio, while it was lowered in the other salts as their concentration increased. The K + /Cl − ratio decreased in the presence of all salts except for KCl, the greatest decrease being observed in NaCl. The Mg 2+ /Cl − ratio decreased with increasing concentrations of salts, other than for MgCl 2 . A similar situation was seen for the Ca 2+ /Cl − ratio, which decreased in each treatment except for CaCl 2 . The NH 4 + /Cl − ratio decreased in all situations as did SO 4 2− /Cl − ratios, where the highest values were detected in SW (Table 8 ).
Table 8.
Cation and anion content against chloride, in seedlings of quinoa treated with different salts, expressed as percentages. *Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). The values correspond to the average of five replicates.
| Treatments | Na /cations | Cl /anions | SO /Cl | K /Cl | NH /Cl | Mg /Cl | Ca /Cl | Na /Cl | |
|---|---|---|---|---|---|---|---|---|---|
| Control | 15 | – | – | – | – | – | – | – | |
| NaCl | 200 mM | 55 | 83 | 18 | 18 | 3 | 15 | 24 | 1.9 |
| NaCl | 400 mM | 65 | 87 | 15 | 11 | 2.9 | 5 | 21 | 1.2 |
| MgCl | 26.73 mM | 17 | 41 | 19 | 26 | 36 | 46 | 63 | 62 |
| MgCl | 53.46 mM | 11 | 85 | 18 | 21 | 18 | 49 | 50 | 4 |
| CaCl | 5.08 mM | 9 | 42 | 16 | 37 | 54 | 23 | 70 | 110 |
| CaCl | 10.16 mM | 2.6 | 78 | 4.7 | 13 | 20 | 9 | 78 | 149 |
| KCl | 5.08 mM | 12 | 13 | 6.4 | 345 | 14 | 23 | 56 | 27 |
| KCl | 10.16 mM | 6 | 71 | 4.0 | 654 | 5 | 21 | 52 | 26 |
| SW | 50 % | 43 | 67 | 48 | 30 | 1.9 | 54 | 11 | 11 |
The activity of the antioxidant enzymes depended on the salt and on the concentrations used (Fig. 5 ). Ascorbate peroxidase activity significantly decreased in the presence of MgCl 2 and KCl. In contrast, it increased in CaCl 2 -, SW- and NaCl-treated seedlings compared with control. POX activity increased in all treatments except for MgCl 2 and KCl. The most significant increase in catalase activity was in NaCl and SW. The same trend was observed for the SOD activity, with the highest values seen in the presence of SW and NaCl.

Effect of different salts on antioxidant enzymatic activities of quinoa seedlings after 21 days of different salt treatments. Mean ± SE ( n = 4–5). Different letters denote significant differences among the treatments ( P ≤ 0.05).
A significant increase in total phenols was observed in seedlings grown with NaCl and SW (Table 9 ). The SW was the most damaging agent, causing a 2-fold increase in the concentration of phenols. The total antioxidant capacity was doubled by NaCl and tripled by SW in respect to the control (Table 9 ).
Table 9.
Total antioxidant activity and total phenol content in quinoa seedlings after 21 days with different salt treatments: A = 200 mM NaCl, 5.08 mM KCl, 5.08 mM CaCl 2 , 26.76 mM MgCl 2 , 50 % SW; B = 400 mM NaCl, 10.16 mM KCl, 10.16 mM CaCl 2 , 53.52 mM MgCl 2 , 100 % SW. Different letters in the same column denote significant differences among treatments ( P ≤ 0.05). Mean ± SE ( n = 4–5).
| NaCl | KCl | CaCl | MgCl | SW | |
|---|---|---|---|---|---|
| Total antioxidant activity (mmol α-tocopherol/g FW) | |||||
| Control | 2.07 ± 0.03 | 2.07 ± 0.03 | 2.07 ± 0.03 | 2.07 ± 0.03 | 2.07 ± 0.03 |
| A | 4.35 ± 0.05 | 1.76 ± 0.02 | 2.06 ± 0.02 | 2.16 ± 0.07 | 6.20 ± 0.03 |
| B | 6.01 ± 0.06 | 1.56 ± 0.02 | 1.82 ± 0.04 | 1.58 ± 0.04 | – |
| Total phenols (mg TAET/g DW) | |||||
| Control | 272 ± 40 | 272 ± 40 | 272 ± 40 | 272 ± 40 | 272 ± 40 |
| A | 635 ± 20 | 322 ± 30 | 252 ± 30 | 222 ± 30 | 1075 |
| B | 840 ± 30 | 312 ± 10 | 240 ± 20 | 239 ± 20 | – |
In the Mediterranean region, besides water scarcity or high coastal soil salinity, it is mainly where saline water is used for irrigation that adverse effects are seen on crops, delaying or preventing germination and seedling growth ( Hegarty 1978 ; Almodares et al. 2007 ). Utilization of halophytes as crops would help in highly salinized zones, where only poor quality water, unsuitable for most agriculture, is available ( Rozema and Flowers 2008 ).
In this context, quinoa a facultative halophyte with exceptional nutritional quality could be useful to recover salinized land and to increase the poor agricultural economy of semiarid regions of the Mediterranean area. Our study focused on germination and seedling growth, because crop establishment depends on a successful germination and seedling emergence. Optimal germination for most halophytes has been reported in non-saline conditions ( Khan et al. 2002 ; Gul et al. 2013 ), and our data conform to these findings, showing toxicity of different salts. Results provided evidence for the existence of both ionic and osmotic effects by different treatments on seeds, depending on the salts used.
Our data clearly demonstrated that SW was the most detrimental solution affecting seed germination and seedling emergence of quinoa, mainly through its IE, confirming previous work showing that germination of halophytes was inhibited more by SW than different chlorides of Na, K, Mg ( Joshi et al. 1995 ). There is little information available on comparative influence of single salts and SW on seed germination of other halophytes ( Joshi et al. 1995 ; Baskin and Baskin 1998 ; Houle et al. 2001 ; Zia and Khan 2002 ; Atia et al. 2006 ; Liu et al. 2006 ). Some authors found NaCl more detrimental than SW and others the opposite ( Tirmizi et al. 1993 ; Zia and Khan 2002 ; Duan et al. 2003 ). Our data showed that the inhibition of different salt solutions on seed germination was in the order of SW > NaCl > KCl > CaCl 2 > MgCl 2 with no significant differences among the treatments in germination rapidity, except for the SW. The greatest negative effects of SW may be due to ion toxicity on germination, as a consequence of a coincident increase in cations and anions. Ion toxicity during germination has been previously demonstrated by Zehra et al. (2013) for the halophytic reed Phragmites karka : the inhibitory effect of different salts was interpreted mainly as an IE.
Although NaCl is the predominant salt in SW, its effects on seed germination and seedling growth were less detrimental than SW itself. The negative effects of SW on seedling growth may be ascribed to the induced accumulation of SO 4 2− (7.67 mmol g −1 DW, at least five times more than the other treatments) in leaves and of SO 4 2− (0.88 mmol g −1 DW) and Cl − (47.97 mmol g −1 DW) in roots. Sulfate is one of the components of sulfur-containing amino acids (cysteine and methionine) and many other compounds (e.g. glutathione or ferredoxin), which play important physiological functions, but when SO 4 2− is present in high concentration, it may affect plant development and crop yield, becoming injurious to plants ( Lianes et al. 2013 ). Lianes et al. (2013) previously showed that when the SO 4 2− is present in the medium, the capacities for ion compartmentalization and osmotic adjustment were reduced in the halophyte Prosopis strombulifera , resulting in water imbalance and symptoms of toxicity due to altered carbon metabolism (e.g. synthesis of sorbitol instead of mannitol, reduced sucrose production and protein content). This inhibition was partially mitigated when SO 4 2− and Cl − were present together in the solution, demonstrating a detrimental effect of the sulphate ion on plant growth ( Reginato et al. 2013 ).
According to Munns (2002) , the time scales for the osmotic and specific ionic component of salinity stress differ significantly, with the osmotic component dominating the first several days. Interestingly, however, comparing seed germination and seedling growth in the different salts, the results suggest that most probably ion toxicity is more detrimental to seedlings compared with the osmotic component of salt stress, as evidenced by the effect of SW treatment. This high salinity tolerance of quinoa, during germination and early seedling growth, may be explained by the existence of a significant gradient in the accumulation of potentially toxic (Na and Cl) and non-toxic essential (K, Mg, Ca, P and S) elements in seeds and also in the different distribution between shoot and root in salt-treated seedlings, as already demonstrated by Koyro and Eisa (2008) . Hence, we suggest that, once the seed's ability to exclude toxic Na + from the developing embryo fails, ion toxicity occurs, and seeds become unviable. The details of the distributions of ions between root and shoot showed differences among treatments; specifically with NaCl in shoot, we observed a significant accumulation of Na + , and little Cl − . In accordance with previous investigations ( Eisa et al. 2000 ), Na + was shown to be preferentially accumulated in shoots thereby the plants avoid excessive ion accumulation in the root tissues ( Koyro 2000 ; Ashraf et al. 2006 ).
Seawater caused an accumulation of Na + and SO 4 2− both in roots and in shoots, and an accumulation of Cl − in roots. Excessive accumulation of ions in halophytes (salt includers) under high substrate salinities (such a full strength SW) can lead to toxic effects in plants ( Munns 2005 ). The cause of injury is probably the salt load exceeding the ability of cells to compartmentalize salts in the vacuole. Salts might then build up rapidly in the cytoplasm inhibiting enzyme activity or alternatively, they might build up in cell walls, dehydrating the cell.
Considering the high energy cost of de novo synthesis of organic osmolytes ( Raven 1985 ), we can suppose that the seedlings tend to use Na + for osmotic adjustment. Hariadi et al. (2011) previously showed in quinoa that accumulation of Na + and K + was responsible for >95 % of cell turgor in old leaves and between 80 and 100 % in young leaves. A further role in the maintenance of turgor was also attributed to Cl − accumulated in roots ( James et al. 2006 ). Our results showed that the Cl − concentration was more than enough to contribute to osmotic adjustment maintaining root turgor as previously demonstrated in seedling of Stylosanthes guianensis by Veraplakorn et al. (2013) . Thus, it appears that the better germination and growth of cv. Titicaca observed in NaCl with respect to the other salts and SW may be achieved by the accumulation of inorganic osmolytes, particularly of Na + in shoots, and of Cl − in roots. The differences in ion uptake and distribution may be ascribed to properties of the roots. Roots have a high degree of plasticity, enabling plants to cope with a wide range of soil constraints ( Ho et al. 2005 ; Panuccio et al. 2011 ). Root morphology is a compromise among costs of resource capture, transport and efficiency ( Malamy 2005 ). Some morphological modifications at the individual root level can affect the structural and physiological characteristics of the entire root system and this can change water uptake and nutrient supply by plants. Specific root length, indicating root functionality ( Ryser 2006 ), characterizes the economic aspects of a root system, defining the cost-benefit ratio. Generally, under high salinity the costs per root length is minimized because of the growth limiting conditions. SW (50 %) reduced root growth and elongation, suggesting a decrease in photosynthate supply from the shoot. At the highest NaCl concentration, the greatest SRL ratio suggests the plants maximized the effectiveness of roots in water and nutrient uptake ( Fitter 1991 ). At the lowest concentrations of NaCl, KCl, CaCl 2 and MgCl 2 , the high root tissue density and root fineness ratios indicated that the seedlings explored a larger soil volume per unit of root surface area under stress than in its absence. In short, our data suggest that root morphology modifications should not be considered as a simple growth reduction, but rather as an induced reorientation of growth to avoid stress.
The results of this study clearly indicated that salt tolerance in this variety of quinoa is largely conferred by a delicate balance between osmotic adjustment and ion accumulation, showing differences in the ion compartmentalization between root and shoot. The greater negative effect of SW compared with NaCl, MgCl 2 CaCl 2 and KCl used separately suggests an additive and/or an interactive effect of these salts which cause an accumulation of ions in excess or leading to ion toxicity.
Conclusions
In conclusion, the present findings allow us to speculate that quinoa cv. Titicaca is a NaCl-tolerant cultivar of quinoa. Osmotic adjustment to NaCl salinity is largely conferred by inorganic ions, especially Na + , the main osmoregulatory material in the seedlings. The high SRL contributed to a high relative NaCl salinity tolerance in Titicaca, maintaining water and nutrient uptake. Higher SW toxicity may have been caused by SO 4 2− accumulation in seedlings that affected Titicaca germination and growth more than Cl − . Even if salinity reduced the productivity in terms of biomass, there was an increase in the antioxidant compounds, important health-protecting factors in food. On the basis of salt soil classifications currently used in all countries of the world, our results suggest that saline-sodic soils may be suitable for the cultivation of quinoa.
Sources of Funding
The research in the Mediterranea University laboratory and travelling was funded by Fattoria della Piana Company and by COST ( STSM FA0901 ).
Contributions by the Authors
S.S.A. participated in the experiments, M.R.P. and A.M. did the experiments, analysed the data and wrote the manuscript, S.E.J. participated in the writing of the manuscript, acquired the funds for S.S.A. through the COST action ‘Putting Halophytes to Work’, and provided quinoa seed material for the study.
Conflicts of Interest Statement
None declared.
Acknowledgements
The authors thank Carmelo Mallamaci for technical assistance and for taking care of the plants.
Literature Cited
- Abugoch L, Romero N, Tapia C, Silva J, Rivera M. Study of some physicochemical and functional properties of quinoa ( Chenopodium quinoa Willd.) protein isolates. Journal of Agricultural and Food Chemistry. 2008; 56 :4745–4750. [ PubMed ] [ Google Scholar ]
- Adolf VI, Shabala S, Andersen MN, Razzaghi F, Jacobsen S-E. Varietal differences of quinoa's tolerance to saline conditions. Plant and Soil. 2012; 357 :117–129. doi:10.1007/s11104-012-1133-7 . [ Google Scholar ]
- Adolf VI, Jacobsen S-E, Shabala S. Salt tolerance mechanisms in quinoa ( Chenopodium quinoa Willd.) Environmental and Experimental Botany. 2013; 92 :43–54. http://dx.dx.doi.org/10.1016/j.envexpbot.2012.07.004 . [ Google Scholar ]
- Almodares A, Hadi MR, Dosti B. Effects of salt stress on germination percentage and seedling growth in sweet sorghum cultivars. Journal of Biological Sciences. 2007; 7 :1492–1495. [ Google Scholar ]
- Ashraf M, Hameed M, Arshad M, Ashraf Y, Akhtar K. Salt tolerance of some potential forage grasses from Cholistan desert of Pakistan. In: Khan MA, Weber DJ, editors. Ecophysiology of high salinity tolerant plants. Dordrecht: Springer; 2006. pp. 31–54. Task Vegetation Sci 40. [ Google Scholar ]
- Atia A, Hamed KB, Debez A, Abdely C. Salt and seawater effects on the germination of Crithmum maritimum . In: Ozturk M, Waisel Y, Khan MA, Gork G, Verlag B, editors. Biosaline agriculture and salinity tolerance in plants. Switzerland: Springer: 2006. pp. 29–33. [ Google Scholar ]
- Baskin JM, Baskin CC. The annual dormancy cycle in buried weed seeds: a continuum. Biological Science. 1998; 35 :492–498. [ Google Scholar ]
- Beaumont F, Jouve HM, Gagnon J, Gaillard J, Pelmont J. Purification and properties of a catalase from potato tubers ( Solanum tuberosum ) Plant Science. 1990; 72 :19–26. [ Google Scholar ]
- Bonales-Alatorre E, Pottosin I, Shabala L, Chen Z-H, Zeng F, Jacobsen S-E, Shabala S. Differential activity of plasma and vacuolar membrane transporters contributes to genotypic differences in salinity tolerance in a halophyte species, Chenopodium quinoa . International Journal of Molecular Sciences. 2013; 14 :9267–9285. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Burrieza HP, Koyro A-W, Tosar LM, Kobayashi K, Maldonado S. High salinity induces dehydrin accumulation in Chenopodium quinoa Willd. cv. Hualhuas embryos. Plant and Soil. 2012; 354 :69–79. [ Google Scholar ]
- Chen Z, Newman I, Zhou M, Mendham N, Zhang G, Shabala S. Screening plants for salt tolerance by measuring K + flux: a case study for barley. Plant, Cell and Environment. 2005; 28 :1230–1246. [ Google Scholar ]
- Cotruvo JA. Water desalinization processes and associated health and environmental issue. Water Conditioning & Purification. 2005; 1 :13–17. [ Google Scholar ]
- Duan D, Liu X, Feng F, Li C. Effects of salinities on seed germination of halophytes Suaeda salsa . Chinese Agricultural Science Bulletin. 2003; 19 :168–172. [ Google Scholar ]
- Eisa S, Hussin S, Geissler N, Koyro HW. Effect of NaCl salinity on water relations, photosynthesis and chemical composition of quinoa ( Chenopodium quinoa Willd.) as a potential cash crop halophyte. Australian Journal of Crop Science. 2000; 6 :357–368. [ Google Scholar ]
- Fitter AH. Characteristics and functions of root systems. In: Waisel Y, Eshel A, Kafkafi U, editors. Plant roots: the hidden half. New York: Marcel Dekker; 1991. pp. 3–25. [ Google Scholar ]
- Flowers TJ, Galal HK, Bromham L. Evolution of halophytes: multiple origins of salt tolerance. Functional Plant Biology. 2010; 37 :604–612. [ Google Scholar ]
- Glenn EP, Brown JJ. Salt tolerance and crop potential of halophytes. Critical Reviews in Plant Sciences. 1999; 18 :227–255. [ Google Scholar ]
- González JA, Prado FE. Germination in relation to salinity and temperature in Chenopodium quinoa Willd. Agrochimica. 1992; 36 :101–108. [ Google Scholar ]
- Gul B, Ansari R, Flowers TJ, Khan MA. Germination strategies of halophyte seeds under salinity. Environmental and Experimental Botany. 2013; 92 :4–18. [ Google Scholar ]
- Gupta SA, Webb RP, Holaday AS, Allen RD. Overexpression of superoxide dismutase protects plants from oxidative stress. Plant Physiology. 1993; 103 :1067–1073. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hariadi Y, Marandon K, Tian Y, Jacobsen S-E, Shabala S. Ionic and osmotic relations in quinoa ( Chenopodium quinoa Willd.) plants grown at various salinity levels. Journal of Experimental Botany. 2011; 62 :185–193. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hasegawa PM, Bressan RA, Zhu JK, Bohnert HJ. Plant cellular and molecular responses to high salinity. Annual Review of Plant Physiology and Plant Molecular Biology. 2000; 51 :463–499. [ PubMed ] [ Google Scholar ]
- Hegarty TW. The physiology of seed hydration and dehydration, and the relation between water stress and the control of germination: a review. Plant, Cell and Environment. 1978; 1 :101–119. [ Google Scholar ]
- Ho MD, Rosas JC, Brown KM, Lynch JP. Root architectural trade offs for water and phosphorus acquisition. Functional Plant Biology. 2005; 32 :737–748. [ PubMed ] [ Google Scholar ]
- Houle G, More L, Reynolds LE, Siége J. The effect of salinity on different developmental stages of an endemic annual plant Aster laurentianus (Asteraceae) American Journal of Botany. 2001; 88 :62–67. [ PubMed ] [ Google Scholar ]
- Jacobsen S-E, Quispe H, Mujica A. 2001. Scientists and farmer—partners in research for the 21st century. Quinoa: an alternative crop for saline soils in the Andes. CIP Program Report 1999–2000, 403–408.
- Jacobsen S-E, Mujica A, Jensen CR. The resistance of quinoa ( Chenopodium quinoa Willd.) to adverse abiotic factors. Food Reviews International. 2003; 19 :99–109. [ Google Scholar ]
- Jacobsen S-E, Monteros C, Christiansen JL, Bravo LA, Corcuera LJ, Mujica A. Plant responses of quinoa ( Chenopodium quinoa Willd.) to frost at various phenological stages. European Journal of Agronomy. 2005; 22 :131–139. [ Google Scholar ]
- Jacobsen S-E, Monteros C, Corcuera LJ, Bravo LA, Christiansen JL, Mujica A. Frost resistance mechanisms in quinoa ( Chenopodium quinoa Willd.) European Journal of Agronomy. 2007; 26 :471–475. [ Google Scholar ]
- Jacobsen S-E, Liu F, Jensen CR. Does root-sourced ABA play a role for regulation of stomata under drought in quinoa ( Chenopodium quinoa Willd .) Scientia Horticulturae. 2009; 122 :281–287. [ Google Scholar ]
- Jacobsen S-E, Christiansen JL, Rasmussen J. Weed harrowing and inter-row hoeing in organic grown quinoa ( Chenopodium quinoa Willd.) Outlook on Agriculture. 2010; 39 :223–227. [ Google Scholar ]
- James JJ, Alder NN, Muhling KH, Läuchli AE, Shackel KA, Donovan LA, Richards JH. High apoplastic solute concentrations in leaves alter water relations of the halophytic shrub, Sarcobatus vermiculatus . Journal of Experimental Botany. 2006; 57 :139–147. [ PubMed ] [ Google Scholar ]
- Jensen CR, Jacobsen SE, Andersen MN, Núńez N, Andersen SD, Jasmussen L, Mogensen VO. Leaf gas exchange and water relation characteristics of field quinoa ( Chenopodium quinoa Willd.) during soil drying. European Journal of Agronomy. 2000; 13 :11–25. [ Google Scholar ]
- Joshi AJ, Mali BS, Hingalajia H. Halophytes a good source of forage production under salt stress conditions 1. Salvadora persica . In: Khan MA, Ungar IA, editors. Biology of salt tolerant plants. Karachi, Pakistan: Department of Botany, University of Karachi; 1995. pp. 353–360. [ Google Scholar ]
- Kader MA. A comparison of seed germination calculation formulae and the associated interpretation of resulting data. Journal and Proceeding of the Royal Society of New South Wales. 2005; 138 :65–75. [ Google Scholar ]
- Kader MA, Jutzi SC. Effects of thermal and salt treatments during imbibition on germination and seedling growth of sorghum at 42/19 °C. Journal of Agronomy and Crop Science. 2004; 190 :35–38. [ Google Scholar ]
- Khan MA, Gul B, Weber DJ. Effect of temperature, and salinity on the germination of Sarcobatus vermiculatus . Biologia Plantarum. 2002; 45 :133–135. [ Google Scholar ]
- Koyro HW. Effect of high NaCl-salinity on plant growth, leaf morphology, and ion composition in leaf tissues of Beta vulgaris ssp. maritima. Journal of Applied Botany. 2000; 74 :67–73. [ Google Scholar ]
- Koyro HW, Eisa SS. Effect of salinity on composition, viability and germination of seeds of Chenopodium quinoa Willd. Plant and Soil. 2008; 302 :79–90. [ Google Scholar ]
- Larcher W. Physiological plant ecology. 2nd totally rev. edition edn. Berlin and New York: Springer; 1980. p. 303. [ Google Scholar ]
- Lianes A, Bertazza G, Palacio G, Luna V. Different sodium salts cause different solute accumulation in the halophyte Prosopis strombulifera . Plant Biology. 2013; 15 :118–125. [ PubMed ] [ Google Scholar ]
- Liu X, Qiao H, Li W, Tadano T, Khan MA. Comparative effect of NaCl and seawater on seed germination of Suaeda salsa and Atriplex central asiatica . In: Ozturk M, Waisel Y, Khan MA, Gork G, editors. Biosaline agriculture and salinity tolerance in plants. Switzerland: Birkhauser; 2006. pp. 45–53. [ Google Scholar ]
- Malamy JE. Intrinsic and environmental response pathways that regulate root system architecture. Plant, Cell and Environment. 2005; 28 :67–77. [ PubMed ] [ Google Scholar ]
- Maughan PJ, Turner TB, Coleman CE, Elzinga DB, Jellen EN, Morales JA, Udall DJ, Fairbanks DJ, Bonifacio A. Characterization of salt overly sensitive 1 (SOS1) gene homologous in quinoa ( Chenopodium quinoa Willd.) Genome. 2009; 52 :647–657. [ PubMed ] [ Google Scholar ]
- Morais MC, Panuccio MR, Muscolo A, Freitas H. Does salt stress increase the ability of the exotic legume Acacia longifolia to compete with native legumes in sand dune ecosystems. Environmental and Experimental Botany. 2012a; 82 :74–79. [ Google Scholar ]
- Morais MC, Panuccio MR, Muscolo A, Freitas H. Salt tolerance traits increase the invasive success of Acacia longifolia in Portuguese coastal dunes. Plant Physiology and Biochemistry. 2012b; 55 :60–65. [ PubMed ] [ Google Scholar ]
- Munns R. Comparative physiology of salt and water stress. Plant, Cell and Environment. 2002; 25 :239–250. [ PubMed ] [ Google Scholar ]
- Munns R. Tansley review: genes and salt tolerance: bringing them together. New Phytologist. 2005; 167 :645–669. [ PubMed ] [ Google Scholar ]
- Munns R, Termaat A. Whole-plant responses to salinity. Functional Plant Biology. 1986; 13 :143–160. [ Google Scholar ]
- Munns R, Tester M. Mechanisms of salinity tolerance. Annual Review of Plant Biology. 2008; 59 :651–681. [ PubMed ] [ Google Scholar ]
- Munns R, Schachtman D, Condon A. The significance of a two-phase growth response to salinity in wheat and Barley. Australian Journal of Plant Physiology. 1995; 22 :561–569. [ Google Scholar ]
- Muscolo A, Sidari M, Santonoceto C, Anastasi U, Preiti G. Response of four genotypes of lentil to salt stress conditions. Seed Science and Technology. 2007; 35 :497–503. [ Google Scholar ]
- Muscolo A, Sidari M, Panuccio MR, Santonoceto C, Orsini F, De Pascale S. Plant responses in saline and semiarid environments: an overview. The European Journal of Plant Science and Biotechnology. 2011; 5 :1–11. [ Google Scholar ]
- Muscolo A, Panuccio MR, Heshel A. Ecophysiology of Pennisetum clandestinum : a valuable salt tolerant grass. Environmental and Experimental Botany. 2013; 92 :55–63. [ Google Scholar ]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant and Cell Physiology. 1981; 22 :867–880. [ Google Scholar ]
- Panda SK, Singha LB, Khan MH. Does aluminum phytotoxicity induce oxidative stress in greengram ( Vigna radiate )? Bulgarian Journal of Plant Physiology. 2003; 29 :77–86. [ Google Scholar ]
- Panuccio MR, Logoteta B, De Lorenzo F, Muscolo A. Root plasticity improves salt tolerance in different genotypes of lentil ( Lens culinaris ) Ecological Questions. 2011; 14 :95–97. [ Google Scholar ]
- Prasad KN, Yang B, Yang SY, Chen YL, Zhao MM, Ashraf M. Identification of phenolic compounds and appraisal of antioxidant and antityrosinase activities from litchi ( Litchi sinensis Sonn.) seeds. Food Chemistry. 2009; 116 :1–7. [ Google Scholar ]
- Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Analytical Biochemistry. 1999; 269 :337–341. [ PubMed ] [ Google Scholar ]
- Raven JA. Regulation of pH and generation of osmolality in vascular plants: a cost–benefit analysis in relation to efficiency of use of energy, nitrogen and water. Phytologist. 1985; 101 :25–77. [ PubMed ] [ Google Scholar ]
- Reginato M, Sosa L, Llanes A, Hampp E, Vettorazzi N, Reinoso H, Luna V. Growth responses and ion accumulation in the halophytic legume Prosopis strombulifera are determined by Na 2 SO 4 and NaCl. Plant Biology. 2013; 1 :1–10. [ PubMed ] [ Google Scholar ]
- Rozema J, Flowers TJ. Crops for a salinized world. Science. 2008; 322 :1478–1480. [ PubMed ] [ Google Scholar ]
- Ruiz-Carrasco K, Antognoni F, Coulibaly AK, Lizardi S, Covarrubias A, Martínez EA, Molina-Montenegro MA, Biondi S, Zurita-Silva A. Variation in salinity tolerance of four lowland genotypes of quinoa ( Chenopodium quinoa Willd.) as assessed by growth, physiological traits, and sodium transporter gene expression. Plant Physiology and Biochemistry. 2011; 49 :1333–1341. [ PubMed ] [ Google Scholar ]
- Ruiz KB, Biondi S, Oses R, Acuña-Rodríguez IS, Antognoni F, Martinez-Mosqueira EA, Coulibaly A, Canahua-Murillo A, Pinto M, Zurita-Silva A, Bazile D, Jacobsen S-E, Molina-Montenegro MA. Quinoa biodiversity and sustainability for food security under climate change. A review. Agronomy for Sustainable Development. 2014; 34 :349–359. [ Google Scholar ]
- Ryser P. The mysterious root length. Plant and Soil. 2006; 286 :1–6. [ Google Scholar ]
- Sanchez HB, Lemeur R, Van Damme P, Jacobsen S-E. Ecophysiological analysis of drought and salinity stress of quinoa ( Chenopodium quinoa Willd .) Food Reviews International. 2003; 19 :111–119. [ Google Scholar ]
- Schleiff U, Muscolo A. Fresh look at plant salt tolerance as affected by dynamics at the soil/root-interface using Leek and Rape as model crops. The European Journal of Plant Science and Biotechnology. 2011; 5 :27–32. [ Google Scholar ]
- Sevengor S, Yasar F, Kusvuran S, Ellialtioglu S. The effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidative enzymes of pumpkin seedling. African Journal of Agricultural Research. 2011; 6 :4920–4924. [ Google Scholar ]
- Shabala L, Mackay A, Tian Y, Jacobsen S-E, Zhou D, Shabala S. Oxidative stress protection and stomatal patterning as components of salinity tolerance mechanism in quinoa ( Chenopodium quinoa Willd) Physiologia Plantarum. 2012; 146 :26–38. [ PubMed ] [ Google Scholar ]
- Shabala S, Hariadi Y, Jacobsen S-E. Genotypic difference in salinity tolerance in quinoa is determined by differential control of xylem Na+ loading and stomatal density. Journal of Plant Physiology. 2013; 170 :906–914. [ PubMed ] [ Google Scholar ]
- Shannon MC, Noble CL. Genetic approaches for developing economic salt tolerant crops in agricultural salinity assessment and management. NY: American Society of Civil Engineering; 1990. p. 161. [ Google Scholar ]
- Sidari M, Santonoceto C, Anastasi U, Preiti G, Muscolo A. Variations in four genotypes of lentil under NaCl-salinity stress. American Journal of Agriculture and Biological Science. 2008; 3 :410–416. [ Google Scholar ]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965; 16 :144–158. [ Google Scholar ]
- Sun Y, Liu F, Bendevis M, Shabala S, Jacobsen S-E. Sensitivity of two quinoa ( Chenopodium quinoa Willd.) varieties to progressive drought stress. Journal of Agronomy and Crop Science. 2014; 200 :12–23. [ Google Scholar ]
- Tirmizi SAS, Khan KM, Qadir SA. Study on salt tolerance of Hippophae rhamnoides L. during germination. Pakistan Journal of Science and Industrial Research. 1993; 36 :252–257. [ Google Scholar ]
- Trognitz BR. Prospects of breeding quinoa for tolerance to abiotic stress. Food Reviews International. 2003; 19 :129–137. [ Google Scholar ]
- Ungar I. Effect of salinity on seed germination, growth, and ion accumulation of Atriplex patula (Chenopodiaceae) American Journal of Botany. 1996; 83 :604–607. [ Google Scholar ]
- Veraplakorn V, Nanakorn M, Kaveeta L, Suwanwong S, Bennett IJ. Variation in ion accumulation as a measure of salt tolerance in seedling and callus of Stylosanthes guianensis . Theoretical and Experimental Plant Physiology. 2013; 25 :106–115. [ Google Scholar ]
- Yeo AR, Flowers TJ. Salinity resistance in rice ( Oryza sativa L.) and a pyramiding approach to breeding varieties for saline soils. In: Turner NC, Passioura JB, editors. Effect of drought on plant growth. Salts in soils. Melbourne, Australia: CSIRO; 1986. pp. 161–173. [ Google Scholar ]
- Zehra A, Gul B, Ansari R, Alatar AA, Hegazy AK, Khan MA. Interactive effect of salt, light and temperature on seed germination and recovery of a halophytic grass. Phragmites karka . Pakistan Journal of Botany. 2013; 45 :725–736. [ Google Scholar ]
- Zhu JK. Plant salt stress. In: O'Daly A, editor. Encyclopedia of life sciences. Chichester: John Wiley & Sons, Ltd; 2007. pp. 1–3. doi:10.1002/9780470015902.a0001300.pub2 . [ Google Scholar ]
- Zia S, Khan MA. Comparative effect of NaCl and seawater on seed germination of Limonium stocksii . Pakistan Journal of Botany. 2002; 34 :345–350. [ Google Scholar ]
ORIGINAL RESEARCH article
Effects of temperature and salinity on seed germination of three common grass species.

- 1 State Key Laboratory of Grassland Agro-Ecosystems, Key Laboratory of Grassland Livestock Industry Innovation, Ministry of Agriculture and Rural Affairs, College of Pastoral Agriculture Science and Technology, Lanzhou University, Lanzhou, China
- 2 Plants and Ecosystems (PLECO), Department of Biology, University of Antwerp, Wilrijk, Belgium
Temperature and salinity significantly affect seed germination, but the joint effects of temperature and salinity on seed germination are still unclear. To explore such effects, a controlled experiment was conducted, where three temperature levels (i.e., 15, 20, and 25°C) and five salinity levels (i.e., 0, 25, 50, 100, and 200 mmol/L) were crossed, resulting in 15 treatments (i.e., 3 temperature levels × 5 salinity levels). Three typical grass species ( Festuca arundinacea , Bromus inermis , and Elymus breviaristatus ) were used, and 25 seeds of each species were sown in petri dishes under these treatments. Germination percentages and germination rates were calculated on the basis of the daily recorded germinated seed numbers of each species. Results showed that temperature and salinity significantly affected seed germination percentage and germination rate, which differed among species. Specifically, F. arundinacea had the highest germination percentage, followed by E. breviaristatus and B. inermis , with a similar pattern also found regarding the accumulated germination rate and daily germination rate. Generally, F. arundinacea was not sensitive to temperature within the range of 15–25°C, while the intermediate temperature level improved the germination percentage of B. inermis , and the highest temperature level benefited the germination percentage of E. breviaristatus . Moreover, F. arundinacea was also not sensitive to salinity within the range of 0–200 mmol/L, whereas high salinity levels significantly decreased the germination percentage of B. inermis and E. breviaristatus . Thus, temperature and salinity can jointly affect seed germination, but these differ among plant species. These results can improve our understanding of seed germination in saline soils in the face of climate change.
Introduction
Seed germination is a fundamental stage in the life cycle of a plant ( Bewley, 1997 ; Nimbalkar et al., 2020 ). Seed germination is significantly affected by both physical and biological factors such as temperature and species identity ( Larsen et al., 2004 ; Bewley et al., 2013 ; Zhang et al., 2020 ). Soil salinization is one of the major drivers of soil degradation ( Zhang et al., 2015a ; Gorji et al., 2017 ), and it can significantly affect seed germination and the following stages such as seedling establishment ( Khan and Gulzar, 2003 ; Qu et al., 2008 ). Over 900 Mha land is impacted by salinity in the whole world ( Rengasamy, 2006 ; Shiade and Boelt, 2020 ). Climate change such as extreme warming is expected to be more frequent in the future ( Khan and Qaiser, 2006 ; Blackport and Screen, 2020 ; Bai et al., 2021 ). Such change could significantly affect seed germination ( Walck et al., 2011 ; Mondoni et al., 2012 ). Soil salinization could become more serious in the face of climate change because global warming generally increases evaporation, which can promote soil salinization ( Utset and Borroto, 2001 ). Therefore, salinity and temperature would jointly affect seed germination, especially in the arid and semi-arid areas of northeastern China, where the soil salinization area covers over 70% of the total land area ( Wang et al., 2011 ). Moreover, several species are facing population reductions due to human disturbances and climate change ( Richmond et al., 2007 ; Ureta et al., 2012 ; Gu et al., 2018 ). Thus, exploring seed germination under the ongoing soil salinization and global warming is important in assessing the stability of plant community.
Theoretically, the seed germination of each species has an optimal temperature, under which seeds could germinate better than under other temperatures. Previous studies found that salinity decreased seed germination of some species compared with non-saline conditions ( Khan and Gulzar, 2003 ; Qu et al., 2008 ). However, the impact of salinity on seed germination might be modified by temperature, as Gorai and Neffati (2007) found that negative effects of salinity on seed germination were less severe at the optimum temperature, as the additional environmental stress at low or high temperatures would thus be alleviated ( Al-Khateeb, 2006 ). Yet, Khan and Ungar (2001) found that the effect of salinity was stronger at lower temperatures, while Delesalle and Blum (1994) revealed that such effect was stronger at higher temperatures. Finally, Khan and Ungar (1998) showed that the effect of salinity was not affected by temperature in their experiment. Thus, the joint effects of salinity and temperature on seed germination are still unclear ( Fernandez et al., 2015 ; Lin et al., 2018 ).
In response to local salinity and suboptimal temperatures, plant species developed different strategies, including adjusting germination percentage or germination rate through modifying seed dormancy and/or seed viability ( Ungar, 1995 ; Khan et al., 2001 ; Khan and Ungar, 2001 ; Shahba et al., 2008 ; Guan et al., 2009 ). Such responses can further alter seedling establishment and seedling growth ( Gu et al., 2018 ; Del Vecchio et al., 2021 ). Exploring the effects of salinity and temperature on seed germination may shed light on understanding the mechanisms of species coexistence. However, studying such effects under natural conditions is difficult since (1) soil conditions such as temperature and salinity vary spatially and temporally ( Hermans et al., 2016 ), which makes it difficult to keep a constant level of temperature or salinity. (2) Other environmental variables such as radiation and soil moisture hamper separating the roles of temperature and salinity from these factors ( Khan and Ungar, 1997 ; De Boeck et al., 2015 ; Borja et al., 2016 ; Bhatt et al., 2020 ). (3) Some particular species in a community such as halophytes and xerophytes may skew the results, where halophytes can modify their strategies (e.g., reduce seed germination percentage or delay the start of germination under the high level of salinity) to adapt to different salinity levels ( Gulzar and Khan, 2001 ; Khan and Gul, 2006 ; El-Keblawy et al., 2020 ), and xerophytes can grow well under conditions with a large variation of temperature ( Zhang et al., 2015b ).
To explore the joint effects of temperature and salinity on seed germination of grass species with less confounding factors ( Figure 1 ), a controlled experiment was thus conducted. Three typical grass species ( Festuca arundinacea , Bromus inermis , and Elymus breviaristatus ) widely used as forage species ( Lu et al., 2008 ) that can be potentially grown in saline soils were exposed to three levels of temperature and five levels of salinity. Specifically, (1) we expect seed germination in general to be the highest at the intermediate level of temperature (20°C), which is thought to be closest to the optimal temperature for seed germination for such grasses ( Romo and Eddleman, 1995 ; Lu et al., 2008 ; Zhang et al., 2013 ). (2) We assume that seed germination would consistently decrease with increasing salinity ( Wu et al., 2015 ; Zhang and Dai, 2019 ). (3) We anticipate that the intermediate (and supposed optimum) temperature level would alleviate the negative effects of salinity on seed germination ( Gorai and Neffati, 2007 ).
Figure 1. The expected effects of temperature (three levels: low, medium, and high) and salinity (five levels: no, low, medium, high, and extreme) on seed germination, where “+” and “–” refer to the positive and negative effect, respectively. More “+” or “–” indicates a stronger effect.
Materials and Methods
Experimental design.
To explore the effects of temperature and salinity on seed germination, an experiment was conducted at the Yuzhong Campus of Lanzhou University, China (104°09′44″N, 35°56′55″E) from 6 April to 25 April 2021. Three levels of temperature (i.e., 15, 20, and 25°C) and five levels of salinity (i.e., NaCl concentration 0, 25, 50, 100, and 200 mmol/L) were created to simulate the future climatic conditions. Note that these temperature and salinity levels were set in line with previous studies ( Lu et al., 2008 and Zhang et al., 2013 for temperature levels; Yang et al., 2009 and Li et al., 2019 for salinity levels). Three target grass species ( F. arundinacea , B. inermis , and E. breviaristatus ) were exposed to these 15 treatments. A recent study reported that different varieties of a species responded differently to salinity stress ( Shiade and Boelt, 2020 ). However, this study aimed to explore the responses of seed germination of different species to the joint effects of temperature and salinity, not of varieties of specific species. Seeds of the three species used in our experiment were bought from a commercial company (Best, Beijing, China). Further information can be found in Table 1 . Twenty-five seeds of each species were applied in each treatment. All seeds were evenly sown in petri dishes with two sheets of filter paper (diameter 7 cm). The filter paper was saturated with saline solutions (around 5 mL) and kept stable during the experiment.
Table 1. Information of the seeds applied in this experiment.
Three incubators (LRH-250-G, Illuminating Incubator) were used, and each of them was set at one of the three applied temperature levels. Petri dishes with the five salinity levels were randomly stored in each of these chambers. These petri dishes were covered with lids at the beginning of the experiment, and they were removed after the germination of the seeds since lids impeded the growth of these seedlings. Five replicates were used per treatment, resulting in 225 petri dishes (i.e., 3 species × 3 temperature levels × 5 salinity levels × 5 replications) in total. Note that the seed germination test was conducted according to the rules of the International Seed Testing Associations ( ISTA, 2018 ), and the germinated seeds in each petri dish were daily recorded. Seeds were treated as germinated when the radicle was more than 2 mm long ( Shiade and Boelt, 2020 ). This experiment was ended when there was no additional germination for 3 days.
Data Analysis and Statistics
Germination percentage (GP) was calculated by dividing the germinated seed number by the total seed number in each petri dish along the experimental period. Accumulated germination rate (AGR) and daily germination rate (DGR) in each petri dish were calculated by the following two equations:
AGR = (∑ G P i )/ i , where i is the day after seed set in these chambers;
DGR = the newly germinated seed number per day/25 in each petri-dish.
To explore the seed germination during the experiment, four separate analyses were conducted. First, repeated-measures ANOVA was used to explore the differences of GP, AGR, and DGR among the target species. Second, repeated-measures ANOVAs were applied to investigate the effects of temperature, species, and their interactions on the GP. Third, repeated-measures ANOVAs were employed to test the effects of salinity, species, and their interactions on the GP. A significant effect of species was found in the second and third analyses. Thus, separate repeated-measures ANOVAs analyses were conducted for each species, where temperature (or salinity), time, and their interaction were treated as variables. Fourth, MANOVA was performed to examine the impacts of temperature, salinity, species and their interactions on the GP, AGR at the last day of the experiment, and the average DGR during the experiment. Note that time (i.e., the germination date) was treated as an extra factor in these analyses except the last one.
Curve estimations were conducted to explore the relationships between salinity and GP separated by temperature, where linear, quadratic, power, and exponential curves were tested. A better model was identified with a lower Akaike Information Criterion (AIC) and a significant P -value. All statistics were performed with SPSS 23.0 ( IBM Corp, 2015 ).
In the first analysis, GP, AGR, and DGR varied within species, germination date, and species × germination date interaction ( Table 2 and Figure 2 ). On average, the GP of F. arundinacea was higher than that of E. breviaristatus and B. inermis , and the GP of E. breviaristatus was in turn higher than that of B. inermis ( Figure 2A ). Such a pattern was also found for AGR ( Figure 2B ) and DGR ( Figure 2C ). B. inermis germinated faster at the beginning of the experiment, while its germination decreased faster than the other two species during the experiment ( Figure 2C ). The interaction effect between species and germination date was likely caused by the convergence of the seed germination ( Figure 2 ).
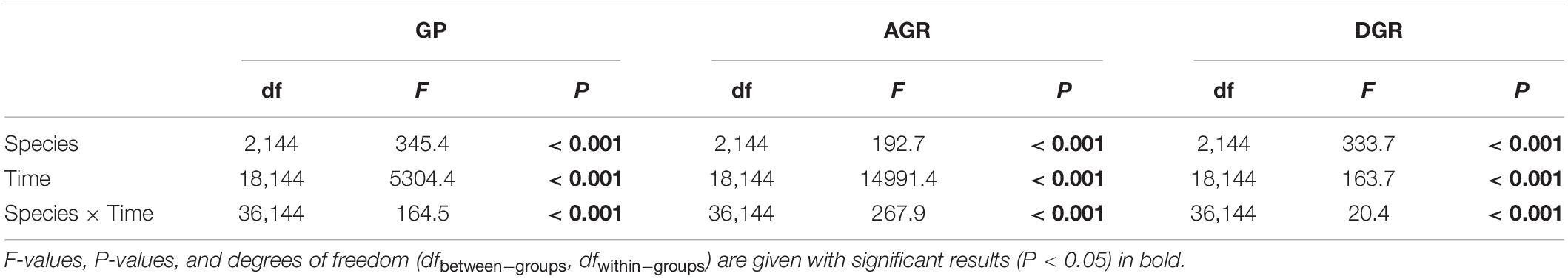
Table 2. Effects of species, time, and their interaction in repeated-measures ANOVA of germination percentage (GP), accumulated germination rate (AGR), and daily germination rate (DGR).
Figure 2. The germination percentage (A) , accumulated germination rate (B) , and daily germination rate (C) of the three target grass species ( Festuca arundinacea , Bromus inermis , and Elymus breviaristatus , labeled as red, orange, and blue color, respectively) along time (i.e., the germination date). Note that these figures are derived from the average data of the three temperature levels and five salinity levels.
In the second analysis, on investigating the effects of species, temperature, and their interaction on GP, the three target species demonstrated different responses ( Table 3 and Figure 3 ). The GP of F. arundinacea was not sensitive to the relatively high levels of temperature ( Figure 3A ). The GP of B. inermis was highest at the intermediate temperature level ( Figure 3B ), and the GP of E. breviaristatus was highest at the highest temperature level in this study ( Figure 3C ).
Table 3. Effects of species, temperature, time, and their interactions in repeated-measures ANOVA of germination percentage, which was separated by species since it was a significant factor.
Figure 3. Seed germination percentages of Festuca arundinacea (A) , Bromus inermis (B) , and Elymus breviaristatus (C) under different temperatures as a function of salinity levels. Note that all these significant equations are non-linear, so P -values are given.
In the third analysis, on testing the effects of species, salinity, and their interaction on GP, the three target species likewise showed different patterns ( Table 4 and Figure 3 ). The GP of F. arundinacea was not sensitive to relatively low levels of salinity. However, the other two species showed a different pattern, where the higher salinity levels decreased the GP of B. inermis , while the intermediate level of salinity increased. The GP of E. breviaristatus consistently decreased with increasing salinity levels. Moreover, the intermediate temperature level (i.e., 20°C) × lowest salinity level (i.e., 0 mmol/L) generated the highest GP for F. arundinacea , while the highest temperature level (i.e., 25°C) × lowest salinity level (i.e., 0 mmol/L) generated the highest GP for B. inermis and E. breviaristatus ( Figure 4 ).
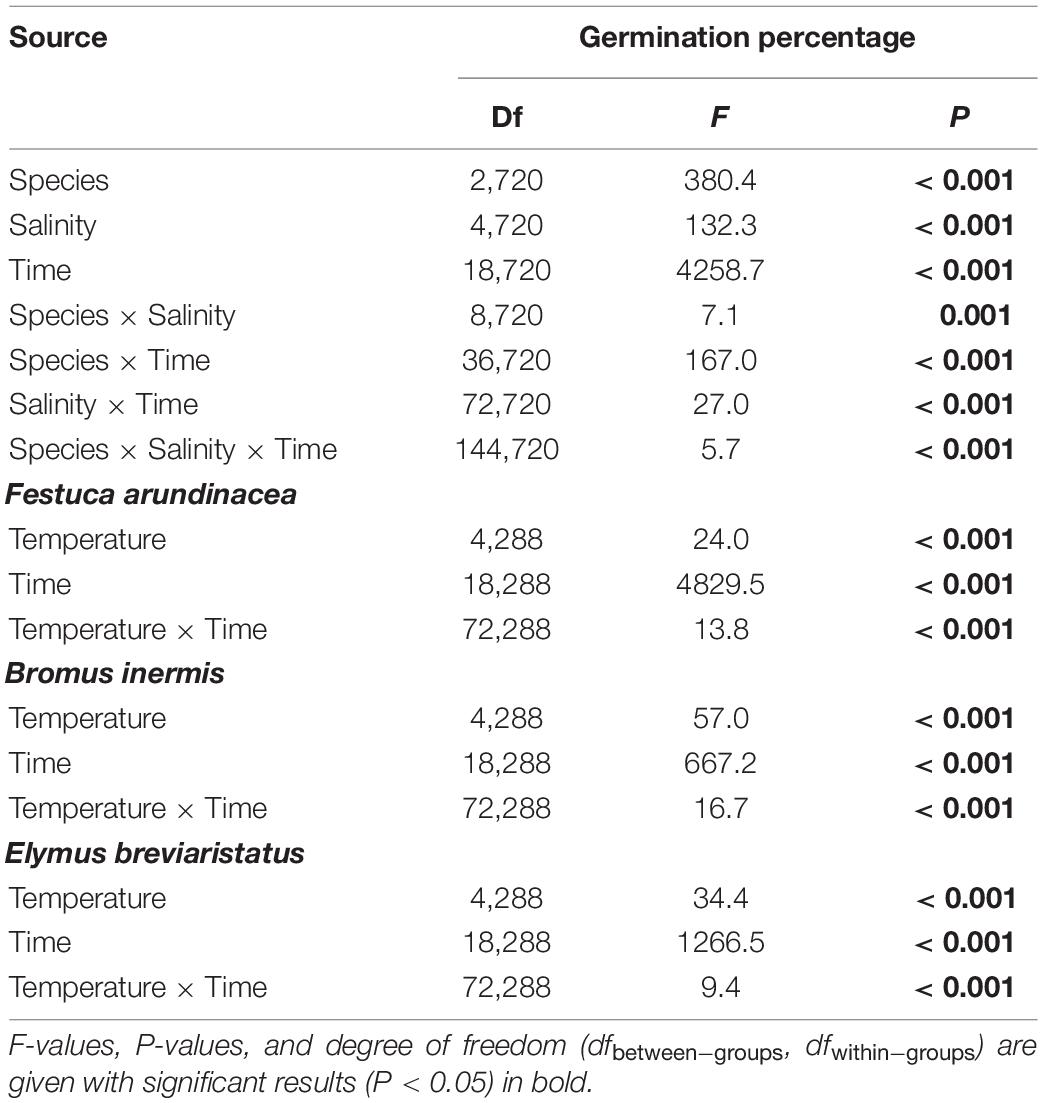
Table 4. Effects of species, salinity, time, and their interactions in repeated-measures ANOVA of germination percentage, which was separated by species since it was a significant factor.
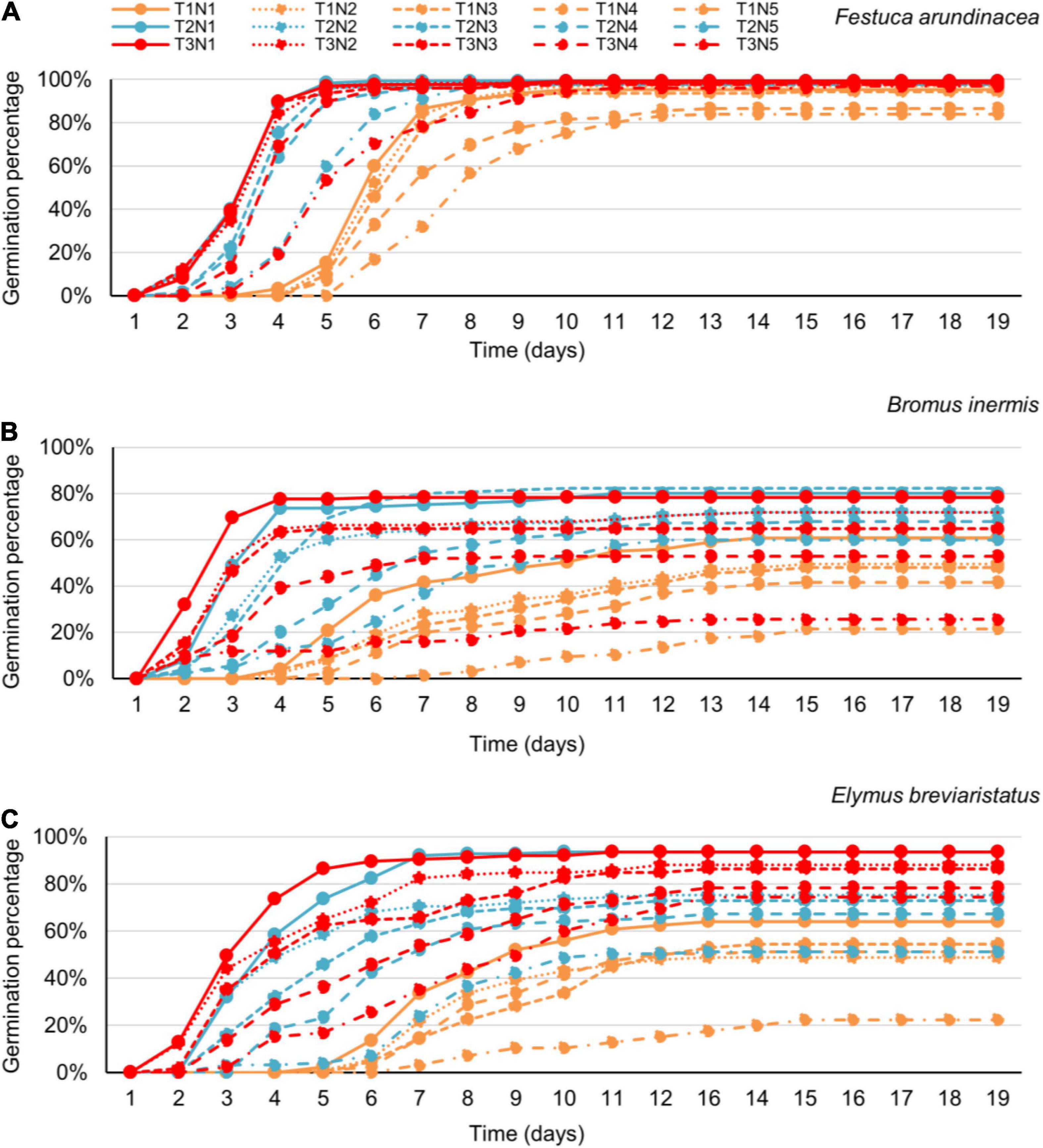
Figure 4. The joint effect of temperature and salinity on seed germination of Festuca arundinacea (A) , Bromus inermis (B) , and Elymus breviaristatus (C) as a function of time (i.e., the germination date). Note that T1–T3 refer to the three temperature levels, that is, 15, 20, and 25°C, respectively, while N1–N5 reflect the five salinity levels, that is, 0, 25, 50, 100, and 200 mmol/L, respectively.
Finally, exploring the effects at the last day of the experiment, species, temperature, salinity, species × temperature, species salinity, and species × temperature × salinity significantly affected GP, AGR, and DGR ( Table 5 and Figure 4 ), while there were no significant temperature × salinity effects at this measurement data.
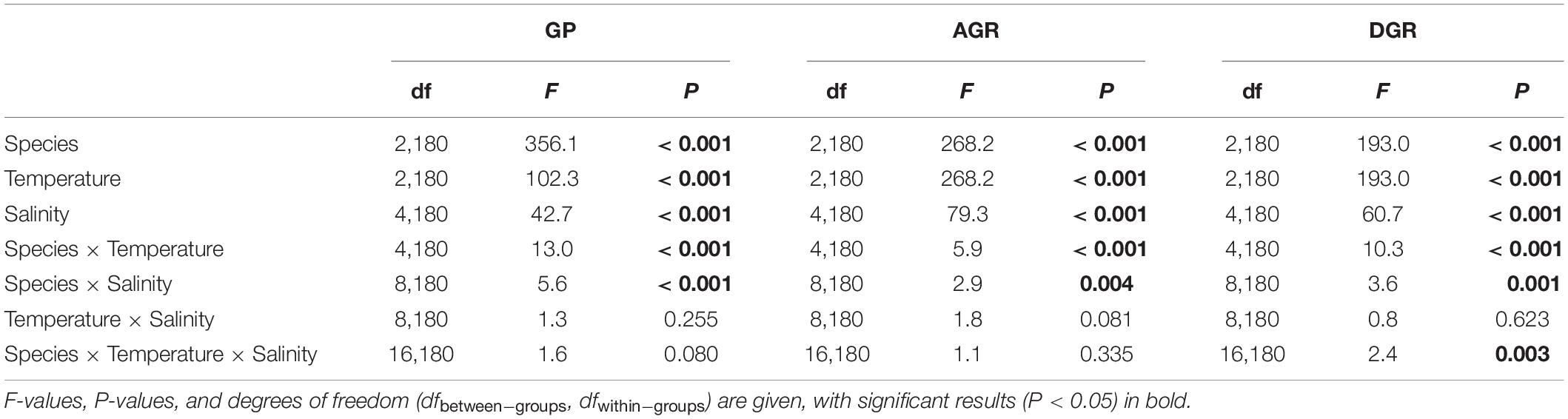
Table 5. Effects of temperature, salinity, species, and their interactions in MANOVA of germination percentages (GP), accumulated germination rate (AGR), and daily germination rate (DGR).
The first hypothesis stated that seed germination would be the highest at the intermediate level of temperature. This was partly supported as such a pattern was found in one of the target plant species (i.e., B. inermis , Figure 3B ), where lower germination was found at lower temperatures. This is partly consistent with the finding of Ao et al. (2014) , where seed germination of B. inermis was low at lower temperatures. Note that such a pattern was not found in the other two target species. For F. arundinacea , temperature levels in this study may have all been in the optimal temperature range of this species ( Lu et al., 2008 ), while for E. breviaristatus , the optimal temperature of seed germination might have been higher than the temperature levels we set ( Figure 3C ).
Our second hypothesis aimed to test whether seed germination would be reduced at higher levels of salinity. This was supported as seed germination of the three target species was generally lower at higher salinity levels, even though they responded inconsistently to the salinity gradient ( Figure 3 ). Such results are in line with previous studies on the target species F. arundinacea ( Shiade and Boelt, 2020 ), B. inermis ( Yang et al., 2009 ). and E. breviaristatus ( Li et al., 2019 ), and on other species such as Helianthus annuus ( Wu et al., 2015 ), Oryza sativa ( Xu et al., 2011 ), and Zea mays ( Khodarahmpour et al., 2012 ). Such results could be related to the effects of ion toxicity on seed germination ( Panuccio et al., 2014 ). The different responses of plants to salinity are likely caused by the genetic traits of these species ( Vu et al., 2015 ; Chamorro et al., 2017 ) and their growing conditions ( Mira et al., 2017 ).
The last hypothesis focused on the joint effects of salinity and temperature on seed germination, and we expected that the negative effect of salinity on seed germination would be alleviated at the intermediate level of temperature. This was supported by our findings in one of the three target species ( B. inermis , Figure 3B ), where the germination percentage of B. inermis at the intermediate temperature level was higher than at the other two temperature levels, and the germination percentage decreased more slowly with increasing salinity compared with the other two temperature levels. This is in line with the finding of Gorai and Neffati (2007) , where the negative effect of salinity on seed germination was alleviated at the optimum temperature. However, the other two species did not show such a pattern.
Results of this study should be interpreted and extrapolated with caution because of the following two reasons. One is that NaCl solutions in this study might evaporate at different rates when they were set under different temperatures during the experiment ( Sayer et al., 2017 ), and this may affect the ultimate salinity level and thus the ensuing results. The other is that each level of temperature was kept constant during the experiment in this study, while previous studies found that variation of temperature can benefit seed germination ( Liu et al., 2013 , 2017a ; Spindelböck et al., 2013 ; Burghardt et al., 2016 ). Moreover, soil resources such as soil temperature and salinity vary a lot even at a short distance in natural conditions ( Maestre et al., 2003 ; Lundholm, 2010 ). Thus future studies on seed germination should consider the heterogeneous distributions of these factors, potentially in combination with other aspects of soil heterogeneity (e.g., Liu et al., 2017b , c , 2019 ; Liu and Hou, 2021 ).
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
YL designed the study, conducted the analyses, and wrote the first draft of the manuscript. SZ collected the data. All authors contributed significantly to the manuscript.
This work was supported by the Key Research and Development Program of Forestry and Grassland Administration of Ningxia. Hui Autonomous Region, China “Study on Construction Mode and Key Technology of Grassland Ecological Civilization Demonstration Area in Ningxia Hui Autonomous Region”. YL holds a start-up fund from Lanzhou University (508000-561119213).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Yan Zhang, Sixia Liu, and Qingyu Du for the experimental assistance. We would also like to thank Zhixia Ying for her valuable comments on the earlier versions of this manuscript.
Al-Khateeb, S. A. (2006). Effect of salinity and temperature on germination, growth and ion relations of Panicum turgidum Forssk. Bioresour. Technol. 97, 292–298. doi: 10.1016/j.biortech.2005.02.041
PubMed Abstract | CrossRef Full Text | Google Scholar
Ao, M., Miura, R., and Tominaga, T. (2014). Germination characteristics of four common perennial grasses of Inner Mongolian grassland, China. Grassl. Sci. 60, 9–14. doi: 10.1111/grs.12043
CrossRef Full Text | Google Scholar
Bai, H., Xiao, D., Wang, B., Liu, D., Feng, P., and Tang, J. (2021). Multi-model ensemble of CMIP6 projections for future extreme climate stress on wheat in the North China plain. Int. J. Climatol. 41, E171–E186.
Google Scholar
Bewley, J. D. (1997). Seed germination and dormancy. Plant Cell 9, 1055–1066.
Bewley, J. D., Bradford, K., and Hilhorst, H. (2013). Seeds: Physiological of Development, Germination and Dormancy , 3rd Edn, New York, NY: Springer, 407.
Bhatt, A., Gairola, S., Caron, M. M., Santo, A., Murru, V., El-Keblawy, A., et al. (2020). Effects of light, temperature, salinity, and maternal habitat on seed germination of Aeluropus lagopoides (Poaceae): an economically important halophyte of arid Arabian deserts. Botany 98, 117–125.
Blackport, R., and Screen, J. A. (2020). Insignificant effect of Arctic amplification on the amplitude of midlatitude atmospheric waves. Sci. Adv. 6:eaay2880. doi: 10.1126/sciadv.aay2880
Borja, J., Fernando, A. O. S., Alessandra, F., Peter, P., and Lucy, E. C. (2016). Seed germination traits can contribute better to plant community ecology. J. Veg. Sci. 27, 637–645. doi: 10.1111/jvs.12375
Burghardt, L. T., Edwards, B. R., and Donohue, K. (2016). Multiple paths to similar germination behavior in Arabidopsis thaliana . New Phytol. 209, 1301–1312. doi: 10.1111/nph.13685
Chamorro, D., Luna, B., and Moreno, J. M. (2017). Germination responses to current and future temperatures of four seeder shrubs across a latitudinal gradient in western Iberia. Am. J. Bot. 104, 83–91. doi: 10.3732/ajb.1600278
De Boeck, H. J., Vicca, S., Roy, J., Nijs, I., Milcu, A., Kreyling, J., et al. (2015). Global change experiments: challenges and opportunities. Bioscience 65, 922–931.
Del Vecchio, S., Mattana, E., Ulian, T., and Buffa, G. (2021). Functional seed traits and germination patterns predict species coexistence in Northeast Mediterranean foredune communities. Ann. Bot. 127, 361–370. doi: 10.1093/aob/mcaa186
Delesalle, V. A., and Blum, S. (1994). Variation in germination and survival among families of Sagittaria latifolia in response to salinity and temperature. Int. J. Plant Sci. 155, 187–195.
El-Keblawy, A., Elnaggar, A., Tammam, A., and Mosa, K. A. (2020). Seed provenance affects salt tolerance and germination response of the habitat-indifferent Salsola drummondii halophyte in the arid Arabian deserts. Flora 266:151592. doi: 10.1016/j.flora.2020.151592
Fernandez, I. C. D., Luque, E. G., Mercado, F. G., and Marrero, J. M. (2015). Germination responses of Limonium insigne (Coss.) Kuntze to salinity and temperature. Pak. J. Bot. 47, 807–812.
Gorai, M., and Neffati, M. (2007). Germination responses of Reaumuria vermiulata to salinity and temperature. Ann. Appl. Biol. 151, 53–59.
Gorji, T., Sertel, E., and Tanik, A. (2017). Monitoring soil salinity via remote sensing technology under data scare conditions: a case study from Turkey. Ecol. Indic. 74, 384–391.
Gu, R., Zhou, Y., Song, X., Xu, S., Zhang, X., Lin, H., et al. (2018). Effects of temperature and salinity on Ruppia sinensis seed germination, seedling establishment and seedling growth. Mar. Pollut. Bull. 134, 177–185. doi: 10.1016/j.marpolbul.2017.08.013
Guan, B., Zhou, D., Zhang, H., Tian, Y., Japhet, W., and Wang, P. (2009). Germination responses of Medicago ruthenica seeds to salinity, alkalinity, and temperature. J. Arid. Environ. 73, 135–138. doi: 10.1016/j.jaridenv.2008.08.009
Gulzar, S., and Khan, M. A. (2001). Seed germination of a halophytic grass Aeluropus lagopoides . Ann. Bot. 87, 319–324.
Hermans, T., Oware, E., and Caers, J. (2016). Direct prediction of spatially and temporally varying physical properties from time-lapse electrical resistance data. Water Resourc. Res. 52, 7262–7283. doi: 10.1002/2016wr019126
IBM Corp (2015). IBM SPSS Statistics for Windows (Version 23.0) . New York, NY: IBM.
ISTA (2018). International Rules for Seed Testing. Bassersdotf: International Seed Testing Association.
Khan, M. A., and Gul, B. (2006). “Halophyte seed germination,” in Ecophysiology of High Salinity Tolerant Plants , eds M. Khan and D. Weber (Dordrecht: Springer), 11–30. doi: 10.1007/1-4020-4018-0_2
Khan, M. A., Gul, B., and Weber, D. J. (2001). Seed germination in relation to salinity and temperature in Sarcobatus vermiculatus . Biol. Plant. 45, 133–135.
Khan, M. A., and Gulzar, S. (2003). Germination responses of Sporobolus ioclados : a saline desert grass. J. Arid. Environ. 53, 387–394. doi: 10.1006/jare.2002.1045
Khan, M. A., and Qaiser, M. (2006). Sabkha Ecosystems. Berlin: Springer, 129–153.
Khan, M. A., and Ungar, I. A. (1997). Effect of light, salinity and thermoperiod on seed germination of halophytes. Can. J. Bot. 75, 835–841. doi: 10.1139/b97-093
Khan, M. A., and Ungar, I. A. (1998). Seed germination and dormancy of Polygonum aviculare L. as influenced by salinity, temperature, and gibberellic acid. Seed Sci. Technol. 26, 107–117.
Khan, M. A., and Ungar, I. A. (2001). Alleviation of salinity stress and the response to temperature in two seed morphs of Halopyrum mucronatum (Poaceae). Aust. J. Bot. 49, 777–783.
Khodarahmpour, Z., Ifar, M., and Motamedi, M. (2012). Effects of NaCl salinity on maize ( Zea mays L.). at germination and early seedling stage. Afr. J. Biotechnol. 11, 298–304.
Larsen, S. U., Bailly, C., Côme, D., and Corbineau, F. (2004). Use of the hydrothermal time model to analyse interacting effects of water and temperature on germination of three grass species. Seed Sci. Res. 14, 35–50.
Li, J., Ma, Z., Liu, Z., Qiao, A., Deng, Y., Wang, W., et al. (2019). Salt resistance of six alpine grass species in Qinghai Province. Pratacult. Sci. 36, 442–449.
Lin, J., Hua, X., Peng, X., Dong, B., and Yan, X. (2018). Germination responses of ryegrass (annual vs. perennial). seed to the interactive effects of temperature and salt-alkali stress. Front. Plant Sci. 9:1458. doi: 10.3389/fpls.2018.01458
Liu, K., Baskin, J. M., Baskin, C. C., Bu, H., Du, G., and Ma, M. (2013). Effect of diurnal fluctuating versus constant temperatures on germination of 445 species from the eastern Tibet Plateau. PLoS One 8:e69364. doi: 10.1371/journal.pone.0069364
Liu, X., Xu, D., Yang, Z., and Zhang, N. (2017a). Geographic variations in seed germination of Dalbergia odorifera T. Chen in response to temperature. Ind. Crops Prod. 102, 45–50.
Liu, Y., Bortier, M. F., De Boeck, H. J., and Nijs, I. (2017b). Root distribution responses to three-dimensional soil heterogeneity in experimental mesocosms. Plant Soil 421, 353–366.
Liu, Y., De Boeck, H. J., Wellens, M. J., and Nijs, I. (2017c). A simple method to vary soil heterogeneity in three dimensions in experimental mesocosms. Ecol. Res. 32, 287–295. doi: 10.1007/s11284-017-1435-6
Liu, Y., De Boeck, H. J., Li, Z., and Nijs, I. (2019). Unimodal relationship between three-dimensional soil heterogeneity and plant species diversity in experimental mesocosms. Plant Soil 436, 397–411. doi: 10.1007/s11104-019-03938-w
Liu, Y., and Hou, F. (2021). Effects of three-dimensional soil heterogeneity on seed germination in controlled experiments. J. Plant Ecol. 14, 1–9. doi: 10.1093/jpe/rtaa070
Lu, H., Shen, J., Jin, X., Hannaway, D. B., Daly, C., and Halbleib, M. D. (2008). Determining optimal seeding times for tall fescue using germination studies and spatial climate analysis. Agric. For. Meteorol. 148, 931–941.
Lundholm, J. T. (2010). Relative importance of available energy, environmental heterogeneity, and seed availability for seedling emergence on a limestone pavement. Bot. Botaniq. 88, 1045–1056. doi: 10.1139/b10-077
Maestre, F. T., Cortina, J., Bautista, S., Bellot, J., and Vallejo, R. (2003). Small-scale environmental heterogeneity and spatiotemporal dynamics of seedling establishment in a semiarid degraded ecosystem. Ecosystems 6, 630–643.
Mira, S., Arnal, A., and Perez-Garcia, F. (2017). Habitat-correlated seed germination and morphology in populations of Phillyrea angustifolia L. (Oleaceae). Seed Sci. Res. 27, 50–60.
Mondoni, A., Rossi, G., Orsenigo, S., and Probert, R. J. (2012). Climate warming could shift the timing of seed germination in alpine plants. Ann. Bot. 110, 155–164. doi: 10.1093/aob/mcs097
Nimbalkar, M. S., Pawar, N. V., Pai, S. R., and Dixit, G. B. (2020). Synchronized variations in levels of essential amino acids during germination in grain Amaranth. Braz. J. Bot. 43, 481–491. doi: 10.1007/s40415-020-00624-5
Panuccio, M. R., Jacobsen, S. E., Akhtar, S. S., and Muscolo, A. (2014). Effect of saline water on seed germination and early seedling growth of the halophyte quinoa. AoB Plants 6, lu047. doi: 10.1093/aobpla/plu047
Qu, X., Huang, Z., Baskin, J. M., and Baskin, C. C. (2008). Effect of temperature, light and salinity on seed germination and radicle growth of the geographically widespread halophyte shrub Halocnemum strobilaceum . Ann. Bot. 101, 293–299. doi: 10.1093/aob/mcm047
Rengasamy, P. (2006). World salinization with emphasis on Australia. J. Exp. Bot. 57, 1017–1023. doi: 10.1093/jxb/erj108
Richmond, C. E., Wethey, D. S., and Woodin, S. A. (2007). Climate change and increased environmental variability: demongraphic responses in an estuarine harpacticoid copepod. Ecol. Model. 209, 189–202. doi: 10.1016/j.ecolmodel.2007.06.023
Romo, J. T., and Eddleman, L. E. (1995). Use of degree-days in multiple-temperature experiments. J. Range Manag. 48, 410–416.
Sayer, A. H., Al-Hussaini, H., and Campbell, A. N. (2017). Experimental analysis of the temperature and concentration profiles in a salinity gradient solar pond with, and without a liquid cover to suppress evaporation. Solar Energy 155, 1354–1365.
Shahba, M. A., Qlan, Y. L., and Lair, K. D. (2008). Improving seed germination of saltgrass under saline conditions. Crop Sci. 48, 756–762. doi: 10.2135/cropsci2007.07.0382
Shiade, S. R. G., and Boelt, B. (2020). Seed germination and seedling growth parameters in nine tall fescue varieties under salinity stress. Acta Agric. Scand. Sec. B Soil Plant Sci. 70, 485–494.
Spindelböck, J. P., Cook, Z., Daws, M. I., Heegaard, E., Måren, I. E., and Vandvik, V. (2013). Conditional cold avoidance drives between-population variation in germination behavior in Calluna vulgaris . Ann. Bot. 112, 801–810. doi: 10.1093/aob/mct142
Ungar, I. A. (1995). Seed germination and seed-bank ecology of halophytes. Seed Dev. Germin. 9, 599–628. doi: 10.1201/9780203740071-23
Ureta, C., Martorell, C., Hortal, J., and Fornoni, J. (2012). Assessing extinction risks under the combined effects of climate change and human disturbance through the analysis of life-history plasticity. Perspect. Plant Ecol. Evol. Syst. 14, 393–401. doi: 10.1016/j.ppees.2012.09.001
Utset, A., and Borroto, M. (2001). A modelling-GIS approach for assessing irrigation effects on soil salinization under global warming conditions. Agric. Water Manag. 50, 53–63. doi: 10.1016/s0378-3774(01)00090-7
Vu, W. T., Chang, P., Moriuchi, K. S., and Friesen, M. L. (2015). Genetic variation of transgenerational plasticity of offspring germination in response to salinity stress and the seed transcriptome of Medicago truncatula . BMC Evol. Biol. 15:59. doi: 10.1186/s12862-015-0322-4
Walck, J. L., Hidayati, S. N., Dixon, K. W., Thompson, K., and Poschlod, P. (2011). Climate change and plant regeneration from seed. Glob. Chang. Biol. 17, 2145–2161.
Wang, H., Wu, Z., Chen, Y., Yang, C., and Shi, D. (2011). Effects of salt and alkali stresses on growth and ion balance in rice ( Oryza sativa L.). Plant Soil Environ. 57, 286–294.
Wu, G., Jiao, Q., and Shui, Q. (2015). Effect of salinity on seed germination, seedling growth, and inorganic and organic solutes accumulation in sunflower ( Helianthus annuus L.). Plant Soil Environ. 5, 220–226. doi: 10.17221/22/2015-pse
Xu, S., Hu, B., He, Z., Ma, F., Feng, J., Shen, W., et al. (2011). Enhancement of salinity tolerance during rice seed germination by presoaking with hemoglobin. Int. J. Mol. Sci. 12, 2488–2501. doi: 10.3390/ijms12042488
Yang, H., Huang, Z., Baskin, C. C., Baskin, J. M., Cao, Z., Zhu, X., et al. (2009). Responses of caryopsis germination, early seedling growth and ramet clonal growth of Bromus inermis to soil salinity. Plant Soil 316, 265–275. doi: 10.1007/s11104-008-9778-y
Zhang, H., McGill, C. R., Irving, L. J., Kemp, P. D., and Zhou, D. (2013). A modified thermal time model to predict germination rate of ryegrass and tall fescue at constant temperatures. Crop Sci. 53, 240–249. doi: 10.2135/cropsci2012.02.0085
Zhang, Q., and Dai, W. (2019). “Plant response to salinity stress,” in Stress Physiology of Woody Plants , ed. W. Dai (Boca Raton: CRC Press), 155–173. doi: 10.1201/9780429190476-7
Zhang, R., Luo, K., Chen, D., Baskin, J., Baskin, C., Wang, Y., et al. (2020). Comparison of thermal and hydrotime requirements for seed germination of seven Stipa species from cool and warm habitats. Front. Plant Sci. 11:560714. doi: 10.3389/fpls.2020.560714
Zhang, T., Qi, J., Gao, Y., Ouyang, Z., Zeng, S., and Zhao, B. (2015a). Detecting soil salinity with MODIS time series VI data. Ecol. Indic. 52, 480–489. doi: 10.1016/j.ecolind.2015.01.004
Zhang, T., Song, J., Fan, J., and Feng, G. (2015b). Effects of saline-waterlogging and dryness/moist alternations on seed germination of halophyte and xerophyte. Plant Spec. Biol. 30, 231–236. doi: 10.1111/1442-1984.12056
Keywords : germination percentage, germination rate, grass species, salinity, temperature
Citation: Liu Y, Zhang S, De Boeck HJ and Hou F (2021) Effects of Temperature and Salinity on Seed Germination of Three Common Grass Species. Front. Plant Sci. 12:731433. doi: 10.3389/fpls.2021.731433
Received: 27 June 2021; Accepted: 12 November 2021; Published: 10 December 2021.
Reviewed by:
Copyright © 2021 Liu, Zhang, De Boeck and Hou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY) . The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yongjie Liu, [email protected]
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.

IMAGES
COMMENTS
Experiments were conducted with 5 different water salinity. Germination percentage (GP), germination index (GI), mean germination time (MGT), seedling vigor index (SVI), ion leakage (Il), radicula ...
Two different experiments were carried out in a growth chamber (Green line WRS 96-85, KW, Scientific Equipment, Italy) (temperature of 25 ± 1 °C in the dark with a relative humidity of 70 %) to characterize the responses of quinoa to salt stress. Seed germination and biochemical responses were studied in the first experiment, while ...
Salinity is the major environmental stress source that restricts on agricultural productivity and sustainability in arid and semiarid regions by a reduction in the germination rate and a delay in the initiation of germination and subsequent seedling establishment. Salt negatively effects the crop production worldwide. Because most of the cultivated plants are salt-sensitive glycophytes.
Sample hypothesis: The more salt added to the water, the fewer seeds will germinate. The radish seeds will not germinate at all in a solution with more than 3 teaspoons of salt in 8 oz. of water. When soil has too much salt, crops won't grow well. This experiment studies how salt affects seed germination. Klinkow CCO Public domain via Pixaby.
Graph 1 - Preliminary Results of Seed Germination The concentrations of salinity tested for this experiment is 0%, 2%, 4% and 6%. As predicted, the most percentage of germination is from the concentration with no salinity.
To ensure the accuracy of tests on salinity stress response, Pre-germination experiments were carried out to obtain the germplasm seeds with high vigor that the germination rate exceeded 99% for ...
temperature, salinity level, pH of water, and more affect germination and growth rate (Overhiser 2019). Knowing this, we aimed to determine how the salinity concentration present in the growth medium would affect the germination rate of mung beans. In this experiment, members performed the same experiment with the same measurements.
Knowing this, we aimed to determine how the salinity concentration present in the growth medium would affect the germination rate of mung beans. In this experiment, members performed the same experiment with the same measurements. Each group member exposed 25 mung bean seeds to varying salinity treatment groups: 0, 40, 80 and 120 mM/ L.
Temperature and salinity significantly affect seed germination, but the joint effects of temperature and salinity on seed germination are still unclear. To explore such effects, a controlled experiment was conducted, where three temperature levels (i.e., 15, 20, and 25°C) and five salinity levels (i.e., 0, 25, 50, 100, and 200 mmol/L) were ...
estimation of growth and yield potential under salt conditions [13]. Recent studies underline that salinity stress during germination and early growth crucially affect crop growth [14], while inhibition and/or delay of seed germination and seedling growth due to salt stress is correlated with decreased yield in soybean [15-18].
This experiment investigates the effects of different salt solutions on the germination and growth of brassica rapa seeds. Students can use this experiment to study the effects of salt on seed germination, and, if time permits, can continue looking at the growth and development of plants in a saline environment.
Our experiments differ from those of Ungar (1979) and Philipupillai and Ungar (1984) in several aspects. The above authors analyzed a response of two seed morphs of S. europaea only to NaCl and only at the seed stage. The focus of this study was to understand how variations in salinity affect dimorphic seed germination and plant growth.
Although S. alterniflora has a higher tolerance to salinity than other halophytic plants, its germination rate was observed to be repressed in experiments on seed germination with a high salinity condition, suggesting that S. alterniflora suffers from salinity stress in typical habitats (Yuan and Shi, 2009, Li et al., 2010, Xiao et al., 2016).
Germination experiment - The type of salt used in the study was NaCl (Merck life science Catalogue number 106404). We have performed a Petri dish experiment as a complete randomized design in 6*1 factorial (six salt treatments: 0 mM (distilled water), 25 mM, 50 mM, 100 mM, 150 mM and 200 mM) and one chilli variety. 5 ml of the respective ...
Experiment 1: seed germination. Germination conditions and experimental design . Seeds were surface-sterilized for 20 min in 20 % (v/v) sodium hypochlorite, rinsed and soaked for 1 h in distilled water. ... Ungar I. Effect of salinity on seed germination, growth, and ion accumulation of Atriplex patula (Chenopodiaceae) American Journal of Botany.
Temperature and salinity significantly affect seed germination, but the joint effects of temperature and salinity on seed germination are still unclear. To explore such effects, a controlled experiment was conducted, where three temperature levels (i.e., 15, 20, and 25°C) and five salinity levels (i.e., 0, 25, 50, 100, and 200 mmol/L) were ...
An experiment was conducted to. investigate the effect of salinity on germination and seedling growth of mungbean genotypes. The. experiment comprised two factors viz. genotypes (BARI Mung-6 ...
atory of Biological sciences to see the efects of salinity on germination and early seedling.Background: It is estimated that the world 20% of farming land and 50% of cropland is salt stressed and salinity decre. ses the germination of seed, retards the growth of plant and so it reduces the yield of crop.Objective: The major objective of this ...
The response of three bean cultivars (Phaseolus vulgaris L.) to equimolar NaCl and Na2SO4 salinity at germination and early seedling growth was investigated. Seeds were germinated and grown in Petri plate on filter paper with solution of the ... The experiment was conducted in Petri plate on filter paper beds in a thermostat. 20 seeds were sown ...
the germination experiment. Effect of salt on germination and recovery germination Salt solutions, consisting of 0 (control), 21,66,110, 155, 200, 267, 334, and 445 mmol/litre NaCl, were prepared using half-strength Hoagland solution. After being surface sterilised with 0.01% HgCl2 solution for 3 min and rinsed in tap water, 100 seeds
Four salinity levels control (0.66 dS m-1), 4, 8 and 12 dS m-1 respectively were used as treatment in the experiments. The experiments were laid out in a factorial Completely Randomized Design ...
Laboratory and greenhouse experiments were conducted to determine the effect of ... germination in both acidic and alkaline soils. These results suggest that under field conditions, if moisture and (or) salinity are not limiting ... L'achèvement de la germination était inhibé par la diminution du potentiel osmotique et l'accroissement de ...