
- History & Society
- Science & Tech
- Biographies
- Animals & Nature
- Geography & Travel
- Arts & Culture
- Games & Quizzes
- On This Day
- One Good Fact
- New Articles
- Lifestyles & Social Issues
- Philosophy & Religion
- Politics, Law & Government
- World History
- Health & Medicine
- Browse Biographies
- Birds, Reptiles & Other Vertebrates
- Bugs, Mollusks & Other Invertebrates
- Environment
- Fossils & Geologic Time
- Entertainment & Pop Culture
- Sports & Recreation
- Visual Arts
- Demystified
- Image Galleries
- Infographics
- Top Questions
- Britannica Kids
- Saving Earth
- Space Next 50
- Student Center

- Is mathematics a physical science?
- Why does physics work in SI units?


Robert Millikan
Our editors will review what you’ve submitted and determine whether to revise the article.
- The Nobel Prize - Biography of Robert A. Millikan
- American Institute of Physics - Biography of Robert Andrews Millikan
- Robert Andrews Millikan - Student Encyclopedia (Ages 11 and up)

Robert Millikan (born March 22, 1868, Morrison, Illinois , U.S.—died December 19, 1953, San Marino , California) was an American physicist honored with the Nobel Prize for Physics in 1923 for his study of the elementary electronic charge and the photoelectric effect .
Millikan graduated from Oberlin College (Oberlin, Ohio) in 1891 and obtained a doctorate at Columbia University in 1895. In 1896 he became an assistant at the University of Chicago , where he became a full professor in 1910. During his time in Chicago as an assistant professor, he wrote for high-school and college students several physics textbooks that entered widespread use.

In 1909 Millikan began a series of experiments to determine the electric charge carried by a single electron . He began by measuring the course of charged water droplets in an electric field . The results suggested that the charge on the droplets is a multiple of the elementary electric charge, but the experiment was not accurate enough to be convincing. He obtained more precise results in 1910 with his famous oil-drop experiment in which he replaced water (which tended to evaporate too quickly) with oil . Millikan varied the electric voltage between two metal plates as an oil drop fell between them until the drop stopped falling. When the drop was stationary , the downward force of gravity on the drop equaled the upward electrical force on the charges in the drop, and then Millikan could measure how much charge the drop had.
In 1916 he took up with similar skill the experimental verification of the equation introduced by Albert Einstein in 1905 to describe the photoelectric effect , in which electrons are ejected from a metal plate when light falls on it. The photoelectric effect had puzzled physicists, but Einstein described the energy of the ejected electron as equal to h f - φ, where h is Planck’s constant, f is the frequency of the light, and φ is a property of the metal called the work function. Einstein’s description of the photoelectric effect as a quantum phenomenon was controversial, but Millikan’s measurements proved Einstein’s theory and obtained an accurate value of Planck’s constant . When the United States entered World War I in 1917, he became vice chairman of the National Research Council in Washington, D.C., where he helped scientists apply their research to the war effort. He returned to Chicago in 1919.
In 1921 Millikan left the University of Chicago to become director of the Norman Bridge Laboratory of Physics at the California Institute of Technology (Caltech) in Pasadena . There he undertook a major study of the radiation that the physicist Victor Hess had detected coming from outer space. Millikan proved that this radiation is indeed of extraterrestrial origin, and he named it “ cosmic rays .” As chairman of the executive council of Caltech from 1921 until his retirement in 1945, Millikan turned that school into one of the leading research institutions in the United States.
- Structure of Atom
- Oil Drop Experiment
Milliken's Oil Drop Experiment
The Millikens Oil Drop Experiment was an experiment performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the charge of an electron. This experiment proved to be very crucial in the physics community.
Millikens Oil Drop Experiment Definition
In the experiment, Milliken allowed charged tiny oil droplets to pass through a hole into an electric field. By varying the strength of the electric field the charge over an oil droplet was calculated, which always came as an integral value of ‘e.’
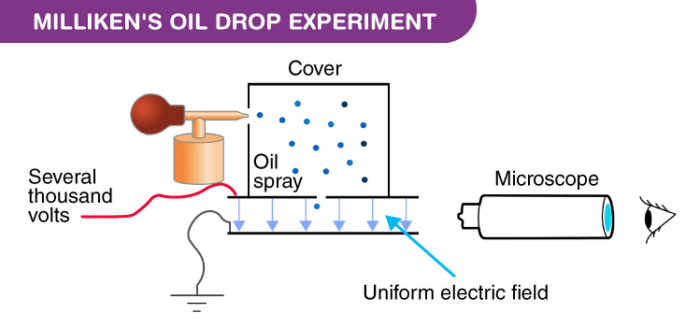
Apparatus of the Milliken’s Oil Drop Experiment
The apparatus for the experiment was constructed by Milliken and Fletcher. It incorporated two metal plates held at a distance by an insulated rod. There were four holes in the plate, out of which three were there to allow light to pass through them and one was there to allow viewing through the microscope.
Ordinary oil wasn’t used for the experiment as it would evaporate by the heat of the light and so could cause an error in the Millikens Oil Drop Experiment. So, the oil that is generally used in a vacuum apparatus which is of low vapour pressure was used.
Milliken’s Oil Drop Experiment Procedure
- Oil is passed through the atomizer from where it came in the form of tiny droplets. They pass the droplets through the holes present in the upper plate of the apparatus.
- The downward motions of droplets are observed through a microscope and the mass of oil droplets, then measure their terminal velocity.
- The air inside the chamber is ionized by passing a beam of X-rays through it. The electrical charge on these oil droplets is acquired by collisions with gaseous ions produced by ionization of air.
- The electric field is set up between the two plates and so the motion of charged oil droplets can be affected by the electric field.
- Gravity attracts the oil in a downward direction and the electric field pushes the charge upward. The strength of the electric field is regulated so that the oil droplet reaches an equilibrium position with gravity.
- The charge over the droplet is calculated at equilibrium, which is dependent on the strength of the electric field and mass of droplet.
Milliken’s Oil Drop Experiment Calculation
F up = F down
F up = Q . E
F down = m.g
Q is an electron’s charge, E is the electric field, m is the droplet’s mass, and g is gravity.
One can see how an electron charge is measured by Millikan. Millikan found that all drops had charges that were 1.6x 10 -19 C multiples.
Milliken’s Oil Drop Experiment Conclusion
The charge over any oil droplet is always an integral value of e (1.6 x 10 -19 ). Hence, the conclusion of Millikens Oil Drop Experiment is that the charge is said to be quantized, i.e. the charge on any particle will always be an integral multiple of e.
Frequently Asked Questions – FAQs
What did millikan’s oil drop experiment measure.
Millikan oil-drop test, the first simple and persuasive electrical charge calculation of a single electron. It was first conducted by the American physicist Robert A. in 1909. He discovered that all the drops had charges that were simple multiples of a single integer, the electron’s fundamental charge.
What is the importance of Millikan’s oil drop experiment?
The experiment with Millikan is important since it defined the charge on an electron. Millikan used a very basic, very simple system in which the behaviour of gravitational, electrical, and (air) drag forces were controlled.
What did Millikan conclude after performing his oil drop experiment?
An integral multiple of the charge on an electron is the charge on every oil decrease. About an electric force. In a relatively small amount, the charge and mass of the atom must be condensed.
Why charges are quantized?
Charges are quantized since every object’s charge (ion, atom, etc.) Charge quantization, therefore, implies that no random values can be taken from the charge, but only values that are integral multiples of the fundamental charge (proton / electron charge).
Can charge be created or destroyed?
The Charge Conservation Law does not suggest that it is difficult to generate or remove electrical charges. It also means that any time a negative electrical charge is produced, it is important to produce an equal amount of positive electrical charge at the same time so that a system’s overall charge does not shift.
For more information about quantum physics , download BYJU’S-The learning app to play store and app store.

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below
Request OTP on Voice Call
| CHEMISTRY Related Links | |
Leave a Comment Cancel reply
Your Mobile number and Email id will not be published. Required fields are marked *
Post My Comment
Register with BYJU'S & Download Free PDFs
Register with byju's & watch live videos.
- Subscriber Services
- For Authors
- Publications
- Archaeology
- Art & Architecture
- Bilingual dictionaries
- Classical studies
- Encyclopedias
- English Dictionaries and Thesauri
- Language reference
- Linguistics
- Media studies
- Medicine and health
- Names studies
- Performing arts
- Science and technology
- Social sciences
- Society and culture
- Overview Pages
- Subject Reference
- English Dictionaries
- Bilingual Dictionaries
Recently viewed (0)
- Save Search
- Share This Facebook LinkedIn Twitter
Related Content
More like this.
Show all results sharing these subjects:
Millikan's oil-drop experiment
Quick reference.
An experiment begun in 1909 by Robert Millikan to determine the charge on an electron. Between two horizontal oppositely charged plates Millikan introduced a fine spray of oil droplets. He first measured the mass of the oil drops by measuring their rate of fall under the influence of gravity and against the air resistance. Then using X-rays to ionize the air he noted the changed rate of fall of droplets that were attracted to the lower plate after these droplets had acquired a charge from the ionized air. These figures enabled Millikan to conclude that the acquired charge was always a simple multiple of a basic unit. He found this basic unit to be 4.774 × 10 −10 esu, which was the value for the charge on an electron used until it was improved on in 1928.
From: Millikan's oil-drop experiment in A Dictionary of Physics »
Subjects: Science and technology — Physics
Related content in Oxford Reference
Reference entries.
View all related items in Oxford Reference »
Search for: 'Millikan's oil-drop experiment' in Oxford Reference »
- Oxford University Press
PRINTED FROM OXFORD REFERENCE (www.oxfordreference.com). (c) Copyright Oxford University Press, 2023. All Rights Reserved. Under the terms of the licence agreement, an individual user may print out a PDF of a single entry from a reference work in OR for personal use (for details see Privacy Policy and Legal Notice ).
date: 12 September 2024
- Cookie Policy
- Privacy Policy
- Legal Notice
- Accessibility
- [81.177.182.174]
- 81.177.182.174
Character limit 500 /500

- Collections

- APS Journals
Landmarks —Millikan Measures the Electron’s Charge

Landmarks articles feature important papers from the archives of the Physical Review journals.
Researchers now routinely isolate single electrons in quantum dots, but a century ago the state-of-the-art charge-trapping device was a droplet of clock oil. Robert Millikan’s oil drop experiment provided the first clear measurement of the fundamental electric charge and thus helped cement the notion that nature is “grainy” at the smallest level. The first results came out in 1910, but the seminal work was a 1913 paper in the Physical Review . Millikan reported a value for the fundamental electric charge that was within half a percent of today’s accepted value. The experiment helped earn Millikan a Nobel prize in 1923 but has been a source of some controversy over the years.
J. J. Thomson discovered the electron in 1897 when he measured the charge-to-mass ratio for electrons in a beam. But the value of the charge and whether it was fundamental remained open questions. Thomson and others tried to measure an irreducible electric charge by looking at clouds of water droplets. Using various techniques, they estimated the smallest charge that a droplet could hold, but the results were not entirely convincing because they relied on averages over many particles of various sizes. “The evidence for a unitary charge was at the time very ambiguous,” says science historian Gerald Holton of Harvard University.
At the University of Chicago in the 1900s, Millikan and his graduate students realized that ramping up the electric field would disperse a water cloud, so that only a few droplets remained. He decided to try isolating single droplets, but it soon became clear that single water droplets evaporated too quickly to make reliable measurements. One of his students, Harvey Fletcher, found that long-lasting droplets could be made with a light oil that was used for lubricating clocks.
The oil drop experiment that Millikan and Fletcher designed had two chambers. In the upper chamber, an atomizer (like that used in perfume bottles) dispersed a fine mist of micron-sized oil droplets. Individual droplets would fall through a pinhole into the lower chamber, which consisted of two horizontal plates, with one held 16 millimeters above the other. The air in this chamber was ionized with x rays , so that ions or free electrons could be captured on the falling droplets. A small window on the side allowed the scientists to observe the droplets through a telescope. The droplets fell slowly enough—due to atmospheric drag—that the researchers could measure their downward speed by eye, using horizontal lines in the telescope. From this speed, they could estimate the size and mass of each droplet.
They then applied a high voltage across the plates and measured the upward speed of the droplet, to determine the electric force and ultimately the charge. Multiple measurements on a single droplet could be performed by repeatedly turning the electric field on and off. The droplets had various amounts of charge on them (and they would often gain or lose charge during an observation), but the data showed that the charge was indeed quantized into integer multiples of a unit charge.
In 1910 Millikan published the first results of these experiments [1] (Fletcher was not included as an author, based on a deal the two struck [2] ). Millikan then made several improvements, including an empirical estimate of the drag forces. The culmination of this effort, reported in 1913, was a value of the fundamental charge with an error bar of just 0.2 percent. The precision acquired was so great that “other experiments did not improve on his result until a decade later,” Holton says.
But Felix Ehrenhaft of the University of Vienna repeatedly challenged Millikan’s results, based on his own measurements of “sub-electron” charges on small metal particles. The dispute lasted for many years—known as the “Battle over the Electron”—but eventually most physicists sided with Millikan.
In more recent years, historians who have examined Millikan’s lab notes have said that he discarded some of the measurements to boost the evidence of a fundamental charge. But David Goodstein of the California Institute of Technology in Pasadena believes these accusations of fraud are unwarranted. He has analyzed the notes and says that Millikan excluded droplets because their observations were incomplete, not because their implied charge didn’t match his expectations [3] . “Millikan’s oil drop experiment is a classic example of outstanding physics done by one of the giants of his era,” Goodstein says.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.
- R. A. Millikan, “The Isolation of an Ion, a Precision Measurement of its Charge, and the Correction of Stokes’s Law,” Science 32 , 436 (1910) ; first reported at the American Physical Society meeting, 23 April 1910, Phys. Rev. (Series I) 30 , 656 (1910)
- H. Fletcher, “My work with Millikan on the oil‐drop experiment,” Phys. Today 35 , 43 (1982)
- D. Goodstein, “In Defense of Robert Andrews Millikan,” Am. Sci. 89 , 54 (2001)
More Information
Focus story on Millikan’s measurement of Planck’s constant
article by Gerald Holton on the Millikan-Ehrenhaft Dispute
article about the ethics of Millikan’s handling of data
Millikan Nobel Prize: Nobel lecture, biography, and other information
On the Elementary Electrical Charge and the Avogadro Constant
R. A. Millikan.
Phys. Rev. 2 , 109 (1913)
Published August 1, 1913
Subject Areas
Related articles.
Gamma-Ray Burst Tightens Constraints on Quantum Gravity
An analysis of the brightest gamma-ray burst ever observed reveals no difference in the propagation speed of different frequencies of light—placing some of the tightest constraints on certain violations of general relativity. Read More »
Flavor Profiling the Highest-Energy Neutrinos
A way to determine the flavors of ultrahigh-energy cosmic neutrinos observed by future detectors could help scientists understand the origin of these elusive particles. Read More »
First Direct Detection of Electron Neutrinos at a Particle Collider
Electron neutrinos produced by proton–proton collisions at the LHC have been experimentally observed. Read More »
Sign up to receive weekly email alerts from Physics Magazine .

- Why Does Water Expand When It Freezes
- Gold Foil Experiment
- Faraday Cage
Oil Drop Experiment
- Magnetic Monopole
- Why Do Fireflies Light Up
- Types of Blood Cells With Their Structure, and Functions
- The Main Parts of a Plant With Their Functions
- Parts of a Flower With Their Structure and Functions
- Parts of a Leaf With Their Structure and Functions
- Why Does Ice Float on Water
- Why Does Oil Float on Water
- How Do Clouds Form
- What Causes Lightning
- How are Diamonds Made
- Types of Meteorites
- Types of Volcanoes
- Types of Rocks

Who Did the Oil Drop Experiment?
The Oil Drop Experiment was performed by the American physicist Robert A Millikan in 1909 to measure the electric charge carried by an electron . Their original experiment, or any modifications thereof to reach the same goal, are termed as oil drop experiments, in general.
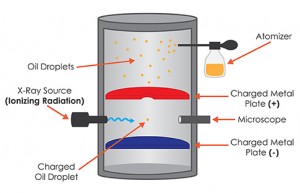
What is the Oil Drop Experiment?
In the original version, Millikan and one of his graduate students, Harvey Fletcher, took a pair of parallel horizontal metallic plates. A uniform electric field was created in the intermediate space by applying a potential difference between them. The plates were held apart by a ring of insulating material. The ring had four holes, three for allowing light to illuminate the setup, and the fourth one enabled a microscope for viewing. A closed chamber with transparent walls was fitted above the plates.
At the beginning of the experiment, a fine mist of oil droplets was sprayed into the chamber. In modern setups, an atomizer replaces the oil droplets. The oil was so chosen such that it had a low vapor pressure and capable of charging. Some of the oil drops became electrically charged by friction as they forced their way out of the nozzle. Alternatively, charging could also be induced by incorporating a source of ionizing radiation , such as an X-Ray tube, in the apparatus. The droplets entered the space between the plates and raised or fell, according to the requirement, by varying plate voltage.
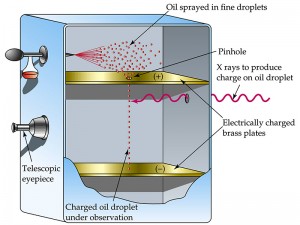
In terms of the present-day arrangement, when the electric field is turned off, the oil drops fall between the plates under the action of gravity only. The friction with the oil molecules in the chamber makes them reach their terminal velocity fast. The terminal velocity is the constant speed that a freely falling object eventually reaches when the resistance of the medium through which it is falling prevents further acceleration . Once the field is turned on, the charged drops start to rise. This motion happens since the electric force directed upwards is stronger than the gravitational force acting downwards. One charged drop is selected and kept at the center of the field of view of the microscope after allowing all other drops to fall by alternately switching off the voltage source. The experiment is conducted with this drop.
Theory and Calculations
First, the oil drop is allowed to fall in the absence of an electric field, and its terminal velocity, say v 1 , is found out. Using Stokes’ law, the drag force acting on the drop is calculated using the following formula.
Here r is the radius of the drop and ɳ, the viscosity of air.
The weight of the drop, w’, which is the product of its mass and acceleration due to gravity g, is given by the equation,
where ρ is the density of the oil.
However, what we need here is the apparent weight w of the drop in the air given by the difference of the actual weight and the upthrust of the air. We can express w by the following formula.
Here ρ air denotes the density of air.
When the drop attains terminal velocity, then it has no acceleration. Hence, the total force acting on it must be zero. That means,
The above equation can be used to find out the value of r. Once r is calculated, the value of w can easily be found out from equation (i) marked above.
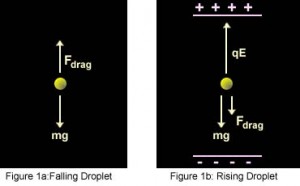
Now after turning on the electric field between the plates, the electric force F E acting on the drop is,
Where E is the electric field and q the charge on the oil drop. For parallel plates, the formula for E is,
Here V is the potential difference and d the distance between the plates. That implies,
Now if we adjust V to make the oil drop remain steady at a point, then
Thus, the value of q can be calculated. By repeatedly applying this method to multiple oil droplets, the electric charge values on individual drops were always found to be integer multiples of the smallest value. This lowest charge could be nothing but the charge on the elementary particle, electron. By this method, the electronic charge was calculated to be approximate, 1.5924×10 −19 C, making an error of 1% of the currently accepted value, 1.602176487×10 −19 C. All subsequent research pointed to the same value of charge on the fundamental particle.
Millikan was able to measure both the amount of electric force and magnitude of electric field on the tiny charge of an isolated oil droplet and from the data determine the magnitude of the charge itself. Millikan’s oil drop experiment proved that the electric charge is quantized in nature. The electric charge appears in quanta of magnitude 1.6 X 10 -19 C in oil droplets.
Robert Millikan’s Oil Drop Experiment Animation
Millikan’s oil drop experiment and the atomic theory.
Until the time of the Oil Drop Experiment, the world had little or no knowledge of what is present inside an atom . Earlier experiments by the English Physicist J.J. Thomson had shown that atoms contain some negatively charged particles of masses significantly smaller than that of the hydrogen atom. Nevertheless, the exact value of the charge carried by these subatomic particles remained in the dark. The very existence of these particles was not accepted by many due to a lack of concrete evidence. Thus, the atomic model was shrouded in mystery. In this scenario, with Millikan’s groundbreaking effort to quantify the charge on an electron, the atomic theory came of age in the early years of the twentieth century.
Controversy about the Oil Drop Experiment and Discovery
Robert Millikan was the sole recipient of the Nobel Prize in Physics in 1923 for both his work in this classic experiment and his research in the photoelectric effect . Fletcher’s work on the oil drop project, however, was not recognized. Many years later, the writings of Fletcher revealed that Millikan wished to take the sole credit for the discovery in exchange for granting him a Ph.D. and helping him secure a job after his graduation.
The beauty of the oil drop experiment lies in its simple and elegant demonstration of the quantization of charge along with measuring the elementary charge on an electron that finds widespread applications to this day. With the progress of time, considerable modifications have been made to the original setup resulting in obvious perfection in the results. Still, no substantial deviation from the results of the classical experiment could yet be found.
- Robert Millikan and Harvey Fletcher conducted the oil drop experiment to determine the charge of an electron. The experiment was the first direct and riveting measurement of the electric charge of a single electron.
- They suspended tiny charged droplets of oil between two metal electrodes by balancing downward gravitational force with upward drag and electric forces.
- They later used their findings to determine the mass of the electron.
- Kentchemistry.com
- Physics.utah.edu
- Nobelprize.org
- Ffden-2.phys.uaf.edu
- Chem.libretexts.org
Article was last reviewed on Thursday, February 2, 2023
Related articles

Leave a Reply Cancel reply
Your email address will not be published. Required fields are marked *
Save my name, email, and website in this browser for the next time I comment.
Popular Articles

Join our Newsletter
Fill your E-mail Address
Related Worksheets
- Privacy Policy
© 2024 ( Science Facts ). All rights reserved. Reproduction in whole or in part without permission is prohibited.

- Previous Article
- Next Article
Remembering the oil-drop experiment
- Reprints and Permissions
- Cite Icon Cite
- Search Site
Michael F. Perry; Remembering the oil-drop experiment. Physics Today 1 May 2007; 60 (5): 56–60. https://doi.org/10.1063/1.2743125
Download citation file:
- Ris (Zotero)
- Reference Manager
In 1910 Robert Millikan of the University of Chicago announced that he and Harvey Fletcher had developed a successful means of isolating and measuring the charge of an electron. This exciting breakthrough, which became known as the oil-drop experiment, was a major contribution to physics. Millikan received worldwide recognition for the experiment, including the Nobel Prize in Physics in 1923. He published a great deal regarding the implications of the measurements and ultimately became one of the most influential physicists of the 20th century.
Fletcher enjoyed a distinguished career in the field of communication acoustics, but his contribution to the oil-drop experiment was largely forgotten. His version of the story remained untold until 1982, when after his death Physics Today published an excerpt from his unpublished 1967 autobiography 1 detailing his involvement with Millikan on the famous experiment (see Physics Today , June 1982, page 43). While it offered...
PERSONAL SUBSCRIPTION
Purchase an annual subscription for $25. A subscription grants you access to all of Physics Today 's current and backfile content.

Citing articles via
- Online ISSN 1945-0699
- Print ISSN 0031-9228
- For Researchers
- For Librarians
- For Advertisers
- Our Publishing Partners
- Physics Today
- Conference Proceedings
- Special Topics
pubs.aip.org
- Privacy Policy
- Terms of Use
Connect with AIP Publishing
This feature is available to subscribers only.
Sign In or Create an Account

- Simple Search
- Advanced Search




IMAGES
VIDEO
COMMENTS
Millikan oil-drop experiment, first direct and compelling measurement of the electric charge of a single electron. It was performed originally in 1909 by the American physicist Robert A. Millikan, who devised a method of measuring the minute electric charge that is present on many of the droplets in an oil mist.
Millikan's setup for the oil drop experiment. The oil drop experiment was performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the elementary electric charge (the charge of the electron). [1] [2] The experiment took place in the Ryerson Physical Laboratory at the University of Chicago.[3] [4] [5] Millikan received the Nobel Prize in Physics in 1923.
In 1909 Millikan began a series of experiments to determine the electric charge carried by a single electron. He began by measuring the course of charged water droplets in an electric field. The results suggested that the charge on the droplets is a multiple of the elementary electric charge, but the experiment was not accurate enough to be ...
The Millikens Oil Drop Experiment was an experiment performed by Robert A. Millikan and Harvey Fletcher in 1909 to measure the charge of an electron. This experiment proved to be very crucial in the physics community. ... It was first conducted by the American physicist Robert A. in 1909. He discovered that all the drops had charges that were ...
Millikan's oil-drop experiment was performed by Robert Millikan and Harvey Fletcher in 1909. It determined a precise value for the electric charge of the electron, e.The electron's charge is the fundamental unit of electric charge because all electric charges are made up of groups (or the absence of groups) of electrons.
Measuring of the charge of the electron. Oil drop experiment. Robert A. Millikan.. (1909). q=1.5924(17)×10−19 C. Shot noise experiment. First proposed by Walter H. Schottky. In terms of the Avogadro constant and Faraday constant. =.
Oil drop experiment. Robert A. Millikan.. (1909). ... Robert A. Millikan "for his work on the elementary charge of electricity and on the photoelectric effect". ROBERT ANDREWS MILLIKAN 1868-1953 University of Chicago 9/23/2019 4 On January 26, 1982, he was honored by the United States Postal Service
1. Oil drop experiment. Robert A. Millikan.. (1909). e=1.5924(17)×10−19C 2. Shot noise experiment. First proposed by Walter H. Schottky 3. In terms of the Avogadro constant and Faraday constant 𝒆= 𝑵𝑨; F- Faraday constant, N A - Avagadro constant. Best uncertainty ~1.6 ppm. 4. From Josephson ( = 𝒆 )and von Klitzing 𝑹 = 𝒆
An experiment begun in 1909 by Robert Millikan to determine the charge on an electron. Between two horizontal oppositely charged plates Millikan introduced a fine spray of oil droplets. He first measured the mass of the oil drops by measuring their rate of fall under the influence of gravity and against the air resistance. Then using X-rays to ...
Robert Millikan's oil drop experiment provided the first clear measurement of the fundamental electric charge and thus helped cement the notion that nature is "grainy" at the smallest level. The first results came out in 1910, but the seminal work was a 1913 paper in the Physical Review. Millikan reported a value for the fundamental ...
Measuring of the charge of the electron. Oil drop experiment. Robert A. Millikan.. (1909). q=1.5924(17)×10−19. Shot noise experiment. First proposed by Walter H. Schottky. In terms of the Avogadro constant and Faraday constant. =. F- Faraday constant,
The Oil Drop Experiment was performed by the American physicist Robert A Millikan in 1909 to measure the electric charge carried by an electron. Their original experiment, or any modifications thereof to reach the same goal, are termed as oil drop experiments, in general. Oil Drop Experiment.
In 1909, Robert Millikan and Harvey Fletcher developed an experiment to determine the fundamental charge of the electron. This was achieved by measuring the charge of oil drops in a known electric field. If all electrons have the same charge, then the measured charge on the oil drops must be multiples of the same fundamental constant.
Tools. In 2018, we celebrated the sesquicentennial birthday of Robert A. Millikan, a Nobel laureate in physics who worked among the greats such as Niels Bohr and Albert Einstein. His name, however, is perhaps not as widely known. He was born in 1868 in Morrison, IL, and moved with his family to the small town of Maquoketa, IA, at age nine.
In 1910 Robert Millikan of the University of Chicago announced that he and Harvey Fletcher had developed a successful means of isolating and measuring the charge of an electron. This exciting breakthrough, which became known as the oil-drop experiment, was a major contribution to physics. Millikan received worldwide recognition for the experiment, including the Nobel Prize in Physics in 1923.
The Millikan Oil Drop Experiment. An experiment performed by Robert Millikan in 1909 determined the size of the charge on an electron. He also determined that there was a smallest 'unit' charge, or that charge is 'quantized'. He received the Nobel Prize for his work. We're going to explain that experiment here, and show how Millikan was able to ...
Robert Andrews Millikan (March 22, 1868 - December 19, 1953) was an American experimental physicist who won the Nobel Prize for Physics in 1923 for the measurement of the elementary electric charge and for his work on the photoelectric effect.. Millikan graduated from Oberlin College in 1891 and obtained his doctorate at Columbia University in 1895. In 1896 he became an assistant at the ...
From 1909 until the spring of 1912, Millikan reports, he spent every available moment in the laboratory on his oil-drop experiment. His first comprehensive, though to some extent preliminary, results were published in September 1910 in the journal Science as "The Isolation of an Ion, a Precision Measurement of Its Charge, and the Correction ...
Add a one-line explanation of what this file represents. Summary [edit]Description: Yoshkar-Ola, Mari El Republic, Russia
Mari El is notable for being one of the last strongholds of organized paganism in Europe. While many of the Mari people have converted to Russian Orthodoxy, a sizable number practice the Marla Faith, which combines Christianity with significant native shamanistic traditions. The Russian and Soviet governments have been suspicious of Finno-Ugric nationalism within Mari El (and Udmurtia ...
Measuring of the charge of the electron. Oil drop experiment. Robert A. Millikan.. (1909). e=1.5924(17)×10−19. Shot noise experiment. First proposed by Walter H. Schottky. In terms of the Avogadro constant and Faraday constant =. F- Faraday constant, NA- Avagadro constant. Best.
Flag of Yoshkar-Ola is the capital city of the Mari El Republic, Russia. Vector illustration. Download a free preview or high-quality Adobe Illustrator (ai), EPS, PDF, SVG vectors and high-res JPEG and PNG images.