

The Biology Corner
Biology Teaching Resources

Investigation – Cellular Respiration Virtual Lab

Students in AP Biology investigate cellular respiration by placing peas or other living organisms in respirometers. After submerging the devices, students then measure the rate or respiration by collecting data on water movement in the pipets .
As an alternative to this lab, I created a virtual version that can be completed at home.
The lab is hosted at Olabs Ministry of Electronics and Technology .
This virtual version takes less time and money. Students can change variables in the simulator, like the number of seeds and the temperature of the chamber. They read the respirometer after two minutes and record how much the water in the tube has increased.
The faster the rate of respiration, the more displacement of the water in the tube. This occurs due to the oxygen being consumed during the process.
The worksheet is set up as a CER (claim, evidence, reasoning) and provides minimal instructions on how to solve the experimental questions. I wouldn’t technically call it an “inquiry lab,” but it does give students the opportunity to explore variables.
Students can complete the worksheet either as a handout or online. You can even have students use Google sheets to graph the data, though it’s not necessary. They can fairly easily note trends in data without a graph. I usually place respiration directly after the unit on cells where students learn about organelles and their jobs. You could also pair this activity with a unit on photosynthesis because they are examining how seeds use oxygen.
Shannan Muskopf
Please log in to save materials. Log in
Cellular Respiration Lab (online lab)
This online cellular respiration lab activity is written for students who are unable to attend an in-person lab.
Name:
Cellular Respiration (online lab activity)
Copyright © 2020 by Jeff Carmichael, Ph.D.
University of North Dakota
Learning Objectives:
After completing this lab you should be able to:
- Make predictions about fermentation rates based on hypotheses
- Distinguish between aerobic and anaerobic respiration
- Generate date on fermentation rates and interpret that data
- Calculate and compare mass specific metabolic rates
Energy is required by all living organisms for metabolism. Where does that energy come from? The process of cellular respiration involves the breakdown of complex organic molecules (e.g., sugars and other nutrients). The energy released from the breaking of bonds in these molecules is used to generate adenosine triphosphate (ATP). The ATP can then be used to drive a number of cellular metabolic reactions in living cells (e.g., move muscles, transport molecules across membranes, produce action potentials in neurons). Although the complete set of reactions is quite complex, the general reaction below summarizes the overall process of aerobic cellular respiration.
C 6 H 12 O 6 + 6O 2 6CO 2 + 6H 2 O + ATP + Heat
Note that aerobic respiration requires oxygen. Cells are able to produce a lot of ATP when oxygen is present. Look up the process of respiration in your textbook and familiarize yourself with glycolysis, the Krebs Cycles (citric acid cycle) and the electron transport chain . Determine how they are connected and which portion of respiration produces the most ATP.
Most living cells are also able to produce ATP through anaerobic respiration (fermentation). However, this process only yields a few molecules of ATP per glucose (most of the energy is retained in alcohol which is formed as a byproduct of fermentation). Note that fermentation is less efficient than aerobic respiration. This lab will explore both aerobic and anaerobic respiration in various organisms.
Part 1- Anaerobic Respiration (fermentation)
One form of anaerobic respiration well known by most involves the use of yeast in the production of bread, beer, and other products. Yeast produces CO 2 and alcohol as byproducts of anaerobic respiration (or, fermentation). This activity will investigate the effect of various factors on the rate of fermentation in yeast.
- ? Make some predictions: What impact do you think each of the factors below will have on the rate of fermentation in yeast? Explain your reasoning.
Temperature:
Addition of Pyruvate (an intermediate compound formed as a result of glycolysis):
Addition of NaF (sodium fluoride- the active ingredient in toothpaste, also toxic to many organism):
Addition of Glucose:
Procedure that was followed in lab:
Add the contents of the five treatments shown in the table below in small beakers. Mix well. Then, add to five separate fermentation tubes.
10 ml
5 ml
15 ml
10 ml
5 ml
15 ml
5 ml
10 ml
15 ml
5 ml
10 ml
15 ml
15 ml
15 ml
Place treatment 1 in the refrigerator and treatments 2 – 5 in the 40 o C incubator for 30 minutes. After 30 minutes measure the height of the bubble (CO 2 ) in each tube and record your results in the table below.
The images below show results of the five treatments. Note: if CO 2 was produced within a fermentation tube, you will see a “bubble” of air toward the top of the tube.

- ? Which treatment served as a control? Explain your reasoning.
- ? Based on your results, what was the effect of glucose, NaF, and Pyruvate on respiration? How did temperature effect the rate of respiration? Are these results what you predicted? Explain these results .
Part 2- Aerobic Respiration in Plants and Animals
Aerobic respiration consumes oxygen and produces carbon dioxide. The rates of aerobic respiration varies among organisms and is determined by numerous factors. In this experiment you will measure the rate of oxygen consumption and carbon dioxide production in germinated and un-germinated seeds and compare these with animals (worms).
- ? Which do you hypothesize will produce more carbon dioxide on a per weight basis, germinated or ungerminated seeds? Explain your reasoning.
- ? Which do you hypothesize will produce more carbon dioxide on a per weight basis, germinated seeds or worms? Explain your reasoning.
The CO 2 and O 2 measurements of the ungerminated seeds will be setup as a demonstration .
- Obtain 2 plastic BioChambers, O 2 probes, CO 2 probes, labquest modules, germinated seeds, and worms.
- Weigh about 10 g of ungerminated seeds. Record the exact mass below.
9.6 = Mass of germinated seeds (g).
- Weigh about 10 g of germinated seeds. Record the exact mass below.
9.1 = Mass of germinated seeds (g).
- Place the seeds in BioChambers.
- Obtain 4 worms and record their combined mass below.
9.3 = Mass of worms (g).
- Place the worms in a separate BioChamber.
- Connect the oxygen and carbon dioxide probes to each biochamber as indicated by your instructor.
- Choose New from the File menu.
- On the Meter screen, tap Length. Change the data-collection length to 900 seconds.
- Now change both the oxygen and carbon dioxide sensors to report their measurements in parts per trillion (PPT). Tap Sensors, Change Units, choose CO 2 and oxygen, then choose PPT.
- Begin data collection (click the green arrowhead) for both seeds and worms.
- When data collection has finished (after about 10 minutes), graphs of oxygen and carbon dioxide gasses vs. time will be displayed.

Final CO 2 Levels (%):
Ungerminated seeds = 2
Germinated seeds = 6
Worms = 20
- ? Now calculate CO 2 production on a per weight basis for the germinated seeds and the worms. Simply divide final CO 2 levels by the weight of the samples.
Ungerminated seeds = % CO 2 / g
Germinated seeds = % CO 2 / g
Worms = % CO 2 / g
- ? Are these results what you predicted? How do the respiratory rates of the ungerminated and germinated seeds compare? Which produced more CO 2 on a per weight basis—the plants or animals? How could this experiment be improved to provide a more accurate comparison between living plant and animal tissue (think about the structure of sunflower seeds)? Explain .
Part 3- Aerobic Respiration in Humans
This activity will be a simple demonstration that compares the CO 2 and O 2 levels in the air you breathe in versus the air you exhale.
Imagine you are at rest and breathing normally. Now imagine you are at rest, but hold your breath as long as you comfortably can.
- ? What do you predict would be the relative levels of CO 2 and O 2 in the air you breathe in versus the air you exhale when breathing normally and the air you exhale after holding your breath? Write your hypotheses here.
Now lets examine CO 2 and O 2 levels in the air people inhale and exhale.
Procedure followed in lab:
- Obtain a large plastic BioChamber. Make sure the lid is off and GENTLY turn it upside down and wave it through the air (this will remove any residual CO 2 that may be present from its previous use).
- Next add the lid and CO 2 and O 2 probes and measure the gas levels for about 3 minutes. Record the CO 2 and O 2 after 3 minutes below.
AMBIENT GAS LEVELS:
CO 2 = 395 ppm O 2 = 20.5 %
- Now measure the CO 2 and O 2 levels in the air you exhale while breathing normally. Remove the BioChamber lid and use a straw to gently exhale a single breath into the BioChamber. Add the lid and CO 2 and O 2 probes and measure the gas levels for about 3 minutes. Record the CO 2 and O 2 after 3 minutes below.
GAS LEVELS DURING NORMAL EXHALATION:
CO 2 = 575 ppm O 2 = 15.5 %
- Remove the BioChamber lid and GENTLY invert and wave through the air to remove residual CO 2 .
- Now measure the CO 2 and O 2 levels in the air you exhale after holding your breath. Remove the BioChamber lid. Hold you breath as long as you COMFORTABLY are able (be sure to breathe normally if you feel dizzy or light-headed). Use a straw to gently exhale a single breath into the BioChamber. Add the lid and CO 2 and O 2 probes and measure the gas levels for about 3 minutes. Record the CO 2 and O 2 after 3 minutes below.
GAS LEVELS AFTER HOLDING BREATH:
CO 2 = 700 ppm O 2 = 12.5 %
- ? Compare the levels of CO 2 and O 2 in the three measurements recorded. How do the results compare with your hypotheses? Explain these results. Consider the fact that you are releasing carbon (in the form of CO 2 ) every time you exhale. Where does that carbon come from?
- ? Consider the oxygen consumed during aerobic respiration. Why do we need that oxygen? What, specifically, does it do?
Version History

Want to create or adapt books like this? Learn more about how Pressbooks supports open publishing practices.
6 Cellular Respiration
Introduction: cellular respiration.
Your body is a chemical machine; and like any machine, it uses energy to do work. The process of doing work in your body involves two essential stages: converting energy taken in as food into a usable form, and then using that energy to carry out the chemical processes that make you alive. Work includes everything your body does; from maintaining your internal temperature and regulating your heartbeat to reading this introduction, contracting muscles, digesting food and excreting wastes. Metabolism is the sum of all of the chemical processes carried out by your body (work). Metabolic rate is the rate (amount per unit of time) at which your body expends energy to do this work.
Our cells cannot use the energy in food directly. Instead, they need to convert that energy into a useable form. Adenosine triphosphate (ATP) is the energy source that all organisms use in just about every cellular process requiring energy. Our cells transfer the energy stored in organic molecules to ATP through a process called cellular respiration .
During cellular respiration, proteins called enzymes bind with substrate molecules (like the sugar glucose) and help to break the molecules apart. In doing so, the cells harvest the energy stored within chemical bonds. Through several enzyme-mediated chemical reactions, this energy is transferred and stored in molecules of ATP. When our cells need energy to do any form of work, ATP is broken down and the stored energy is released and used to fuel chemical reactions. In other words, we eat food, our cells convert food molecules to ATP, and ATP is the fuel that is burned when our cells are doing work. Without enzymes, none of this would be possible.
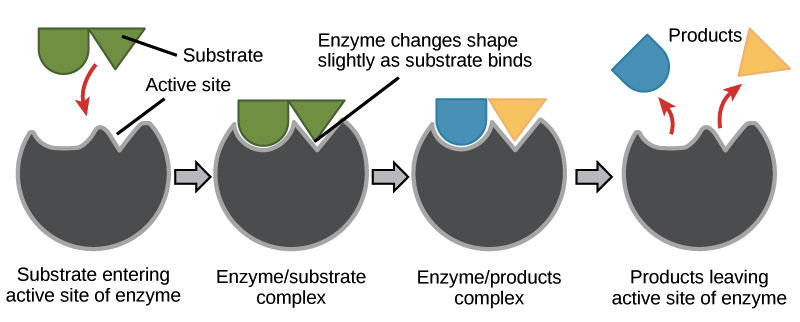
Enzymes are specific ; they only bind to certain substrate molecules, and not all cells have the same enzymes. Additionally, not all organisms have the same enzymes. For example, we all know that termites eat wood. However, they cannot actually metabolize the cellulose found in plant cell walls because termites don’t produce cellulase (the necessary enzyme). Instead, microbes in a termite’s gut that have cellulase break down the cellulose into a useable form of glucose that the termite can metabolize. For another example; when a person lacks the enzyme lactase, their body cannot break down the sugar lactose, which is found in dairy products. The lactose instead passes undigested into the large intestine where bacteria metabolize it (they have lactase), producing gas and digestive discomfort. We call this condition lactose intolerance.
Aerobic respiration involves the complete oxidation (removing electrons) of organic molecules, like glucose. In this process, oxygen is the final electron acceptor in a series of enzyme-catalyzed chemical reactions. The chemical formula for aerobic respiration is:
C 6 H 12 O 6 + 6O 2 → 6CO 2 + 6H 2 O + energy (heat + 36 ATP)
Now, this might appear to be a simple and straightforward process. But what the simple chemical equation is missing is all the stuff that that arrow represents. So let’s take a look at what goes on behind that deceptively simple looking arrow.
Cellular Respiration: What’s really going on?
Cellular respiration starts out with glycolysis , in which glucose (or another similar sugar) is oxidized and broken into 2 product molecules (the word glycolysis actually means breaking sugar). During glycolysis, glucose is brought into the cytoplasm of a cell where it is basically attacked by enzymes that steal a couple electrons. The process of stealing or harvesting electrons is called oxidation; hence the other name for cellular respiration, oxidative metabolism . In order to accomplish this task, these enzymes use the energy from 2 molecules of ATP, and hand the stolen electrons to an electron carrier called nicotinamide adenine dinucleotide ( NAD + for short). Accepting 2 electrons reduces each NAD + molecule into NADH (we’ll come back to these later).

After a bit of atomic rearrangement and several more steps, the glucose is split into 2 molecules of pyruvate . This whole process involves 10 chemical reactions and a bunch of enzymes, but it produces 4 ATP molecules in the process, for a net gain of 2 ATP molecules.
Next, the pyruvate molecules are brought into the intermembrane space inside the mitochondria where they are further oxidized. Removal of another electron from each pyruvate releases some carbon dioxide as waste, and 2 more NAD + molecules are reduced to NADH. The remaining 2-carbon molecules (called an acetyl group) are combined with another coenzyme (Coenzyme A) to form 2 molecules of acetyl-CoA , which is transported across the mitochondrial inner membrane into the matrix. Once acetyl-CoA is in the matrix, the 2-carbon molecule is released and enters the Krebs cycle, and Coenzyme A returns to the intermembrane space to go get more acetyl groups.
The Krebs cycle is another really complicated process involving many enzymes and chemical reactions. But put simply, it goes like this. The 2-carbon molecule is added to a leftover 4-carbon molecule from the previous cycle to form a new, 6-carbon molecule. That 6-carbon molecule gets oxidized over and over, and several NAD + molecules get reduced to NADH. There’s also another electron-carrying coenzyme called FAD that that gets reduced into FADH 2 along the way. As electrons are harvested, CO 2 is released as a waste product and another ATP gets built. At the end of the Krebs cycle, the leftover 4-carbon molecule is combined with an incoming 2-carbon molecule and the whole things happens again.
Alright, well so far we haven’t made much ATP. We only got 2 from glycolysis and 1 from each of the acetyl groups brought into the Krebs cycle. That’s only 4 ATP form a whole glucose molecule! I thought this was supposed to run all of our life processes! Remember all those NADH and FADH 2 molecules? Well we just made 12 of them (10 NADH, 2 FADH 2 ), and each one has 2 high energy electrons ready to get used.
NADH and FADH 2 each carry their electrons to the third part of cellular respiration, the electron transport chain (ETC). The ETC is a series of enzymes that transport electrons from one enzyme to another in a chain. Along the way, each enzyme uses a bit of the energy from these electrons to pump free protons (also called hydrogen ions) out of the matrix and into the intermembrane space. At the end of this whole process, the electrons that were harvested all along the way are combined with an oxygen molecule and a couple free protons to form water.
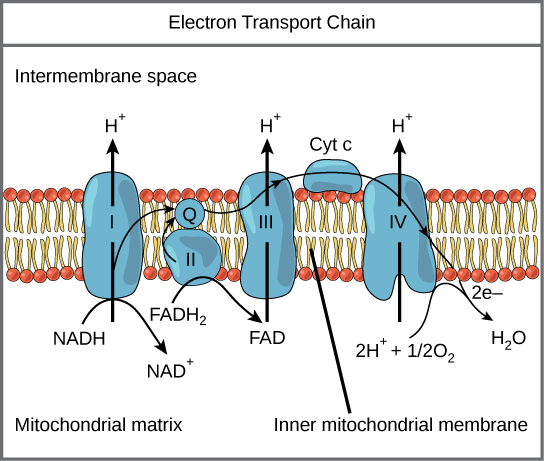
But we’re not done yet. The active transport of protons creates a concentration gradient with lots of protons outside the matrix and few protons inside the matrix. Of course, the universe doesn’t like concentration gradients and the protons are driven to equalize the disparity by re-entering the matrix. Since they are charged particles, the ions can’t pass through the inner membrane directly. Instead, they have to move through a very important enzyme called ATP synthase . This enzyme uses the movement of protons through it to attach phosphate molecules and adenosine diphosphate molecules; thus, synthesizing ATP. Most of the ATP produced during cellular respiration is synthesized by ATP synthase (on average 28-30 ATP in eukaryotes).
Wow! I can’t believe we represent that entire process with a simple arrow!
Aerobic versus Anaerobic respiration
We humans (along with all the other animals) are aerobes , which means we simply must have oxygen to survive. Without oxygen, we have no final electron acceptor for our electron transport chain, and cellular respiration shuts down. Without cellular respiration, we don’t make enough ATP and after a while, well, we die. But some organisms are able to survive without any oxygen through the process of anaerobic respiration . Just like aerobic respiration, anaerobic respiration involves the oxidation of sugars and ATP is produced. However, instead of using oxygen as the final electron acceptor, anaerobes use different molecules in its place. For example, methanogens are anaerobic prokaryotes in the Domain & Kingdom Archaea. They use carbon dioxide (CO 2 ) as their electron acceptor at the end of the ETC and produce methane (CH 4 ) as a product.
Fermentation is another form of anaerobic respiration, and involves the incomplete oxidation of sugars. In fermentation, glucose is broken down through glycolysis, and a small amount of ATP is produced. However, in the absence of oxygen further oxidation of pyruvate is not possible. If the organism can’t use a different substitute electron acceptor, the only way to keep producing ATP (and keep staying alive) is to keep doing glycolysis. But if there’s no molecule to take the electrons from the ETC, then the whole thing backs up and NADH get stuck holding electrons with nowhere to go. So the solution is for NADH to dump its electrons and go back to get more from the next glucose molecule, thus allowing glycolysis to continue.
There are several forms of fermentation and different critters use different forms. For example, when you exercise, you often use up ATP faster than you can take in oxygen. Under these conditions, your muscles switch from aerobic respiration to fermentation. Lucky for us, NADH is perfectly happy to give its electrons back to pyruvate. Reduction of pyruvate produces lactate, which is converted into lactic acid. Lactic acid buildup within muscle cells is a common contributor to soreness after exercise, this process allows us to produce enough ATP to stay alive until we can get oxygen back into our cells and return to aerobic respiration.
Yeasts are a type of single-celled fungi that use fermentation as their preferred form of cellular respiration. After glycolysis, pyruvate gets converted into acetaldehyde which serves as an electron acceptor for NADH. Reduction of acetaldehyde produces ethanol and carbon dioxide as products. We humans utilize this process in the production of bread and beverages such as beer and wine.
The chemical formula for ethanol fermentation is:
Suppose we wanted to measure the metabolic rate of yeasts. What products could we measure in this process?
Measuring cellular respiration
We can’t measure cellular respiration directly (e.g., how fast every cell in your body produces ATP from food molecules). However, we can measure the results of cellular respiration. For example, we can measure the amount of heat given off by an organism, the rate of consumption of chemical reactants, or rate of production of chemical products during cellular respiration. The rate of consumption or production of these materials is directly proportional to an organism’s metabolic rate. For example, the more O 2 is used up, or CO 2 is produced over a set amount of time, the higher the metabolic rate.
Experimental Design: Standardizing Units of Measurements
Standardizing units is one way of controlling for extraneous variables that might affect the results of an experiment. For example, in lab this week, we will be using the same volume of solutions in each treatment of our fermentation experiment. That way our estimations of metabolic rate are comparable as a rate of CO 2 production from a fixed volume of yeast and carbohydrate solutions.
However, we can’t always control the amount of “subject” in an experiment; therefore, we sometimes take mathematical steps to control for a variable. For example, different organisms don’t consume energy at the same rate. Does a 3-ton elephant use the same amount of energy as a 20-gram mouse; or more/less? But is this really a fair comparison? The elephant has more cells in its body, so you would clearly expect it to consume more energy as a whole. If we want to compare metabolic rates between these organisms, we need to standardize our units of measurement. In other words, we need to find a unit of measurement that minimizes the effect ( controls for the variable ) of number of cells in the body. We can do this by dividing the whole-body metabolic rate of each organism by its body weight. This way we are comparing the rate of energy consumption per each unit of mass (or number of cells, assuming most cells weigh about the same). If we are using a group of animals, we simply divide the whole group metabolic rate by the weight of the group of animals. Now we have the per gram metabolic rate and we can compare these values for any organisms we want regardless of number or body size.
Doing the math
Familiarize yourself with the following equations. You will use them to estimate the metabolic rates of our organisms in Experiment 2. For each group of worms, you will calculate the whole-body (whole group) production rate of CO 2 per gram of body weight. In order to have enough cells doing respiration, our experiment will use 15 mealworms at a time. Thus, the whole-body respiration rate will represent the average respiration rate per individual in each trial.
Equation for whole body respiration rate of animals:

Equation for per-gram respiration rate of animals:

Experiment 1: Fermentation
We know that enzymes are essential for cellular respiration to take place, and that different enzymes help metabolize different food molecules. We also know that organisms can lack certain enzymes (e.g., lactose intolerance) causing them to not metabolize certain foods. Yeasts are single-celled fungi that metabolize sugars through fermentation and produce ethanol and carbon dioxide gas. We can estimate the yeasts’ rate of cellular respiration by measuring the amount of CO 2 produced over a period of time. We will use this understanding to examine cellular respiration in yeasts and try to determine which of six carbohydrate substrates yeast can best metabolize.
Our research question for this experiment is: Which of these carbohydrates can yeast best utilize to perform cellular respiration?
We will use the following carbohydrate substrates in solution:
● 10% glucose – monosaccharide, hexagonal sugar
● 10% fructose – monosaccharide, pentagonal sugar, often called fruit-sugar
● 10% galactose – monosaccharide, similar to glucose but with slightly different shape
● 10% sucrose – disaccharide (glucose + fructose), often called table-sugar
● 10% lactose – disaccharide (galactose + glucose) component in milk.
● 1% starch – polysaccharide chain of glucose molecules
● Distilled H 2 O – control
- Obtain a CO 2 sensor and a 250 mL respiration chamber.
- Press the power button on the CO 2 sensor, red light will start blinking.
- Open the graphical analysis app on your phone or wireless device. Select New Experiment → Wireless Devices and select the discovered sensor that matches your sensor’s ID code. Touch Done . The sensor’s light will change to flashing green. ( Be sure your app is connected to your sensor ).
- Label a small respiration chamber (250 mL) with your team’s assigned carbohydrate treatment, and then add 10 mL of yeast slurry and 20 mL of the carbohydrate solution to the chamber and swirl to mix the solutions.
- Insert the CO 2 sensors into the chambers and then wait 5 minutes for the yeasts to begin to metabolize the sugars.
- To set up the time for your experiment, touch Mode on the app screen. Touch End Collection , then touch Duration & set the Duration to 600 seconds. Touch Done .
- Allow at least 3 minutes for the sensor to warm up. Touch the symbol and select Meter . Record starting CO 2 ppm in Table 5.1, then touch Collect to start recording data. You can see your data graph and values by tapping the symbol again and selecting graph or graph and table.
- After 10 minutes, record final CO 2 ppm in Table 6.1.
- The graph will display a line fit over the data in the format y=mx+b, where y is CO 2 concentration (ppm), x is time (s) and m=the rate of change in CO 2 ppm per second.
- Multiply the m value (slope of the line) by 60 to convert the time value to minutes.
- Record the CO 2 Production rate (ppm/min) in Table 5.1.
- Repeat steps 4-9 twice to obtain 3 total replicates with your carbohydrate treatment, and add your team’s data to the class data spreadsheet.
Experiment 2: Aerobic Respiration
You are all no doubt familiar with insects, those crunchy critters, that run about under our feet and occasionally fly directly into our eyeballs. What you might not be aware of is how they work. As far as basic metabolic functions go, they differ very little from animals we are more familiar with: mice, frogs, elephants, us humans. However, insects do differ in some key ways.
Insects are invertebrates , which means they lack an internal segmented backbone (spine). They are also in the Class Arthropoda (literally “jointed feet”). So, like all arthropods, instead of an internal support structure for muscles and other squishy parts to hold on to, insects rely on an external skeleton (or exoskeleton). The exoskeleton is composed primarily of chitin (carbohydrate polymer also used by fungi for cell walls) and consists of many hard plates held together by softer connecting membranes. Inside, insects have an open circulatory system. Unlike your blood, which is contained in vessels and separated from other fluids like lymph & interstitial fluids (the watery fluid between squishy parts), arthropods have hemolymph. Hemolymph is a sort of mixture between blood and other body fluids that flows openly throughout the body cavity. It is collected from the body by vessels that return it to the heart where it is pumped back into the body cavity. Instead of the hemolymph carrying oxygen to cells and carbon dioxide away, like your blood does, insects have a tracheal system for getting oxygen in and carbon dioxide out. Since they lack lungs, insects have pores in their exoskeleton that open into air-filled tubes called trachea .
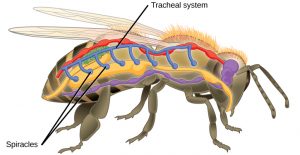
These breathing tubes pass through body tissues, and branch into smaller and smaller tubes called tracheoles . At their finest, tracheoles are so numerous that every cell in an insect’s body resides within 2-3 cells of a tracheole. Oxygen and carbon dioxide diffuse in and out of the cytoplasm directly with the air in the tracheoles.
Ok, so what does this have to do with cellular respiration? In Experiment 2 of this week’s lab, we will be using insects to assess the effects of temperature on cellular respiration rates. We will account for differences in insect body size by calculating respiration rate per gram of body mass. This way we can control for (and negate) the compounding effects of different critters having more or less cells in their bodies. We will use two temperature treatments and look for patterns in the data to help us understand how environment plays a part in regulating metabolic processes.
Since insects are animals, they harvest energy from food molecules primarily through aerobic oxidative metabolism (AKA: cellular respiration), just like you. Unlike you, insects are ectotherms . This means that their body temperature is highly influenced by the temperature of their environment. In most ectotherms, as body temperature changes there is often an observable change in cellular respiration rate. We will thus explore the effects of temperature on respiration rate in mealworms. Mealworms are often raised as food for pet animals (e.g., lizards, bats, hedgehogs), and are apparently very nutritious. They also appear to be able to eat and digest polystyrene (Styrofoam) without detrimental impacts.

- Obtain a CO 2 sensor, a 250 mL respiration chamber, and a plastic tub.
- Weigh your respiration chamber and record its mass.
- Obtain 15 mealworms from the colony, add them to the respiration chamber, and weigh both together. Record the chamber + animal mass, and calculate the mass of your animals.
- Place the chamber in the correct treatment conditions for 10 minutes to allow the animals to come to treatment temperature. For cold treatment, bury the chamber in ice inside the tub.
- Press the power button on the CO 2 sensor; the red light will start blinking.
- Open the graphical analysis app on your phone or wireless device. Select New Experiment → Wireless Devices and select the discovered sensor that matches your sensor’s ID code. Touch Done . The sensor’s light will change to flashing green. ( Make sure your app is connected to your sensor ).
- To set up the time for your experiment, touch Mode on the app screen. Touch End Collection , then touch Duration & set the Duration to 600 seconds . Touch Done.
- After the 10 minute “temperature adjustment,” touch the symbol and select Meter . Record starting CO 2 ppm, then touch Collect to start recording data. You can see your data graph and values by tapping the symbol again and selecting graph or graph and table.
- After 10 minutes, record final CO 2 ppm and calculate whole-body respiration rate, and per gram respiration rate (see above) for your mealworms/treatments.
- Repeat steps 3-9 with new mealworms to obtain 2 replicates with your treatment.
- Add your replicate data to the class spreadsheet.
Biology I: Introduction to Cell and Molecular Biology Lab Guidebook Copyright © by Alex Urquhart is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License , except where otherwise noted.
Share This Book

Measurement of Respiration and Effect of Temperature
Learning Objectives
After completing the lab, the student will be able to:
- Measure the consumption of oxygen during respiration.
- Measure the effect of environmental conditions on respiration in pea seeds.
Activity 2: Pre-Assessment
- Students stain corn seeds over a period of several days after the seeds are soaked with water to promote germination with iodine. Iodine stains starch blue. The students observe that the amount of starch decreases during germination. Can you explain this observation? Which metabolic process uses up starch?
- What kind of biological catalysts are involved in the reactions of respiration? If the rate of a chemical reaction doubles with the temperature, would you expect that rates of respiration to increase continuously with temperature?
- Discuss the answers to questions 1 and 2 with the class.
Activity 2: Measurement of Respiration and Effect of Temperature on Respiration Rate
Imagine that you plan to monitor respiration in a whole organism, such as a small invertebrate or a seedling. You may decide to follow the disappearance of the reactants, either glucose or oxygen. Your second choice is to measure the formation of the products, either water or carbon dioxide. In this laboratory, you will design experiments to assess the effect of environmental conditions on the process of cellular respiration.
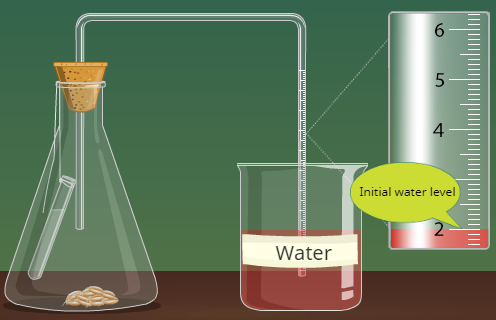
In respiration, oxygen is consumed and CO2 is released. In this experiment, we will measure the disappearance of oxygen. A respirometer consists of an enclosed chamber in which the studied organism is placed and a graduated pipette with which we measure changes in the gas volumes. The CO2 gas that forms will be removed by adding Ca(OH)2, which reacts with carbon dioxide to form the insoluble salt CaCO3, calcium carbonate.
While measuring the changes in the amount of gas produced, you will consider the ideal gas law equation which can be stated as
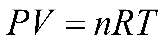
P represents the atmospheric pressure in mmHg, V is the volume of the gas in liters, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature in degrees Kelvin. In the respirometer, pressure remains constant as the gas produced displaces water in the tube. We will set up the respirometers in a water bath to minimize fluctuations in temperature.
In this experiment, you will use pea seeds. In a seed, like the yellow peas shown in Figure 8.2, a tough coat protects the plant embryo. Nutrients in the form of starch and lipids surround the embryo and support its germination , or growth from seed, until the appearance of photosynthetic structures. Seeds are normally dormant , that is metabolically inactive, until the environmental conditions helpful for growth are available. In order to bring the seeds to an active state, (out of dormancy), the seeds you will use were soaked in water via a process called imbibition, for 6 to 8 days.

Cellular respiration involves three major sequential stages: glycolysis, the citric acid cycle, and oxidative phosphorylation. Oxygen serves as a terminal electron acceptor. Glycolysis takes place in the cytoplasm whereas mitochondria are the site of the citric acid cycle and the electron transport chain.
All the steps of respiration are mediated by enzymes , biological catalysts—mainly proteins—that lower the activation energy , the energy required to be available in a system before a chemical reaction can take place. Enzymes are not used up by the reactions they catalyzed. The process of respiration responds to the same environmental factors that affect the activity of enzymes. In this activity, you will measure the effect of temperature on respiration rates.
Safety Precautions
- Handle test tubes or glass containers with care; insert the plug by holding the container in a paper towel.
- Use plastic pipettes rather than glass pipettes.
- Wear goggles or safety glasses.
- Wear gloves when working with KOH or lime [Ca(OH) 2 ], which are corrosive chemical compounds.
- Use care while handling hot water. Wear mitts and do not leave boiling water or a hot plate unattended.
- Protect your clothes with an apron.
- Inform your teacher immediately of any broken glassware as it could cause injuries.
- Clean up any spilled fluids to prevent other people from slipping.
- Wash your hands with soap and water after completion of the activity.
For this activity, you will need the following:
- Dried yellow peas
- Glass beads
- Balance and weigh boats
- Paper towels to imbibe seeds
- KOH or lime water
- Food coloring
- Absorbent and non-absorbent cotton
- Drilled rubber stoppers that fit the opening of the test tubes or bottles
- 1-ml plastic pipettes
- Top loading balance
- Thermometers
- Water baths
- Weights such as clamps or hex keys
- Wide glass test tubes or bottles
- Stirring rod
- Hot plate to boil water
For this activity, you will work in pairs .
Structured Inquiry
Step 1: Obtain 25-30 germinating peas, dry peas, and glass beads to start your experiment. Place the germinating peas in a weigh boat and measure their weight. Record the weight in your notebook and then repeat for the dried peas and glass beads.
Step 2: In this activity, you will indirectly measure the rate of respiration of the peas by monitoring the decrease in gas when the peas are placed in the respirometer chamber. What gas will decrease in the chamber as the peas undergo respiration? Hypothesize how much the gas levels will likely change for the germinating seeds, dry seeds, and glass beads. Record your hypotheses and predictions in your notebook.
Step 3: Student-Led Planning: Which of your treatments serve as a control? Is this a positive or negative control? How will this control reveal whether or not the experiment is functioning properly? Write your answers in your notebook.
Step 4: Assemble a respirometer using Figure 8.3 as a guide and following the steps below.
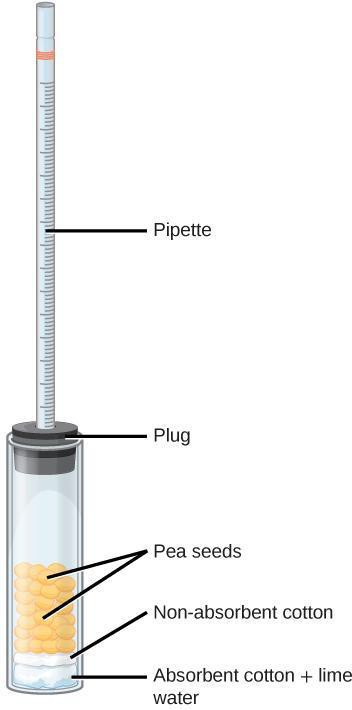
- In a wide test tube (or bottle), drop a pad of absorbent cotton. Pack down the cotton with a stirring rod. Add lime water Ca(OH)2, being careful not to oversaturate the pad or drip the lime water on the side of the tube.
- Insert a thin layer of non-absorbent cotton, pushing down with the glass rod. The cotton protects the seeds from lime water; however, if it is too thick, it will interfere with the diffusion of CO2.
- Plug the test tube with a bored rubber stopper. Add a drop of colored water in a 1-ml graduated pipette and insert the pipette in the hole of the stopper. Adjust the position of the drop by inserting a syringe in the stopper until you can easily read the position of the dye. (The syringe is not shown in Figure 8.3.) Rub some petroleum jelly where the pipette comes into contact with the rubber stopper. The respirometer must be water tight to yield reliable results. It is also possible to wrap the openings with stretchable plastic film.
- You may want to test for leaks by immersing the respirometer with the plug and pipette before filling it with reagents and cotton.
Step 5: Assemble the respirometer containing the control sample in the same manner.
Step 6: Immerse the respirometers with the experimental sample and the control in the water bath. Lining the water bath with a white paper towel will make it easier to read the markings on the pipettes. Make sure that the pipettes are resting across a piece of ribbon or string that spans the width of the water bath, as illustrated in Figure 8.4. The goal is to keep the pipettes out of the water while the test tubes remain submerged.
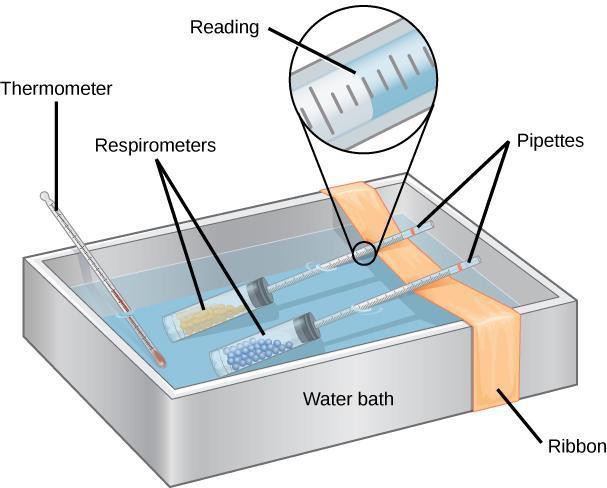
Step 7: Let the respirometers equilibrate for 5–10 minutes.
Step 8: Read the starting volume on the pipette. This is time 0 min. Record the displacement of the colored bead for all samples every 2 minutes for 20 minutes and enter data in a table of measurements.
Step 9: Critical Analysis: Calculate the changes in volume where the reading at time 0 is subtracted from every subsequent reading. Subtract the rate of volume change measured in the control samples to obtain a corrected rate of respiration.
Graph the changes in volume in respirometers as a function of time and calculate the rate of change from the slopes of the line plots. Calculate the rate of change per gram of seed. This will allow you to compare values obtained from different samples. Draw a plot of changes in gas volumes from the data in your table. What measurements will you enter on the axis? What measurements will you enter in the y -axis? Determine the rate of respiration in your experiment. How did you use the data from your control or controls? Did volumes change during the experiment? Which gas caused the change in volume? Do the results support your hypothesis? Can you explain unexpected results? Were the respirometers water-tight at all times? How could you modify the experiment in the future? Write your answers in your notebook.
Guided Inquiry
Step 1: Repeat the steps to set up the respirometers described in the Activity 2 Structured Inquiry. Use three water baths at the following temperatures: 10°C, room temperature (see Structured Inquiry), and 50°C.
Step 2: Hypothesize/Predict: Discuss with your partner what kind of influence temperature might have on metabolic processes. How would the respiration rate measured at 10°C compare to the rate measured at room temperature? Will the rate of respiration be higher at 30°C than room temperature? Do you predict that the rate of respiration will be higher at 50°C than at room temperature or 30°C? Enter your hypotheses in your notebook.
Step 3: Student-Led Planning: You will now measure the rate of respiration at three different temperatures. Discuss with your partner if you need to run the experiment at room temperature again. Decide which control you will set up for this experiment. Make a note of all the steps you will perform, as you did in Activity 2, and create tables for your observations in your lab notebook. You will take readings of the colored water bubble at 2-minute intervals for 20 minutes. Have your teacher approve your experimental procedure before proceeding.
Step 4: Once approved, carry out your experimental procedure, closely monitoring the temperature as you take measurements.
Step 5: Critical Analysis: Graph the changes in gas volumes from the data in your table for all three temperatures for the experimental and control set-up, as you did for the Structured Inquiry. Determine the rate of respiration for each temperature. Because the gas law shows that differences in temperature affect volumes, you must correct for any changes in volume that are a consequence of temperature variations rather than respiration. To do this, subtract changes in volumes measured in the respirometer containing glass beads from the changes in volume measured in the tubes containing germinating seeds held at the same temperature. Do the results support your hypothesis? Explain whether your results support or refute your hypothesis. How could you modify the experiment in the future? Write your ideas in your notebook.
Assessments
- Students record changes in gas released from respirometers containing germinating seeds and dry seeds. They set up their tubes in air rather than in a water bath. A thermometer probe is inserted in each respirometer. The tube that contains germinating seeds shows an increase in temperature. No such increase is recorded in a respirometer that contains dry seeds. What is the reason for the difference in temperature?
- The ideal gas law shows that volume depends on temperature as well as pressure. Why do you set your respirometers in a water bath?
- A classmate insists that there are no mitochondria in leaves because chloroplasts produce ATP through photosynthesis. How would you experimentally disprove this claim?
Lab Manual for Biology Part I Copyright © 2022 by LOUIS: The Louisiana Library Network is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License , except where otherwise noted.
Share This Book
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- HHS Author Manuscripts

A practical guide for the analysis, standardization, and interpretation of oxygen consumption measurements
Ajit s. divakaruni.
1 Department of Molecular and Medical Pharmacology, David Geffen School of Medicine; University of California, Los Angeles. Los Angeles, California 90095. United States of America.
Martin Jastroch
2 Department of Molecular Biosciences, The Wenner-Gren Institute, The Arrhenius Laboratories F3, Stockholm University. SE-106 91 Stockholm, Sweden
AUTHOR CONTRIBUTIONS
Measurement of oxygen consumption is a powerful and uniquely informative experimental technique. It can help identify mitochondrial mechanisms of action upon pharmacologic and genetic interventions, and characterize energy metabolism in physiology and disease. The conceptual and practical benefits of respirometry have made it a frontline technique to understand how mitochondrial function can interface with – and in some cases control – cell physiology. Nonetheless, an appreciation of the complexity and challenges involved with such measurements is required to avoid common experimental and analytical pitfalls. Here we provide a practical guide to oxygen consumption measurements covering the selection of experimental models and instrumentation, as well as recommendations for the collection, interpretation, and normalization of data. These guidelines are provided with the intention of aiding experimental design and enhancing the overall reputability, transparency, and reliability of oxygen consumption measurements.
INTRODUCTION
Measurements of oxygen consumption rates have been central to the recent, resurgent interest in mitochondrial metabolism 1 . In particular, the validation and adoption of microplate-based respirometry has revolutionized the field of bioenergetics by making measurements accessible to non-specialists and enabling studies showing how mitochondrial function is altered in response to growth factor signaling 2 , cytokine stimulus 3 , 4 , and cell activation 5 , 6 . An appreciation that mitochondrial metabolism is central to an array of physiological processes beyond classic ‘metabolic’ tissues 7 , 8 – coupled with turnkey solutions to study respiration in a range of model systems never previously feasible 9 – has resulted in a rapid, broad adoption of the measurements.
The oxygen consumption rate is an integrative and comprehensive readout of cellular metabolism and mitochondrial function. Because respiration is coupled to ATP synthesis 10 , 11 , many processes that either make or consume ATP can be studied with respirometry so long as the experimental conditions allow that process to control the overall oxygen consumption rate. For pathways that generate ATP through oxidative phosphorylation, respiration can be used to assess altered activity of specific enzymes or metabolic nodes by offering isolated mitochondria or permeabilized cells energy substrates that each require different metabolic pathways for oxidation but all terminate in oxygen consumption 12 .
In addition to these conceptual strengths, there are several practical benefits to respirometry. Both major types of commonly used analytical instrumentation – chamber-based platinum electrodes 13 and microplate-based fluorescent readings 9 , 14 – offer a fairly low barrier of entry for the non-specialist. Both platforms also offer real-time, visual readouts that allow rapid experimental iteration unlike destructive, end-point assays that measure gene and protein expression or metabolite levels. The ability to monitor real-time oxygen consumption rates also enables studies measuring acute mitochondrial responses to pharmacologic inhibitors or effectors that can alter the cellular activation state.
As the widespread adoption of this technique has occurred in a relatively short period, the trial-and-error and iteration necessary to develop the best practices remains ongoing. Thus, despite the utility and approachability of these measurements, challenges and complexity remain regarding the design and interpretation of respirometry studies. Several manuscripts have been published detailing the utility of respirometry ranging from conceptual 12 , 15 – 20 to instructional 9 , 13 , 21 – 23 .
As a companion to these, this perspective may help serve as a starting point to foster the reputability, transparency, and trustworthiness of in vitro and ex vivo oxygen consumption data. We discuss considerations for choosing whether isolated mitochondria, cells, or three-dimensional multicellular models is most appropriate for a given question, supplementing existing guidelines 17 , 24 . For each of these systems we review the relative strengths and weaknesses of common measurement platforms (summarized in Table 1 ) and provide recommendations for data collection, normalization, and presentation. Building upon published works that define respiratory parameters and provide rational flowcharts for hypothesis testing 12 , 16 , 25 , we aim to help the novice and experienced user alike in identifying common pitfalls, avoiding data misinterpretation, and establishing shared practices for respirometry data.
Comparison of commonly used measurement platforms
| Chamber-based platinum electrode | Plate-based fluorescence/phosphorescence | |
|---|---|---|
| • Oroboros Instruments • Hansatech Instruments Ltd. • Rank Brothers Ltd. • Strathkelvin Instruments Ltd. | • Agilent Seahorse XF Analyzer • Cayman Oxygen Consumption Rate Assay • Agilent MitoXpress | |
| Range from $1–2K to fully integrated instruments at $40–50K | Assay kits for use with multimode plate reader at ~$400. Seahorse XF Analyzers range from $40K (8-well) to >$200K (96-well). | |
| Single (or dual) chamber setups measure one (or two) technical replicates at a time. Each experiment takes ~15 minutes, and the chamber must be cleaned between runs. | 96-well microplate-based approaches allow several experimental groups, each with multiple replicates, to be assessed simultaneously. Each experiment takes up to 75–90 minutes (for XF Analyzer) and plates are disposable. | |
| Larger chamber volumes require increased amounts of material. Yield from clinical samples or some primary cell preparations may be prohibitively small. | The XF Analyzer reduces the sample material required by orders of magnitude by dramatically reducing the size of the measurement chamber. | |
| Can be multiplexed with electrodes or fiberoptic detectors sensitive to other analytes to measure ROS, pH, Ca , mitochondrial membrane potential, etc. | Simultaneously measures changes in extracellular pH, and recent corrections allow quantitation of lactate efflux. | |
| Easy access to raw experimental data for manual calculation of rates. | Propriety XF analysis software automatically calculates rates. Rates with fluorescent or phosphorescent assay kits are easily calculated manually. | |
| Provides quantitative oxygen consumption rates reliable for very low respiratory rates and at low oxygen tensions | Fluorescent assay kits only provide relative, qualitative comparisons. User-friendly XF Analyzer software is quantitative and matches results from platinum-based electrodes across a range of oxygen consumption rates. | |
| Manual injection of effector compounds allows unlimited additions, which can be helpful for precise titrations. | The XF Analyzer allows up to 4 injections at user-defined time points. For assay kits, the properties of the multimode plate reader used will dictate the injection scheme. | |
| Multiplexed measurements offer multiparametric analysis of isolated mitochondria; Reliable readings of very low oxygen consumption rates; Easy access to raw data avoids calculation artifacts. | Allows simultaneous measurements with several distinct oxidizable substrates across multiple experimental groups (e.g., WT vs. KO) to identify pathway-specific mechanisms of action. Amenable to small sample sizes such as mitochondria isolated from clinical biopsies or specific tissue regions. | |
| Normalization is straightforward, as defined amounts of cells in suspension are assayed. All of the sample material contributes equally to the reading. | Preservation of ECM interactions and cellular structures enhances physiological relevance. Can study small sample sizes such primary cell populations or clinical samples. Concurrent measurements of glycolysis (XF Analyzer) allow calculation of real-time ATP production rates. | |
| Chamber size easily accommodates tissue pieces. | Can be used to assess respiration in single organoids such as individual pancreatic islets or cancer cell spheroids. |
CHOICE OF MODEL SYSTEM AND EXPERIMENTAL DESIGN
Isolated mitochondria, general considerations.
The decision to measure respiration in isolated mitochondria rather than intact cells or multicellular models may be driven by both practical and scientific considerations. Practically, isolating mitochondria is often preferred when studying non-hematopoetic tissues from adult animals. Ample mitochondria can be isolated with relative ease from many adult rodent tissues such as heart, brain, and skeletal muscle 21 , 26 – 28 . Even in tissues where primary cells can be readily isolated, such as liver 29 or adipose tissue 30 , considerations of yield, viability, and sample quality often make isolated mitochondria an attractive choice.
Only in rare cases should mitochondria be isolated from primary or cultured cells, as the yield, purity, and quality are often sub-optimal. A far more useful option is to selectively permeabilize the cellular plasma membrane 31 , 32 . This approach requires less starting material and avoids artifacts generated by the isolation procedure.
Measuring respiration in isolated mitochondria is appropriate when a metabolic phenotype is expected to be driven by changes intrinsic to mitochondria, or when examining drug candidates for a mitochondrial mechanism of action or potential toxicity. The mechanism underlying changes in the respiratory rate can range from altered activity of a single rate-controlling enzyme (e.g. electron transport chain complexes 33 , 34 , mitochondrial dehydrogenases 35 , 36 , inner membrane transporters 37 , 38 , etc.) to changes that globally affect abundance and activity of mitochondrial proteins (e.g. ‘proofreading’ of mitochondrial DNA 39 , cristae density 40 , translation of mitochondrial-encoded proteins 41 , maintenance of inner membrane phospholipid composition 42 , 43 , vitamin and cofactor biosynthesis 44 – 46 , etc.). Oxygen consumption in isolated mitochondria is expected to change when the protein or pathway of interest is a rate-controlling step for substrate oxidation under appropriate assay conditions. For example, reduced activity of pyruvate dehydrogenase (PDH) or respiratory complex I should slow the rate of oxygen consumption when isolated mitochondria are offered pyruvate as an oxidizable substrate, but not succinate, as its oxidation requires neither PDH nor complex I 12 ( Figure 1 ).

(a) Schematic showing how isolated mitochondria or permeabilized cells can be offered multiple oxidizable substrate pairs for pathway-specific analysis. Substrates requiring complex I for oxidation are shown in blue, those bypassing complex I to feed electrons directly to the ubiquinone pool are in red, and those delivering electrons directly to complex IV are in green. Pyr/Mal, pyruvate with malate; Glu/Mal, glutamate with malate; Palm carn/Mal, palmitoyl carnitine with malate; Succ/Rot, succinate with rotenone; G-3-P/Rot, glycerol-3-phosphate with rotenone; Asc/TMPD/AA, ascorbate with TMPD and antimycin A. Proton leak and other processes that consume the membrane potential independently of ATP synthesis are shown with a dashed line. (b) The approach can identify specific metabolic alterations intrinsic to mitochondria. In this hypothetical example, isolated mitochondria from genetically modified knockout (KO) animals show respiratory deficits with complex I-linked substrates but not with others, suggesting gene ablation causes a primary defect in respiratory complex I activity or mitochondrial NAD + homeostasis relative to wild-type (WT) mice. Excess ADP is offered (State 3 respiration) so the respiratory rate is largely set by the rate of substrate oxidation and respiratory chain activity, and minimally restrained by consumption of the membrane potential. Abbreviations are as before. PC, palmitoyl carnitine (c) Microplate-based respirometry also shows the need to pick an appropriate, physiologically relevant set of substrates based on the tissue being studied. As represented here, mouse heart mitochondria can support relatively high rates of long chain fatty acid oxidation compared to glutamate oxidation, but this relationship is flipped in mitochondria isolated from mouse brain.
In some circumstances, indirect alterations to mitochondria may also be detected by respirometry with isolated mitochondria. Changes in cytoplasmic enzyme activity or cell signaling may manifest in altered oxygen consumption rates in isolated mitochondria, but only if there are downstream, direct effects that persist after isolation. Examples include cytoplasmic calcium dysregulation that causes mitochondrial calcium overload 28 , 47 and disrupted iron homeostasis as is observed in models of Friedreich’s Ataxia 48 . Several circumstances that would change the oxygen consumption rate in whole cells or tissues – notably alterations in substrate import 49 or the glycolytic provision of pyruvate to mitochondria 5 – would not be expected to affect respiration in isolated mitochondria. Even changes in cell signaling that directly target mitochondrial proteins (e.g. phosphorylation status of mitochondrial dehydrogenases) quite often do not persist upon organelle isolation and measurement. It is therefore important to consider whether the hypothesized alteration to mitochondria is expected to be present in isolated mitochondria before planning experiments.

Designing experiments
As a testament to their pioneering work, the parameters defining mitochondrial respiration set forth by Chance and Williams over 60 years ago have remained mostly unchanged 50 . These historical definitions of respiratory ‘states’ (e.g. State 3, State 4, etc.) persist today, though slightly altered for practical reasons, and are defined in Table 2 11 , 51 , 52 . Regardless of the measurement platform, isolated mitochondria are almost always offered sufficient ADP and excess oxidizable substrates (e.g. pyruvate with malate, succinate with rotenone, etc.) to stimulate robust rates of oxygen consumption. The selection of what substrates to offer mitochondria should be driven by the experimental question and the physiology of the tissue from which the mitochondria are isolated. Almost all studies are strengthened by comparing multiple, distinct oxidative pathways ( Figure 1 ).
Mitochondrial respiration can be classified by – or partitioned into – different steady-’states’. Their definitions stem from Chance and Williams 50 with some refinement 11 , and are commonly used for measurements with isolated mitochondria and permeabilized cells. For plate-based respirometry, rates should always be corrected for the background signal by addition of electron transport chain inhibitors.
| Term | Definition for respiratory state in mitochondria or permeabilized cells |
|---|---|
| Respiratory rate in the presence of exogenously added substrates – such as pyruvate and malate or succinate with rotenone. With no added ADP present to drive ATP synthesis, the rate is slow and set by other processes that consume the membrane potential (e.g. proton leak or calcium cycling). | |
| Respiration in the presence of oxidizable substrates and ADP. The ADP is usually offered in excess, though in chamber-based setups it can be delivered via ADP-regenerating systems (e.g. hexokinase + glucose). This high rate reflects the capacity of mitochondria to generate ATP. | |
| After ADP is fully converted to ATP (State 4), or the ATP synthase is inhibited with oligomycin (State 4 ), the respiratory rate slows. This rate is similar to State 2 but typically lower, as oligomycin removes any respiration linked to residual ATP turnover in State 2. | |
| Uncouplers such as FCCP, dinitrophenol (DNP), or Bam15 estimate the maximal capacity of mitochondria to oxidize energy substrates. In principle, this rate should equal or surpass the State 3 rate, because any rate limitations associated with ATP synthesis are removed (e.g. ADP/ATP exchange across the inner membrane). The appropriate concentration of uncoupler must be properly determined by titration. | |
| Additional parameters | |
| The P/O ratio is the amount of ATP phosphorylated (P) for every molecule of oxygen consumed (O). Current estimates of maximal P/O ratios are 2.727 for oxidation of NADH and 1.636 for FADH , – . | |
| This metric, defined as the ratio of State 3:State 4(O), is used as an estimate for how tightly mitochondrial substrate oxidation is coupled to synthesis of ATP. It has traditionally been used as a measure of quality control for mitochondrial isolations. |
This control over substrate provision in a reductionist system has its advantages and disadvantages 17 . Stripping away contributions from cell signaling, hormonal control, diffusion gradients, and cytoplasmic metabolism provides a simple, well-defined experimental system. Additionally, the researcher has near-total control over the assay conditions and pathways contributing to the respiratory rate. The measurements are also inherently controlled for changes in mitochondrial content between tissues from different experimental groups.
What is gained in mechanistic insight from a straightforward system, however, sacrifices physiological relevance. The respiratory ‘states’ defined in Table 2 represent extreme conditions that rarely, if ever, exist during healthy physiology. For example, isolated mitochondria given excess ADP and a particular substrate (State 3) does not reproduce physiological circumstances in which the energy demand fluctuates (e.g. cardiac muscle contraction and relaxation, periodic neuronal synaptic activity, etc.) and the availability of energy substrates is a changing, complex mixture rather than one or two carbon sources 53 . Moreover, the experiments can be inherently biased from the a priori selection of the substrate(s) to be studied. For example, reduced oxidation of branched chain amino acids is thought to be associated with insulin resistance and cardiovascular disease 54 , 55 , but these pathways are rarely examined when following traditional respirometry protocols. Changes in mitochondrial morphology and ultrastructure can also be lost upon mitochondrial isolation 56 , and the isolation procedure itself may introduce artifacts of subselection 30 , particularly when tissues from different experimental groups are altered by pathology.
INTACT CELLS
The considerations for choosing to study respiration in cells are straightforward. Practically, it is almost always advised to study respiration in cells when the model systems used for other aspects of the research project are cultured or primary cells. Isolating mitochondria from cells often results in poor quality mitochondria unsuitable for functional analysis, and also disrupts cellular architecture (e.g. neuronal projections or other extensions from cell bodies) that can leave behind important populations of mitochondria.
Measuring respiration in intact cells also preserves interactions mitochondria share with organelles and cellular structures such as the endoplasmic reticulum 57 or lipid droplets 30 . As a result, changes in respiration that could be affected by multiple biological processes – including cell signaling 7 , ion homeostasis 58 , interorganelle communication 30 , 57 , substrate import 49 and mobilization of internal energy stores, etc. – are retained by studying intact cells but likely would be lost upon mitochondrial isolation.
Similarly, a distinct advantage of cell-based respirometry is that it can capture changes driven by alterations in mitochondrial biogenesis or dynamics. Information about mitochondrial content per cell and many ultrastructural changes (i.e. whether mitochondria exist predominantly in fragmented units or a filamentous network) can be reflected in the cellular oxygen consumption rate but are often lost upon mitochondrial isolation. As such, intact cells are generally a more appropriate model to investigate the effects of transcriptional networks that control mitochondrial content 59 or proteins that govern mitochondrial motility and the balance between fusion and fission 60 .
Of course, a drawback with this increased physiological relevance from studying intact cells is that it necessarily restricts the ability to directly offer mitochondria ADP and various substrates, inhibitors, and cofactors for pathway-specific analysis. Cell-permeable substrate analogs may be added to overcome this issue, but these often show slower kinetics of oxidation than the unmodified substrate and likely do not reflect full metabolic rates 61 . However, follow-up analysis by permeabilizing the plasma membrane of cells creates large pores that dilute cytoplasmic contents to the experimental medium, thereby allowing direct substrate provision to in situ mitochondria 31 . These experiments have been historically conducted with plant-based sterol glycosides such as digitonin or saponin 32 , 62 , and more recently with plasma membrane-specific recombinant perfringolysin O (rPFO) that does not disrupt mitochondrial membranes 22 . Regardless of the permeabilization reagent used, these assays enable the rigorous, mechanistic analysis commonly associated with isolated mitochondria to be conducted on cellular samples in response to pharmacologic or genetic manipulation.
As with isolated mitochondria, respirometry in permeabilized cells assesses maximal pathway activity and precludes the ability to assess substrate preference under native, basal conditions. To obtain this information, a powerful experimental technique is to conduct parallel experiments with intact cell respirometry alongside metabolomics and stable isotope tracing 18 . This approach yields information about pathway-specific fluxes under basal conditions and provides a level of depth unmatched by oxygen consumption measurements 63 , 64 . Quantitative fluxes of individual reactions can be modeled from metabolomics and stable isotope tracing data using metabolic flux analysis (MFA) 65 , though this is highly specialized and can be computationally intensive.
Cellular respiration is coupled to ATP synthesis: as the ATP utilization rate of a cell increases or decreases, the rate of oxidative phosphorylation changes correspondingly to match the change in ATP demand. Thus, the basal oxygen consumption rate in cells can reflect alterations in either pathways that generate ATP (i.e. complete oxidation of sugars, amino acids, or fatty acids) or those that consume ATP (e.g. ion homeostasis, biosynthesis, autophagy, motility, etc.) 12 . In addition to altered rates of ATP utilization, cellular respiration also responds to changes in proton leak pathways 66 , 67 and mitochondrial Ca 2+ cycling 68 , 69 .
In most cases, measuring respiration in response to a sequence of chemical effectors can discriminate between these possibilities. This classic experiment to measure oxygen consumption in response to the ATP synthase inhibitor oligomycin and the uncoupler FCCP has been conducted for decades, and long before the advent of microplate-based respirometry 70 , 71 . Use of these effectors for intact cell respirometry experiments is tremendously informative on multiple levels. Notably, despite using similar compounds, the respiratory parameters are termed differently in intact cells ( Table 3 ) than in isolated mitochondria or permeabilized cells ( Table 2 ), as they substantially differ in meaning.
Plate-based oxygen consumption measurements have helped standardize respiratory parameters for intact cells, though the broad experimental framework had been established decades prior. It is almost always most informative to report raw, quantitative rates. Nonetheless, internally normalized parameters can be useful when it is difficult to control for cell number or biomass, such as when working with tissue pieces. This scaling is independent of cell number or sample size, and allows comparisons across different laboratories, experimental platforms, and model systems. Further definitions and interpretations of these parameters, as well as guidelines for calculation, have been previously published 9 , 12 , 16 . Specific points are discussed further in the main text.
| Term | Description of respiratory parameters in intact cells or 3D structures |
|---|---|
| The initial respiratory rate in intact cells or multicellular structures largely reflects the resting ATP demand. In proliferating cells, a substantial portion of this reflects the energetic costs of biosynthesis and cell division. In differentiated cells, the initial rate may be quite low without activation from external, physiologically relevant stimuli. In most cells, roughly 80% of the basal respiratory rate is coupled to ATP synthesis, with the remainder attributable to processes that use the mitochondrial membrane but do not generate ATP . | |
| Oxygen consumption is not completely coupled to ATP synthesis, as a residual respiratory rate persists in the presence of oligomycin. This rate reflects a composite of processes that consume the membrane potential despite ATP synthase inhibition. Changes in proton leak-linked respiration may indicate altered energy expenditure, and can be substantial upon activation of brown adipocytes. | |
| As basal respiratory rates are restrained by the ATP demand of the cell, they often do not accurately reflect the ability of a cell to respond to increased energy requirements. Addition of a titrated amount of protonophore, however, decouples (or ‘uncouples’) respiratory chain activity from cellular ATP requirements. The rate estimates the maximal capacity of mitochondria to transport and oxidize energy substrates. | |
| In microplate-based platforms, mitochondrial respiration should always be corrected by subtracting the rate insensitive to respiratory chain inhibition, usually with the complex I inhibitor rotenone and the complex III inhibitor antimycin A. Apart from experimental conditions where activation of non-mitochondrial oxidases is expected, a substantial component of this rate in the XF Analyzer may be instrument background. | |
| Internally scaled parameters independent of cell number or sample size | |
| The spare respiratory capacity is often calculated as the absolute difference between the basal and uncoupler-stimulated rates of respiration. It can also be presented as a ratio-based parameter (i.e. maximal rate:basal rate) as an internally normalized parameter for the relative ability of cells or 3D structures to respond to an increased energy demand. | |
| The fraction of the basal respiratory rate that is coupled to ATP synthesis (i.e. oligomycin-sensitive respiration:basal respiration) can be represented as a percentage to allow for comparison across model systems. In most cell types this is value is around 80%. An additional, internally normalized metric is the ratio of uncoupler-stimulated respiration to the proton leak-linked respiration, sometimes called the ‘cell respiratory control ratio .’ |
The basal, unstimulated rate of respiration does not reflect the capacity of a cell to respond to an energy demand during activation. Because in vitro assay conditions do not entirely reproduce the in vivo environment, measuring uncoupler-stimulated respiration provides a way to measure the cellular response to an energetic challenge that may not be apparent under resting, basal conditions 58 (discussed further in Box 1 ). Physiologically relevant stimuli, such as membrane depolarizating agents for electrically excitable cells 5 , antigen-coated beads for T cells 6 , or adrenergic stimulation of brown adipocytes or mature cardiomyocytes 72 , can also be used to better understand the energetic response to cell activation. This ability to easily measure the acute response to physiological effectors is a strength of respirometry compared to stable isotope tracing 18 , 63 .
Estimating ‘maximal’ cellular respiratory rates with uncouplers
Oxygen consumption in response to uncouplers – protonophores such as FCCP, DNP, and Bam15 – estimates the maximal activity of the respiratory chain. These compounds decouple (or ‘uncouple’) mitochondrial substrate oxidation from ATP synthesis, relieving any rate limitation imposed by the basal cellular ATP demand.
For standard experiments in intact cells, measuring uncoupler-stimulated respiration can serve two broad purposes. (1) Differences observed in basal respiratory rates could be attributable to changes in either ATP utilization or oxidation of energy substrates. Measuring oxygen consumption independently of ATP synthesis can help distinguish between these two possibilities. (2) Additionally, uncoupled respiration can estimate maximal respiratory rates that may occur in vivo upon cellular activation (e.g. TCR ligation, NMDA receptor activation in neurons, adrenergic stimulation of brown adipocytes or mature cardiomyocytes) that are not reflected in resting, in vitro assays in the absence of physiologically relevant stimulation and microenvironments.
It is critical to properly titrate the concentration of chemical uncoupler to ensure the measurement is a good approximation of the maximal respiratory rate. Importantly, the optimal amount may change upon genetic or pharmacologic modification. Titrations in chamber-based platforms allow several sequential additions to determine the highest achievable rate, and multiple concentrations can be tested in plate-based platforms using sequential additions from the injector ports (or apportioning different measurement groups). Excess uncoupler often results in a steep reduction in the respiratory rate, an observation further discussed elsewhere 12 . As such, while the term ‘maximal respiration’ is a good operational description of the parameter, it is perhaps more likely that this approach measures the highest achievable rate while minimizing the deleterious effects of chemical uncoupling.
In addition to the choice of exogenous effectors, selection of the medium composition is a critical component for intact cell assays. Cells are generally assayed in medium matching their culture medium such as DMEM or RPMI, and supplemented with multiple oxidizable substrates like glucose, glutamine, and pyruvate to obtain maximal rates of uncoupler-stimulated respiration. Although it is possible to more strictly define the experimental medium and substrates offered, caution should be exercised when making broad conclusions about the results. For example, respiration rates in highly glycolytic cells offered only glucose in a simple salts medium may differ substantially from cells in more complete medium supplemented with glutamine, an often-essential substrate to support energy metabolism and anaplerosis in cultured cells 73 . An additional complication for plate-based respirometry is the frequent lack of fatty acids in assay medium, which is an important physiological substrate for cardiac and skeletal muscle but often omitted from studies due to technical or practical reasons.
THREE-DIMENSIONAL, MULTICELLULAR MODELS
With all respirometry studies, there exists a tradeoff balancing simplicity and ease of analysis with the physiological relevance of the model system. This is perhaps most apparent with multicellular models and tissue biopsies, and making these experiments more straightforward remains one of the most significant opportunities to advance the field. Obtaining trustworthy measurements can be technically challenging and results can be difficult to interpret, so these are usually not recommended for introductory experiments.
Three-dimensional (3D) systems such as spheriods 74 , organoids 75 , and tissue slices 76 provide distinct advantages over working with intact cells and isolated mitochondria such as maintaining intercellular communication and microenvironmental gradients that can control metabolic rates. Additionally, given the increased appreciation for metabolic communication between proximal cells, such as between the tumor and stroma 77 or the retina and adjacent epithelial cells 78 , the ability to easily study oxygen consumption in heterogeneous models would be a powerful addition to the current suite of bioenergetic measurements. In certain cases, respirometry studies of whole organisms such as plankton 79 , C. elegans 80 , and even slime mold 81 can link differences in oxygen consumption to organismal energy budgets using instrumentation designed for in vitro assays.
Predictably, this enhanced physiological relevance is accompanied by considerable technical and analytical challenges. These obstacles have precluded the widespread adoption of respirometry to study three-dimensional models at a scale comparable to isolated mitochondria or intact cells. In particular, it can be difficult to determine whether observed changes in oxygen consumption between 3D samples are due to alterations in catabolism or the delivery of oxygen and substrates to the core of the structure. Nonetheless, respirometry in multicellular models and tissue biopsies is possible, and indeed informative when phenotypes are inextricably linked to their three-dimensional structure 82 or it is necessary to determine if an observed phenotype is an artifact of two-dimensional cell culture 83 . Respiratory parameters for multicellular structures are shared with those for intact cells ( Table 3 ).
Regardless of the model system used, in vitro or ex vivo respirometry results can be linked with whole body oxygen consumption measurements in rodent models from indirect calorimetry in metabolic cages or chambers 84 , 85 ( Box 2 ).
Integrating oxygen consumption measurements from the mouse to the molecule
The measurements of in vitro and ex vivo respirometry discussed here can be meaningfully integrated with whole body oxygen consumption measurements to localize molecular mechanisms underlying bioenergetic phenotypes in animal models. When a distinct phenotype can be associated with altered oxygen consumption in rodent models using metabolic cages or chambers 84 , 85 , an integrative analysis using tissue-specific genetic ablation can trace the bioenergetic phenotype to the tissue, cell, and protein(s) involved. An example highlighting this workflow is the discovery of the scaffold protein p62 as a regulator of brown fat thermogenesis. After observing an obese phenotype upon global p62 KO, decreased energy expenditure was only reproduced upon genetic ablation in brown adipose tissue but not in other tissue-specific knockout animals. Respirometry with primary brown adipocytes and isolated mitochondria further localized the effect to an impaired mitochondrial response to β-adrenergic stimulus 123 .
Similarly, multiple studies have used in vitro respirometry as one of several upfront techniques to characterize genes of unknown function 88 , 124 or molecular mechanisms of drug action 31 followed by whole animal studies. Although respirometry monitors the activity of a single enzyme (cytochrome oxidase, or respiratory complex IV), carefully designing experiments allows dozens of upstream or downstream processes to control the overall oxygen consumption rate. As an example, respirometry helped support biochemical and yeast genetic studies identifying the essential components of the mitochondrial pyruvate carrier, MPC1 and MPC2 125 , 126 . Although mouse, whole body knockouts of Mpc1 or Mpc2 are embryonically lethal, mice with liver- or skeletal muscle-specific ablation exhibit lower respiratory exchange ratios (RERs) 37 , 127 , indicating a shift in the animal towards increased fatty acid oxidation upon reduced mitochondrial pyruvate oxidation. Indeed, liver-specific specific ablation protects from diet-induced hyperglycemia and skeletal muscle-specific loss promotes leanness.
INSTRUMENTATION AND EXPERIMENTAL PLATFORMS
As outlined in Table 1 , the major experimental platforms to measure respiration fall into two broad categories: (i) chamber-based setups with platinum, Clark-type electrodes or (ii) microplate-based setups with fluorescent or phosphorescent detection methods. Both instruments have their relative strengths and limitations depending on the model system used and the experimental question.
EXPERIMENTAL THROUGHPUT
A primary benefit of the Seahorse XF Analyzer is that it reduces the sample material required for a single replicate by orders of magnitude relative to platinum-based Clark-type electrodes. This enables analysis of samples from which the mitochondrial yield would be otherwise prohibitively small for meaningful respirometry experiments, such as clinical biopsies 21 . It also allows the same mitochondria from low-yield preparations to be used for additional parallel experiments. When studying cells, reducing the requisite sample material allows the study of small primary cell populations such as those obtained from rodent models (e.g. brain cells isolated from specific regions, neonatal ventricular myocytes, antigen-specific T cells) or clinical samples 86 [e.g. human peripheral blood mononuclear cells (PBMCs)]. By extension, respirometry can easily be conducted on genetically modified samples of immortalized cells as well, where cellular material may be abundant but the volume of expensive reagents required for genetic transformation can be cost-prohibitive.
Additionally, the multi-well format of microplate-based approaches allow simultaneous measurement of 90+ experimental replicates in a single run, providing a throughput unmatched by chamber-based setups. This approach allows oxygen consumption to be used as a straightforward, end-point readout in concentration-response studies of drug candidates that affect cellular energy metabolism 87 . In isolated mitochondria or permeabilized cells, respiratory rates measured in microplates can be assessed on several distinct substrates in different experimental samples (e.g. wild-type vs. genetic modification) with sufficient technical replicates in a single plate.
As highlighted in Figure 1 and illustrated by the dozens of distinct changes that can alter oxygen consumption rates (see general considerations when working with isolated mitochondria), determining whether changes are observed only in one pathway or matched in several pathways is essential for pinpointing the exact mechanism driving a mitochondrial phenotype 29 , 37 , 39 . Simultaneous measurement of different substrates has also helped reveal that mitochondria isolated from different tissues have varying oxidative capacities for different substrates. Although this may be intuitive, it highlights the benefit of measuring several substrates in parallel when using oxygen consumption to characterize mitochondrial function or identify mechanisms of action. It is also often assumed that the rate limitation(s) for respiration lie exclusively within the electron transport chain, but these assays often reveal that substrate transport, oxidation, or even co-factor levels 88 , 89 frequently control the rate of respiration. Put another way, plate-based respirometry has helped show that defining mitochondrial energetics when studying a single respiratory substrate can be inherently limited or biased by the upfront selection of a ‘best’ or ‘representative’ substrate.
MULTIPLEXING WITH OTHER MEASUREMENTS
Platinum-based, Clark-type electrodes provide a cost-effective and customizable approach to conduct respirometry. Despite the lower throughput relative to plate-based approaches, this approach offers far more flexibility for an individual experiment, particularly when working with isolated mitochondria. Oxygen consumption measurements can be multiplexed with fiberoptic detectors or electrodes that report the mitochondrial membrane potential, pH, external calcium concentration, or reactive oxygen species production for simultaneous measurement of multiple mitochondrial parameters in a given sample 90 , 91 . There is also no limit to the number of injections that can be delivered in most chamber-based setups, enabling careful titrations of novel compounds under investigation or uncouplers that require precise titrations to determine the optimal concentration to induce maximal respiratory rates.
On the other hand, multiplexing plate-based respirometry with additional assays almost always requires parallel experiments to measure other aspects of mitochondrial function. Conveniently, though, the limited sample material required for oxygen consumption measurements frequently leaves ample material available for such assays. A notable exception is the simultaneous calculation of extracellular acidification rates with the Seahorse XF Analyzer, which provides additional context to interpret respiratory data and overall energy metabolism when studying intact cells.
These extracellular acidification measurements have been greatly aided by recent corrections that adjust the rates to accurately reflect lactate efflux by accounting for respiratory acidification 92 , 93 . These calibrations can estimate the balance between glycolysis and oxidative phosphorylation, and by extension, can be used to estimate cellular ATP production rates 93 , 94 . When studying isolated mitochondria or permeabilized cells, these pH readings can be valuable for troubleshooting (e.g. verifying pH of reagents) or specialized assays such as measuring ATP hydrolysis 95 .
PRESERVING CELLULAR NETWORKS
Microplate-based platforms have been transformative in enabling researchers to study adherent monolayers of cells without dissociation and stirring of suspension cultures. Cell dissociation is required for chamber-based platforms, and disrupts cellular structures and prevents interactions between cells and extracellular matrix (ECM) proteins. Stirring likely introduces shearing stress from having to spin samples at high speeds (though there may also be shearing stress in the XF Analyzer during the mixing phase). The same dissociated cell suspension is often used for multiple technical replicates with platinum electrode chambers, introducing the possibility of time-dependent effects during prolonged suspension such as alkalinization of bicarbonate-containing medium.
Meaningful respirometry studies in intact neurons were previously impossible without microplate-based platforms 96 , as dislodging adherent cell monolayers separates the cell body from the neurites rich in synaptic mitochondria. Similarly, studying adherent monolayers preserves ECM interactions and likely maintains signaling pathways that regulate energy metabolism during physiological processes such as tumor extravasation or embryogenesis 97 , 98 . Microplates can also readily accommodate hematopoetic cells and those from suspension cultures, as cells can be easily centrifuged onto plates coated with a biological adhesive. Though rare, some cell types such as mature primary white adipocytes will not affix well to microplates even with an adhesive, and are best studied in platinum-based electrode chambers to remain floating and stirred.
ACCOMODATING 3D MULTICELLULAR STRUCTURES
A standout benefit for measuring respiration with Clark-type electrode chambers is that they can accommodate tissue samples. The larger volume of these setups can be a drawback when working with cells or isolated mitochondria – as they require far more sample material that microplate-based approaches – but the increased volume is often advantageous when working with 3D samples. The microchamber formed by the XF Analyzer (2.28 μL in the 96- or 8-well instruments; 7 μL in the 24-well instrument) can present physical limitations that preclude measurement of many types of tissue samples.
Nonetheless, microplate-based analysis has been successfully used to measure respiration in several types of multicellular structures 83 , 99 – 102 , and is necessary when the sample material is limiting or requires seeding onto ECM protein matrices. For some models, the sensitivity of the XF Analyzer enables analysis of individual structures such as spheroids 83 or pancreatic islets 101 , and therefore can provide a better understanding of the factors that drive metabolic heterogeneity. An additional benefit of a microplate-based approach may be the lack of stirring. The need to constantly mix the sample in platinum-based electrode chambers, though it may aid exposure to pharmacologic compounds or exogenous mitochondrial effectors, likely introduces shearing stress that could artificially increase the basal respiratory rate of less rigid 3D structures.
However, there are further challenges and unknowns in addition to geometric constraints associated with studying multicellular models in microplate-based setups. For example, it is unclear to what extent movement of the sample during the XF Analyzer’s mixing cycle (thereby changing its position relative to the measurement sensor) can affect the measurements. This can be largely avoided by seeding 3D structures onto Matrigel or other adhesives as has been done with islets or intestinal crypts 103 .
COLLECTING AND ANALYZING DATA
Data from Clark-type electrodes offer total flexibility for calculating respiratory rates and usually unrestricted access to raw data. Rates can be directly extrapolated from the decreases in oxygen concentration in a large, closed reaction chamber ( Figure 2a ). Some setups provide software which automatically calculate respiratory rates with smoothing and correction algorithms 13 .

Hypothetical experiments with isolated mitochondria are presented. (a) ( left ) Platinum, Clark-type electrodes with closed, glass reaction chambers display linear decreases in oxygen tension, from which the oxygen consumption rate can be directly calculated ( right ). (b) ( left ) In the XF Analyzer, the instrument forms a transient, semi-closed ‘microchamber’ to measure the decrease in oxygen tension over a defined measurement period (almost always 2–3 min) interspersed with regular mixing and reoxygenation of the experimental medium. The reported decrease in oxygen tension is non-linear due to the backflow of oxygen through the polystyrene microplate and into the microchamber. ( right ) A series of empirically-calibrated calculations by the XF Wave software is required to deconvolute the effect of oxygen backflow and generate quantitative oxygen consumption rates from the reported changes in oxygen tension. (c) Scheme of the XF Analyzer well showing how the amount of oxygen back-diffusion into the well changes with the oxygen consumption rate. As the measurement period proceeds, the difference in oxygen tension between the microchamber and the ambient environment increases, driving more oxygen across the microplate and into the chamber. Oligo,, oligomycin.
In microplate-based platforms, the reported oxygen tension values are non-linear due to back-diffusion of oxygen through the plastic microplate and into the measurement chamber. XF ‘Wave’ software, though, reports quantitative rates after a series of adjustments accounting for several factors and calibration to data from Clark-type electrodes 104 . The software aims to be user-friendly, enable rapid analysis and iteration, and lower the barrier of entry for those new to the field. As a result, it shields these corrections from the end user.
These adjustments include a correction for the back-diffusion of oxygen into the plate that progressively increases as O 2 is depleted in the well. This correction deconvolutes biological oxygen consumption from artifacts arising from the use of a semi-closed system ( Figs. 2b , ,c), c ), and the final rates reported by the instrument (“Rate” tab in Wave software) are comparable to those obtained with Clark-type electrodes 104 . Rates of oxygen consumption should therefore never be manually calculated from the non-linear decreases in O 2 values reported by the XF Analyzer (‘Level” tab in Wave software). The reported [O 2 ] has not undergone the necessary corrections – only the ‘rate’ data is corrected, and in a way that is nonobvious to the user.
Although the rates reported by the XF Analyzer are quantitatively trustworthy across a wide range of values, the gas-impermeable measurement chamber of Clark-type electrodes may provide more reliable measurement of very low oxygen consumption rates (e.g. isolated mitochondria when ADP is depleted 21 , ATP synthesis is inhibited, or at low O 2 levels). As an example, a long-used though arguably limiting measurement of mitochondrial function is the respiratory control ratio (RCR; see Table 2 ). The XF Analyzer can often overestimate the RCR in a way that is inconsistent with previous literature and can complicate the cross-comparison of current work with historical data 39 , 47 . This overestimation is perhaps due to slight inconsistency or inaccuracy when reporting low oxygen consumption rates, such as the State 4 o rate, that cause an overestimation of the RCR. Moreover, situations where no appreciable non-mitochondrial respiration is expected still show residual rates upon addition of electron transport chain inhibitors, suggesting very low rates may be quantitatively unreliable in plate-based platforms.
An additional complication unique to microplate-based respirometry stems from varying environmental effects across the multi-well plate when using adherent cell monolayers cultured in humidified CO 2 incubators. Cells grown in wells at the outer rim of the microplate are subject to well-documented differences in temperature and humidity gradients that can cause inconsistent data 105 , 106 . These temperature and evaporative effects are particularly problematic for cell types that require many days in culture, and in all cases, it is strongly recommended that only the inner 60 wells be used and the outer rim filled with medium or PBS to minimize these effects. This arrangement still allows for 12 experimental groups, each with 5 technical replicates.
Lastly, an arguable strength of chamber-based setups – and even bulk fluorescent dyes – relative to the Seahorse XF Analyzer is that the reported oxygen consumption rate represents all the sample material in the chamber. With the XF Analyzer, cells plated directly under the measurement sensor have a greater contribution to the overall rate relative to those at the periphery of the well 93 . This may be an issue when studying intact cells, as unevenly distributed cells may increase the data spread between technical replicates, and growing adherent monolayers to near-confluence could affect cell signaling and basal metabolic rates.
DATA ANALYSIS AND INTERPRETATION
Guidelines for data normalization, isolated mitochondria.
One advantage of studying respiration in isolated mitochondria compared with cells or tissue is the ease with which data can be meaningfully normalized to total mitochondrial protein content. Each mitochondrial preparation necessarily involves a determination of protein concentration in order to add the appropriate amount to the measurement well or chamber, and the particular detection method (e.g. biuret, Bradford, BCA, etc.) and use of detergents in sample preparation can yield different concentrations. Reporting rates in pmol O or O 2 /min/μg mitochondrial protein (or nmol O or O 2 /min/mg) is easy and essential: rates presented this way can indicate the quality of the mitochondrial preparation by comparison with data from other laboratories and previously published work. Indeed, a significant drawback from the use of bulk fluorometric dyes to measure oxygen consumption (e.g. MitoXPress) is that the approach can only measure relative differences without such quantitation 107 .
As a corollary, scaling rates to “1” or “100%” is frequently unnecessary and can very often be misleading ( Figure 3 ). Overly processed data can hide mitochondrial preparations of poor quality with low respiratory rates or high day-to-day variability. This masking of quantitative rates also makes it difficult to discriminate between effects driven by meaningful biological changes or artifacts of calculation ( Figs. 3a , ,b). b ). Similarly, the RCR is a ratio-based parameter that partially relies on the assumption that mitochondrial dysfunction should decrease the ADP-stimulated rate and increase proton leak-associated respiration. However, this is not always the case, particularly when measuring low rates in microplate-based formats.

(a) A hypothetical example is given where a weak inhibitor of pyruvate dehydrogenase (PDHi) reduces the ADP-stimulated respiration rate relative to vehicle controls in isolated mitochondria offered pyruvate with malate, as shown in both a ( left ) Clark-type electrode and ( middle ) Seahorse XF Analyzer. ( right ) Data normalized to 100% of the vehicle control. Mito., isolated mitochondria; Oligo., oligomycin; PDHi, pyruvate dehydrogenase inhibitor. (b) The same experiment conducted with mitochondria from a suboptimal isolation yields different results. With low rates from damage during the isolation, the effect of PDHi is no longer rate-controlling. Presenting percentage-based data relative to vehicle control shows little difference between groups and the confounding effects of the poor isolation cannot be identified. Abbreviations as before. (c) A hypothetical example is presented of two potential cellular phenotypes that may arise from genetic transformation or pharmacologic intervention. ( left ) A reduction in ATP demand (blue) lowers the basal rate of respiration but leaves FCCP-stimulated respiration largely unchanged. Additionally, an increase in oxidative capacity (yellow), as can happen with enhanced mitochondrial biogenesis, has little effect on basal respiration but a dramatic effect on the maximal respiration. ( right ) Calculating the quantitative parameters easily identifies the respective changes. Oligo., oligomycin; Rot/AA, rotenone with antimycin A. (d) Scaling the rates to the initial rate of respiration masks the precise changes, as both present with roughly equal fold-changes in uncoupler-stimulated respiration. Abbreviations as before.
Intact cells
When studying adherent cell monolayers in microplates, normalization of respiration data is one of the most important components of the assay. Genetic interventions or chronic pharmacologic treatments can alter the growth rate of cells in the microplate well, and it is critical to determine if the corresponding changes in the bulk oxygen consumption rate are driven by bioenergetic alterations or merely reflect differences in cell size or number. This is largely not an issue for cellular respiration measured in Clark-type electrode chambers or suspension cells acutely adhered to a microplate well, as a predetermined number of cells are added to the measurement chamber immediately prior to analysis.
Normalizing data to “1” or “100%” is almost always less informative than presenting data in pmol O 2 /min/1×10 3 cells, and kinetic traces should never be scaled in this way unless the initial conditions are identical across measurement groups. Internally normalizing data masks excessive day-to-day variability in cell culture preparations and makes it difficult to discern meaningful changes in oxygen consumption from calculation artifacts. This is particularly true for parameters that integrate multiple pieces of information. Scaling these composite parameters to “1” or “100%” further obscures the information obtained and makes it increasingly difficult to define the precise bioenergetic changes between groups, such as whether changes in spare respiratory capacity are primarily driven by altered basal or uncoupler-stimulated respiration rates ( Figs. 3c , ,d d ).
As with isolated mitochondria, it is also broadly true that the quantitative rates (in pmol O 2 /min/1×10 3 cells) can indicate the quality of the cell preparation, particularly with primary cell preparations. However, while results with isolated mitochondria can be compared with historical data spanning decades, oxygen consumption measurements with adherent cell monolayers are far newer by comparison. They may also vary considerably due to different cell culture conditions across laboratories.
Further complications arise from the use of several distinct approaches to scale respiratory data to cell number that make quantitative cross-comparisons difficult. Whenever possible, adherent cell monolayers should be normalized to cell counts obtained with automated digital microscopy and analysis (i.e. high-content image analysis) 108 . Although these approaches require specialized equipment and training, they provide quantitative readouts with the appropriate resolution. In their absence, cost-effective but less informative approaches have been used such as bulk fluorescence from nuclear stains, total cellular protein, total DNA content, and post hoc cell detachment followed by manual counting with a hemocytometer.
An often-overlooked drawback with non-imaging approaches is their limited sensitivity and linear range of detection. Near-confluent cellular monolayers, as are often suggested with XF Analyzer measurements, very often saturate bulk detection signals or report similar cell densities between groups that may substantially differ. Before any experiment, it is important to determine that the method of normalization can detect subtle differences in cell number (e.g. 10–15%), and does not introduce compounding technical or measurement errors that further cloud interpretation.
Importantly, any normalization parameter used as a surrogate for cell number (i.e. total protein, DNA content, etc.) should be examined in response to the drug treatment or genetic modification under investigation. As an example, loss of the Wolfram Syndrome protein Miner1 causes a substantial expansion in cell size along with an increase in respiratory capacity, cristae density, and mitochondrial Ca 2+ storage 109 . As such, choosing whether to normalize to nuclei or total protein would have profound implications on interpreting the functional consequence of protein ablation.
Cell lifting during the course of the assay may also complicate post hoc normalization efforts, leading to an underestimation of cell number and possible data misinterpretation if an experimental group is differentially susceptible to detachment relative to matched controls. Although there is no perfect solution, several approaches can help such as use of a replicate plate devoted strictly to normalization or conducting cell counts immediately prior to the assay after ensuring the procedure will not affect the functional measurements. Use of a protein- or peptide-based reagent for cell adhesion can be helpful, but use of any plate coating should include a test of whether the adhesive interferes with the normalization method.
3D and multicellular structures
Normalization is one of the bigger challenges when studying respiration in multicellular models and tissue biopsies. For spheroids and other organoids such as pancreatic islets, it is often convenient to present data on a per spheroid/islet basis. However, it is essential that these be controlled for size. Post hoc analysis of cell number, DNA, or protein content after dissociation and sample recovery is a possible solution, though this may compound error in the analysis, especially when needing to dislodge samples from biological adhesives. When possible, tissue samples should be processed with an automated slicer and controlled as best as possible for weight and shape.
The addition of mitochondrial effectors in 3D systems such as oligomycin and FCCP can greatly improve data interpretation. Internally scaled parameters 12 [e.g. ratio of basal to maximal respiration, coupling efficiency, etc.] should be mostly avoided when working with isolated mitochondria or intact cells because it is straightforward to appropriately normalize the data and calculate respiratory rates per cell or microgram of mitochondrial protein. However, given the difficulty associated with normalizing oxygen consumption data from multicellular structures, ratio-based parameters can be a useful way to find differences between experimental groups that are independent of amount of sample material loaded.
RECOMMENDATIONS TO IMPROVE DATA REPUTABILITY
Data collection.
All oxygen consumption measurements in the Seahorse XF Analyzer should be reported only when the system is at steady-state, i.e. a constant rate. Rates that consistently fluctuate or trail down without settling should be interpreted with ample caution. This could be indicative of sub-optimal sample preparation, low signal-to-noise, or any number of technical issues. For all XF experiments, technical replicates should be examined individually prior to data analysis to ensure there are no injection failures or leakage from the injector ports in any measurement well.
Regardless of the platform or model system used, initial studies should ensure a linear dependence of the oxygen consumption rate on the amount of biological material to identify the appropriate dynamic range for the measurements 12 . Recommendations for structuring experiments with appropriate biological and technical replicates are given in Box 3 .
Defining guidelines for biological and technical replicates
The same guidelines regarding biological and technical replicates and their statistical evaluation for other experiments also apply to oxygen consumption measurements. A mitochondrial isolation or cell preparation from each mouse, or each passage of an immortalized cell line, is generally considered a single biological replicate and should be assessed with sufficient technical replicates. There are unique considerations for each measurement platform.
As chamber-based experiments must be conducted with only one or two runs at a time, potential time-dependent effects from hours-long experimentation should be controlled by mirroring the experimental sequence [e.g. WT 1 (replicate 1), KO 1 , KO 2 , WT 2 ]. If the amount of material is not sufficient for technical replicates, increasing the number of biological replicates may be required.
Microplate-based respirometry allows simultaneous measurement of several experimental groups, so temporal effects from sample degradation are often not an issue. Despite the increased throughput, it is almost always recommended to spread different biological replicates over multiple days (and XF cartridges) to control for operational and technical inconsistencies. Microplates offer up to 92 simultaneous measurements (a minimum of 4 of 96 wells are needed as background controls) when studying isolated mitochondria or cell suspensions spun onto the plate immediately prior to use. For adherent cell monolayers cultured in humidified CO 2 incubators, however, it is recommended that the outer rim be filled with medium or PBS. This mitigates effects from varying temperature or humidity in cells grown at the plate edge 105 , 106 , and still accommodates 12 experimental groups with 5 technical replicates per group.
Regardless of the platform, a peer reviewer should be entitled to inquire about the reproducibility of results and request original measurement files during manuscript review.
The quality of any respirometry experiment is largely dictated by the quality of the sample material. When studying isolated mitochondria, isolation protocols have fortunately been established and refined over decades for most rodent tissues 21 , 26 – 30 , 110 . Consulting the established literature prior to beginning work with isolated mitochondria is strongly recommended, as isolations from different tissues frequently require different buffer compositions and centrifugation protocols to optimize function and yield.
Care is required to adhere to seemingly trivial details of the isolation procedure (e.g., keeping sample material near 4°C at all times, routinely checking the pH of isolation buffers, working quickly to extract and mince tissue, disrupting tissue without over- or under-homogenizing the sample, using buffers containing fatty acid-free BSA and chelators of divalent cations, etc.). All efforts should be made to use specialized equipment when disrupting tissue, such as a drill-driven Teflon/PFTE pestle for isolating liver mitochondria or an immersion homogenizer for skeletal muscle mitochondria, as these will significantly improve the yield and quality of the isolation.
The RCR is traditionally used to validate the quality of a mitochondrial preparation, with higher values more indicative of a good preparation. As mentioned previously, however, cross-comparison of data between Clark-type electrodes and XF Analyzers may be problematic as the XF often gives far higher RCR values relative to platinum-based electrodes. Addback of cytochrome c is an additional, platform agnostic functional test for the quality control of a mitochondrial preparation. Exogenously added cytochrome c should not increase the respiratory rate, thereby indicating an intact outer membrane 31 , 111 .
When studying intact cells, subtle changes in the day-to-day quality of the cell preparation that may not alter qualitative findings from destructive, end-point assays (e.g. gene or protein expression, metabolomics etc.) are often reflected in measurements of live cell energetics. For primary cell preparations, seemingly minor details (e.g. small changes in the time required for tissue preparations, changing vendors for the protease used for digestion, etc.) can have surprisingly large effects on the respiratory rate. For immortalized cells, variability can be somewhat mitigated by tightly controlling conditions for subculturing and split ratios, passage number, composition of growth medium, and other technical considerations.
With both cells and mitochondria, decisions that balance sample purity and yield often have functional consequences for respirometry assays. For example, the purity of mitochondrial preparations can be increased by gradient purification (e.g. use of a Percoll gradient to isolate brain mitochondria). However, this increased homogeneity often comes not only with lower yields but also poorer quality data relative to conventional isolations. Similarly, respiration in whole cells is also frequently impaired after purification procedures such as isolation of specific cell populations with flow cytometry that can take hours, and day-to-day inconsistencies of the purifications can cause prohibitive variability in results.
As mentioned previously, the Seahorse XF Analyzer software reports oxygen consumption rates after an opaque correction for several factors including back-diffusion of oxygen from the ambient environment into the measurement microchamber. An additional complication with this automated calculation stems from the requirement that the measurement period be at least two minutes long (the default setting is three minutes) with measurements of oxygen tension taken at 15 second intervals. When respiration rates are very high, the oxygen in the microchamber can be depleted before the measurement time is completed, and the very low [O 2 ] can be limiting for the respiratory rate. This anoxia in the instrument microchamber prior to the end of the measurement period causes the instrument to report artifactually low rates due to oxygen depletion, and can lead to gross data misinterpretation ( Figure 4 ).

(a) At very high respiration rates – as can happen during uncoupler-stimulated respiration in metabolically active cells such as neurons, myocytes, or brown adipocytes – lack of oxygen in the XF measurement well can cause artifacts of calculation. In the hypothetical example, too much isolated mitochondria is loaded into the XF measurement well. Upon addition of ADP, so much oxygen is depleted during the measurement time (minimum of 2 min.) that oxygen availability limits the respiratory rate. The limited O 2 availability and increased back-diffusion of oxygen into the microplate exaggerates the non-linearity of the observed rate. (b) (top ) The XF Wave software uses a composite of the point-to-point rates – calculated from the readings at 15-second intervals shown as squares in (a) – to report the oxygen consumption rate. If the microchamber becomes anoxic, the instrument will report artificially low rates by aggregating the low rates observed during anoxia into the overall average. Artifacts, such as the rate of respiration being roughly similar in the presence or absence of oligomycin, can occur. ( bottom ) Checking the stability of point-to-point rates in the software, as well as observing the oxygen tension levels themselves, can determine whether lack of oxygen availability is an issue during an assay.
Microchamber anoxia should be controlled for whenever initially optimizing respirometry with isolated mitochondria. Mitochondria isolated from different tissues will inevitably have varying oxidative capacities, so each experiment should begin with a titration to find an appropriate concentration of mitochondria that gives a robust signal while avoiding anoxia 21 . For example, mitochondria isolated from liver have a lower maximal oxidative capacity for pyruvate/malate than those isolated from heart or brain, and will require more mitochondria in the microplate well. Additionally, respiratory rates will vary considerably based on the substrate provided, particularly for TMPD with ascorbate (for isolated complex IV activity where respiratory rates should be much higher than complex I- or II-linked substrates). Tips to avoid microchamber anoxia and generating reliable data include adjusting the sample material loaded based on the substrate provided and reducing the measurement time to two minutes.
Although less frequently an issue when studying intact cells, anoxia in the XF Analyzer measurement well can affect measurements of maximal, uncoupler-stimulated respiration. This often occurs with terminally differentiated cells characterized by a high oxidative capacity such as neurons, cardiomyocytes, or brown adipocytes, though the O 2 levels should be checked when working with all cell types. Although the XF24 well plate offers a greater microchamber volume, this does not necessarily minimize the risk of anoxia because it is offset by the increased surface area accommodating more material.
Oxygen depletion during XF measurements well should be checked whenever obtaining rates above 500 pmol O 2 /min in the 8- or 96-well platforms or rates above 1500 pmol O 2 /min in the larger 24-well platform by examining the “Level” option in the XF Wave software. For cells with high respiratory capacities and relatively low basal rates, it may be challenging to find a cell density which yields suitably high inital rates without depleting oxygen when measuring uncoupler-stimulated respiration. In general, an ideal range for initial oxygen consumption rates are around 75–150 pmol O 2 /min but are generally trustworthy above 50 pmol O 2 /min if lower density is required to avoid microchamber anoxia upon addition of FCCP.
When using microplate-based platforms, especially with intact cells or 3D multicellular structures, it is essential to correct all rates for non-mitochondrial respiration and background signal from the instrument by inhibiting the respiratory chain. This is typically done with the complex I inhibitor rotenone and the complex III inhibitor antimycin A. A substantive portion of the initial respiratory rate in whole cells (often 20–35%) is insensitive to respiratory chain inhibition in the XF Analyzer, so correcting for this has a meaningful impact on the reported rates of basal and maximal respiration. The values obtained from the XF Analyzer contrast previous measurements that 90–95% of cellular oxygen consumption is mitochondrial 112 , suggesting some component of the ‘non-mitochondrial’ oxygen consumption rate is instrument background.
As a result, interpreting ‘non-mitochondrial’ rates of respiration is generally only relevant in cells which express abundant amounts of non-mitochondrial oxidases such as the NADPH oxidase. Assessing activity of these enzymes by respirometry can be done with appropriate controls such as activation with phorbol esters and inhibition of NADPH-supported non-mitochondrial oxygen consumption by flavin-targeted compounds such as DPI 113 . In cultured cells, excessively high rates of respiration insensitive to electron transport chain inhibition may indicate contamination with mycoplasma or other bacteria.
Work with 3D structures seeded onto layers of basement membrane mixtures like Matrigel are perhaps more susceptible to high or artificial background signals, which could be due to altered rates of diffusion across the bottom of the microplate compared against the conditions where the algorithm was calibrated 12 , 104 . An additional complication for adding exogenous effectors to multicellular model systems is that differently sized spheroids, organoids, or tissue pieces will likely exhibit different kinetic responses to drugs and mitochondrial effectors. For all measurement platforms, the timing protocols for assays should be empirically determined to ensure enough time has elapsed for the system to reach a new respiratory steady state in response to compound addition. This is particularly important for bulky compounds such as oligomycin that may have kinetic limitations for its diffusion into the core of a 3D structure.
Calculation of respiratory parameters
When working with isolated mitochondria, the most informative respiratory parameters are often oxygen consumption stimulated by ADP phosphorylation (State 3) or an uncoupler such as FCCP or Bam15 (uncoupled or State 3u), as well as oxygen consumption linked to proton leak (State 4; see Table 2 ). It is recommended that the RCR not be presented unless alongside the State 3 and State 4 rates that comprise this ratio. Terms such as basal respiration and spare respiratory capacity, frequently used when studying cell-based systems, are inappropriate when studying isolated mitochondria. These parameters are only informative when considering the energy demand and rate of ATP utilization in intact, live cells, and are therefore meaningless in reductionist systems offered excess ADP.
Respiratory rates in isolated mitochondria should be measured on multiple oxidizable substrates whenever possible. Examining distinct oxidative pathways (e.g. respiration supported by pyruvate/malate, glutamate/malate, succinate/rotenone, ascorbate/TMPD, etc.) helps pinpoint differences between experimental groups 29 . Additionally, these data can also be helpful for judging the quality of a mitochondrial preparation. For example, the relative rates of NADH- vs. QH 2 -linked substrates could indicate whether complex I was damaged during the mitochondrial isolation.
Interpreting respirometry data in cells and 3D models involves an additional layer of complexity compared to isolated mitochondria or permeabilized cells: the basal rate of oxygen consumption largely reflects the cellular rate of ATP utilization, rather than the capacity for oxidation of energy substrates. As a result, increases or decreases in basal respiration do not necessarily reflect improvements or defects in mitochondrial function 18 . For example, a reduction in basal respiration between measurement groups could simply indicate a reduced energy demand in the cell rather than a catabolic defect.
Similarly, an increased basal oxygen consumption between groups may indicate a compensatory response to stress, highlighting the need to interpret basal respiratory rates alongside uncoupler-stimulated rates of respiration that disengage respiratory chain activity from cellular ATP requirements. Figure 5 highlights common phenotypes obtained with intact cell respirometry along with broad guidelines for follow up studies. Box 4 provides additional, specific guidance to determine if changes in proton leak-linked respiration are due to direct alterations in proton permeability of the inner membrane or indirect effects from changes in mitochondrial oxidative capacity.

A description of common phenotypes is given along with their most likely interpretations and suggestions for follow-up experiments with permeabilized cell respirometry. Representative results are presented in the format of Seahorse XF Analyzer kinetic traces for changes in (a) cellular energy demand, (b) substrate transport or oxidation, (c) cell number, or (d) mitochondrial uncoupling. Additionally, examples of common technical problems, and a representative trace, is given in (e). O, oligomycin; F, FCCP; RA, rotenone with antimycin A.
Revealing mechanisms of proton leak and altered energy expenditure with respirometry
There is considerable interest in using oxygen consumption rates as a primary method to identify molecular mechanisms of mitochondrial uncoupling. Respiration linked to mitochondrial proton leak (oxygen consumption in the absence of ATP synthesis; termed ‘State 4’ in isolated mitochondria) is indeed a useful measurement for determining an uncoupling effect. The measurements can reveal genetic pathways or identify pharmacologic compounds that alter the efficiency of mitochondrial energy transduction and even increase whole body energy expenditure through mechanisms such as brown adipose tissue thermogenesis.
In this context, however, it is important to distinguish whether interventions that change proton leak-linked respiration are either (1) direct, genuine changes in proton permeability of the mitochondrial inner membrane, or rather (2) indirect changes in proton leak as a consequence of increased mitochondrial oxidative capacity. This concept is discussed in theory elsewhere 12 , 66 . As a general, practical principle, if proton leak-associated respiration increases but the FCCP-stimulated rate remains unchanged (or is even reduced), then the primary change is likely attributable to increased inner membrane proton conductance. However, if the uncoupler-stimulated respiration rate changes alongside the proton leak rate, the phenotype is likely driven by changes in mitochondrial substrate oxidation and the changes in uncoupling are a secondary consequence.
In addition to directly measuring membrane conductance with patch clamp electrophysiology of mitoplasts 128 , genuine effects of changes in proton permeability in isolated mitochondria can be assessed by simultaneous measurement of State 4 respiration with the mitochondrial membrane potential. This is readily done in chamber-based setups, but can also be conducted with parallel measurements in microplates. In intact cells, the same principle largely holds, though quantitative measurement of the membrane potential is exceedingly specialized 129 . As such, internally normalized parameters identifying the fraction of the total basal respiratory rate that supports ATP synthesis (coupling efficiency) or the ratio of FCCP-stimulated respiration to proton leak respiration (cell respiratory control ratio) may be useful in this specific instance.
Importantly, interpretations of spare respiratory capacity should be viewed in light of the model system used. The calculation is a useful construct to study cells such as myocytes or neurons: differentiated, electrically excitable cells where the resting in vitro respiratory rates are low in the absence of a physiological stimulus and do not reflect the periodic, maximal energy demands experienced in vivo 58 . It has also be applied primary T cells, where maximal rates of respiration may better reflect the capacity to support the energetic and biosynthetic demands of cell activation rather than in vitro , basal rates 114 . However, interpretations of spare respiratory capacity in cancer cells or other proliferating cell models are less clear, as changes in the basal respiratory rates set by the energy demands of cell division and anabolism may be lost or misinterpreted 12 , 16 . Regardless of the model system, it is impossible to directly identify molecular mechanisms of action by calculating spare respiratory capacity as it integrates multiple experimental parameters.
Data presentation
For all model systems and experimental platforms, it is highly recommended to include at least one representative kinetic trace comparing the most relevant experimental groups in addition to bar charts or scatter plots of aggregated biological replicates. This fosters confidence that calculated respiratory parameters are derived from trustworthy data. For the XF Analyzer, it may be appropriate for peer reviewers to also ask for multiple kinetic traces and even the O 2 levels underlying the data. These may be particularly important if there are concerns that data interpretation is confounded by oxygen limitation in the measurement well or other calculation artifacts.
Every effort should be made to report quantitative, appropriately normalized respiratory rates to allow cross-comparison with other laboratories and historical data (i.e. pmol O 2 /min/μg mitochondrial protein for isolated mitochondria or pmol O 2 /min/1×10 3 cells). Quantitative, ‘raw’ rates should always be provided alongside when scaling rates to 1 or 100%, as is often done with concentration-response curves for drugs relative to a vehicle control.
When studying intact cells with the XF Analyzer, it may be helpful to include basal rates of extracellular acidification alongside respiration data even if there is no consideration of glycolysis. This can aid interpretation as to whether altered oxygen consumption rates are due to a global change in energy demand, a shift in the balance between oxidative phosphorylation and glycolysis, or technical issues with the experiment that may present in both readouts such as cells lifting from the plate. Recent adjustments to account for respiratory acidification allow accurate measurements of lactate efflux and estimates of the cellular ATP production rate 93 , 94 , which can help further distinguish between healthy energetic adaptation or bioenergetic dysfunction.
These recommendations are not meant to be pedantic or exclusionary, but rather to improve data consistency and reproducibility across laboratories. For example, a drug candidate or genetic intervention that may weakly, but meaningfully, reduce complex I activity would present very differently in mitochondria from a well-executed isolation with robust oxygen consumption rates compared to a poor isolation. Or, when an observed change in spare respiratory capacity cannot be reproduced, it may be important to determine whether the primary driver of the discrepancy is a change in the basal or maximal respiratory rates to identify the underlying cause. As such, we seek to assist with experimental design and warn of common pitfalls regarding data analysis and interpretation. Additionally, we attempt to merge the transparency and rigor of traditional bioenergetic analyses with the physiological relevance and practicality of current metabolic studies.
The importance of a shared set of best practices is highlighted by the advancing clinical studies targeting metabolism 115 , 116 , and the potential for respirometry to monitor disease states and target engagement. There are also remains a large fraction of unannotated mitochondrial proteins 117 , and oxygen consumption can be an important tool in defining the function of these proteins that may to rewrite our textbook understanding of this organelle 89 , 118 , 119 . Methodologically, the field will inevitably address the challenges associated with conducting respirometry under environmental control and in 3D models and tissue biopsies given our growing appreciation for how the surrounding microenvironment can control metabolism. As progress in metabolic and bioenergetic research is likely to continue at a breakneck pace, establishing a well-accepted set of ground rules for data normalization, analysis, and interpretation of oxygen consumption measurements will be essential to move the field forward.
ACKNOWLEDGEMENTS
ASD is supported by National Institutes of Health Grants R35GM138003, P30DK063491, and P50CA092131, as well as the W.M. Keck Foundation. MJ is supported by the Novo Nordisk Research Fonden (NNF20OC0059646). We would like to thank members of both of our laboratories for their helpful discussions during the preparation of this manuscript, as well as Linsey Stiles (UCLA), Brandon Desousa (UCSF), and Anne Murphy (Cytokinetics, Inc.) for their critical perspective.
ABBREVIATIONS
| FCCP | Carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone |
| ROS | reactive oxygen species |
| DNP | 2,4-dinitrophenol |
| TMPD | , , , -tetramethyl- -phenylenediamine |
COMPETING INTERESTS
The authors declare no current competing interests. ASD has previously served as a paid consultant for Agilent Technologies.
- BiologyDiscussion.com
- Follow Us On:
- Google Plus
- Publish Now

Top 5 Experiments on Respiration | Biology
ADVERTISEMENTS:
List of experiments on Respiration are as follows:
Experiment 1:
Count the number of breaths in a 10-second period every 30 seconds for 10 minutes.
Calculate the rate per minute, and plot a graph of breathing rate against time.
Experiment 5 :
To Show the Effect of Regular Exercise on the Depth of Breathing :
Regular exercise can affect the volume of air a person is able to inspire, and then expire, in one deep breath. This can be demonstrated by using the simple respirometer shown below.
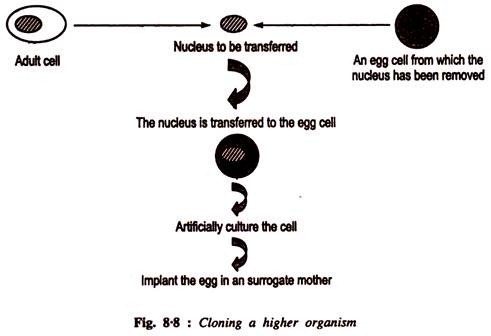
Organise a group of athletes and a group of non-athletes to breathe in as far as they can, then breathe out through the rubber tube as far as they can. Measure the volume of water expelled from the container by each person.
Calculate the average volume per person for the athletes, and the average volume for the non- athletes. Compare the results.
Athletes should be able to exhale a greater volume of air (and thus, be able to breathe more deeply).
The immediate effect of exercise on the depth of breathing may be successfully demonstrated only with a commercial respirometer. A person breathes in and out through a tube connected to a piece of electronic equipment which measures the depth and the frequency of breathing. The respirometer produces a graph of the results.
If a person measured the depth and rate of their breathing before a period of exercise, then every 30 seconds after exercise, the results would be similar to the graph shown below.
Related Articles:
- Term Paper on the Process of Respiration | Life Processes | Biology
- Hyperpnoea: Causes and Effects | Respiration | Humans | Biology
Biology , Experiments , Respiration , Experiments on Respiration
- Anybody can ask a question
- Anybody can answer
- The best answers are voted up and rise to the top
Forum Categories
- Animal Kingdom
- Biodiversity
- Biological Classification
- Biology An Introduction 11
- Biology An Introduction
- Biology in Human Welfare 175
- Biomolecules
- Biotechnology 43
- Body Fluids and Circulation
- Breathing and Exchange of Gases
- Cell- Structure and Function
- Chemical Coordination
- Digestion and Absorption
- Diversity in the Living World 125
- Environmental Issues
- Excretory System
- Flowering Plants
- Food Production
- Genetics and Evolution 110
- Human Health and Diseases
- Human Physiology 242
- Human Reproduction
- Immune System
- Living World
- Locomotion and Movement
- Microbes in Human Welfare
- Mineral Nutrition
- Molecualr Basis of Inheritance
- Neural Coordination
- Organisms and Population
- Photosynthesis
- Plant Growth and Development
- Plant Kingdom
- Plant Physiology 261
- Principles and Processes
- Principles of Inheritance and Variation
- Reproduction 245
- Reproduction in Animals
- Reproduction in Flowering Plants
- Reproduction in Organisms
- Reproductive Health
- Respiration
- Structural Organisation in Animals
- Transport in Plants
- Trending 14
Privacy Overview
| Cookie | Duration | Description |
|---|---|---|
| cookielawinfo-checkbox-analytics | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Analytics". |
| cookielawinfo-checkbox-functional | 11 months | The cookie is set by GDPR cookie consent to record the user consent for the cookies in the category "Functional". |
| cookielawinfo-checkbox-necessary | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookies is used to store the user consent for the cookies in the category "Necessary". |
| cookielawinfo-checkbox-others | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Other. |
| cookielawinfo-checkbox-performance | 11 months | This cookie is set by GDPR Cookie Consent plugin. The cookie is used to store the user consent for the cookies in the category "Performance". |
| viewed_cookie_policy | 11 months | The cookie is set by the GDPR Cookie Consent plugin and is used to store whether or not user has consented to the use of cookies. It does not store any personal data. |

IMAGES
VIDEO
COMMENTS
Some methods involve the use of a redox indicator such as the experiment described below; A redox indicator is a substance that changes colour when it is reduced or oxidised; DCPIP and methylene blue are redox indicators. They are used to investigate the effects of temperature and substrate concentration on the rate of anaerobic respiration in ...
Investigation - Cellular Respiration Virtual Lab. Students in AP Biology investigate cellular respiration by placing peas or other living organisms in respirometers. After submerging the devices, students then measure the rate or respiration by collecting data on water movement in the pipets. As an alternative to this lab, I created a virtual ...
The rates of aerobic respiration varies among organisms and is determined by numerous factors. In this experiment you will measure the rate of oxygen consumption and carbon dioxide production in germinated and un-germinated seeds and compare these with animals (worms).
In Experiment 2 of this week's lab, we will be using insects to assess the effects of temperature on cellular respiration rates. We will account for differences in insect body size by calculating respiration rate per gram of body mass.
Write your answers in your notebook. Step 4: Assemble a respirometer using Figure 8.3 as a guide and following the steps below. Figure 8.3: Assembled respirometer. In a wide test tube (or bottle), drop a pad of absorbent cotton. Pack down the cotton with a stirring rod.
In this experiment, the rate of cellular respiration will be measured by monitoring the consumption of oxygen gas. Many environmental variables might affect the rate of aerobic cellular respiration. Temperature changes have profound effects upon living things. Enzyme-catalyzed reactions are especially sensitive to small changes in temperature.
Revision notes on 5.2.9 Investigating the Rate of Respiration for the AQA A Level Biology syllabus, written by the Biology experts at Save My Exams. ... 1.4.5 Maths Skill: Drawing a Graph for Enzyme Rate Experiments; 1.4.6 Maths Skill: Using a Tangent to Find Initial Rate of Reaction; 1.4.7 Limiting Factors Affecting Enzymes: Temperature;
Designing experiments . Cellular respiration is coupled to ATP synthesis: as the ATP utilization rate of a cell increases or decreases, the rate of oxidative phosphorylation changes correspondingly to match the change in ATP demand. ... Anoxia in the XF microchamber with high respiratory rates causes severe calculation artifacts. (a) At very ...
To investigate the rate of respiration. Control Variables. Number of organisms - 5g of maggots will be used; ... Varying temperature (random error) - repeat experiment but place set-up in a water bath at 30°C for a constant temperature; Simple Respirometer. Advantage - Very simple to set up, minimal number of connections makes a good ...
The fitter the subject, the quicker the pulse rate return to normal. Count the number of breaths in a 10-second period every 30 seconds for 10 minutes. Calculate the rate per minute, and plot a graph of breathing rate against time. Experiment 5: To Show the Effect of Regular Exercise on the Depth of Breathing:
In this experiment, you will. Use a CO 2 Gas Sensor to measure concentrations of carbon dioxide.; Determine the rate of respiration by yeast while using different sugars. Determine which sugars can be used as a food source by yeast.
Indeed, respiration rates often increase in experiments where ADP or mETC uncouplers are added to mimic increased ATP turnover (Noguchi, 2005; ... the relationship among substrate supply, respiratory activity, and product demand at whole-plant to ecosystem scales. Respiration rates of individual plants scale with plant size, ...
The experiments are flying as part of the Polaris Dawn mission which launched aboard a SpaceX Dragon spacecraft and Falcon 9 rocket earlier today. ... respiration rate, and temperature. The technology also provides ultrasound imaging and larynx and throat-focused video camera capabilities, and includes an experimental telemedicine feature that ...