The IRB Approval Process: A Complete Guide

By Logan Pearce
PhD candidate in Social Psychology at Princeton University
10 minute read
Getting Institutional Review Board (IRB) approval is a critical part of conducting psychology research . Since psychologists do research on humans, they must take care to treat their participants with dignity and protect their rights. An IRB is a group of people who deeply consider the study that a psychologist wants to run and determine if it is ethically sound. If the study meets their inspection, then the psychologist receives approval to do the research.
Unfortunately, psychology has a history of unethical studies because there were no regulations as to what types of experiments that psychologists could run. Perhaps the most infamous were the Milgram experiments, where the researchers asked participants to shock another participant until the point of serious injury or even death. The participants were later told that there was actually no other participant in the study and that they hadn’t hurt anyone. However, the participants were deeply distressed at what they had done. These experiments, along with other infamous ones like Zimbardo’s prison experiment, drove the development of IRBs. Now psychologists who are conducting research at the university level must get IRB approval before beginning their research.

Do your own research through Polygence!
Polygence pairs you with an expert mentor in your area of passion. Together, you work to create a high quality research project that is uniquely your own.
As a high school student, whether or not you need to get IRB approval depends on your research goals. You may want to get your research published in a scientific journal or submit your project to a competition (e.g., research competitions for psychology and neuroscience ). In these cases, you need IRB approval. However, if you want to publish your result in a different medium, such as a blog post, then you do not need IRB approval. Regardless of whether you need approval, you should consider the points that I cover below to make sure your research is ethical.
Navigating the IRB approval process can be tricky in the beginning, so here is a detailed list of each part of the IRB approval form. An IRB wants to see that you put thought into every section. I also want to emphasize that you must wait to hear back from the board and receive approval before beginning your research.
What is Included in the List of Researchers?
You’ll need to list all of the researchers involved, no matter how big or how small of a role they have in your study. Different universities have varying requirements for the training that researchers need to complete. For example, I had to complete the Citi Training . Additionally, you will have to indicate one researcher as the PI (Principal Investigator), who takes responsibility for running the study. As a high school student, the PI will most likely be your research mentor.

What is the Study Design?
You need to have all of the study details planned out and communicate these details to the IRB. First, you’ll give a high level description of what participants will do in your study. You’ll also attach a series of documents so that the IRB knows every single detail of the study. For example, if you are conducting an online experiment, you’ll include all of the screens of the experiment, as well as the specific wording you’ll use in recruitment messages and follow-up emails with participants.
In addition to this exact wording, you’ll also provide details of how you will recruit participants. What platform will you use to recruit them? How long will the study take and how will you compensate them for their time? You will also indicate which types of participants will be included or excluded from your study. You may only want to recruit participants of a certain age demographic, and of those participants you’ll exclude those who don’t meet some criteria. Also, as a heads up, participants physically located in the European Economic Area (EEA) are protected by the General Data Protection Regulation (GDPR). The GDPR has stringent rules on what researchers must do to protect participants’ data.
What are Special Populations?
Speaking of participant recruitment, you have to indicate if any of your participants will be members of vulnerable populations, specifically, minors, prisoners, or pregnant women/fetuses. You must take special care to protect members of these populations. If you want to include minors as participants, then the parent/guardian must sign the consent form on behalf of the minor. I will discuss consent forms later in the article. Additionally, if the minors are old enough as determined by the specific IRB you are using, then you must give the minors an assent form , which is “is specifically designed to simply indicate that the minor is willing to participate in the study and understands what he or she will be expected to do as part of the study.”

In regards to prisoners’ rights, you need to make sure not to coerce prisoners. Coercion means giving participants unreasonably high compensation for participating in the study, thereby limiting the amount of agency they have in deciding to participate. Prisoners have limited access to economic resources, so paying them too much money is a type of coercion.
And of course, you want to make sure that pregnant women/fetuses stay safe during your study. For example, I once participated in a study where I received very mild and completely safe electric shocks. If I had been pregnant, I doubt that I would have been able to participate.
Your Project Your Schedule - Your Admissions Edge!
Register to get paired with one of our expert mentors and to get started on exploring your passions today! And give yourself the edge you need to move forward!
How do you Define Risks and Benefits?
You will also indicate the potential risks of participating in your study and how you will mitigate and address those risks. Psychology studies can be distressing, so often there will be emotional risk. To address this risk in an IRB form, the magic words are “this study will cause no more than minimal risk to participants.” In other words, you’ll need to explain why your study, at most, will cause participants no more distress than they would experience in their regular lives. If your study is more than minimal risk, it will be harder for you to get IRB approval. You will need to justify why you are running the study. Less common types of risk for psychology studies are social, physical, and financial.
Relatedly, you’ll also list the potential benefits of the study to the individual and society as a whole. For example, you can describe how your participants will learn something about themselves by participating in your study. You can also discuss how your study will add to the knowledge in your field, help people in the “real world”, or both.
What is a Confidentiality / Privacy Plan ?
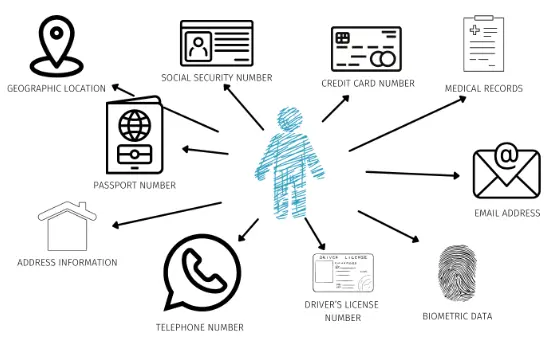
It is very important that you keep your participants’ data confidential and private, and you should communicate to the IRB that you have a specific plan for doing so. Use password protection on any cloud software and physical devices that store data from the study. You may also need to encrypt your files when transferring data, depending on how sensitive your data is. Never release any personally identifying information (PII) about your participants, even if you later make the dataset publicly available for researchers to use. PII includes information such as their name, address, email, phone number, etc. To make sure that I never release PII, I just never collect this information in the first place!
What is Included in a Consent Form?
A consent form tells participants the details of the study and asks them to sign if they want to participate. The form discusses what they should expect to do during the study, how much they will be paid, how long it will take, what risks or benefits they may experience, etc. With a few rare exceptions, all participants 18 or older must sign a consent form before participating in your study. (Parents/guardians must sign on behalf of their minor, as mentioned earlier.) Additionally, in a consent form, you must explicitly say that participation is voluntary and participants can withdraw from the study at any time without penalty. You also have to include information so that participants can contact the researchers if they have a question about or issue with how the research is being conducted. In the IRB form, you will have to answer additional questions about how you will protect the rights of participants if you are getting consent from non-English-speaking subjects or adults who are unable to consent.
What is a Debrief Statement?
At the end of the study, it is nice to tell participants what the study was about! The debrief statement is a few paragraphs written in plain, non-technical language that tells the participants what you were studying. It may or may not be required for your particular study - that depends on the IRB.
However, if you deceive the participants in some way, then the IRB will almost always require that you include a debrief statement. “Deceive” sounds harsh, but the reality is that sometimes you’ll have to *bend the truth* in a consent form or somewhere along the study. For example, you may tell participants that the study is researching a different topic than the one that it is actually researching. Research deception can be uncomfortable, but remember that deception is often necessary from a research perspective. It is important that participants don’t know what you’re studying, or they may alter their behavior. In a debrief for a study that involves deception, you’ll need to explain exactly how you bent the truth and why it was necessary for the study. Participants should also receive information about how to contact the researchers if they have follow-up questions.
Phew, that was a lot! The good news is that once you’ve filled out an IRB form, it becomes very routine to fill out any subsequent ones.
How Does IRB Approval Response Work?
After you’ve submitted an IRB form, here is what to expect from their response. The response time of an IRB varies by institution and by the complexity of the study. They may list the expected response time on their website. Submit your study early so that you have enough time to wait for their response and conduct your study. The IRB may approve your study immediately, or they may also approve it with modifications. (I’ve never gotten a study completely rejected, although I suppose it is possible if you present them with a really unethical study.) For example, I once conducted a study to encourage participants to exercise more. The IRB approved my study with modifications. I needed to include a few lines about how you should contact your doctor before starting a new exercise program to make sure that participants didn’t overdo it and injure themselves. Then, I sent the IRB the revised consent form that included this information, and they fully approved the study.
Of course, after submitting your IRB form, you may realize that you need to change something in your study. This is normal and fine. You simply submit a modification form to the IRB indicating what you would like to change. Remember that you have to wait for their approval of the modification before you run the study with that modification!
Conclusion
It is essential that psychologists at the university level gain approval from an IRB before conducting their research. High school students may or may not need IRB approval depending on their individual goals. Admittedly, filling out IRB forms can feel a bit tedious and monotonous, but protecting participants' rights is important - and when you submit work for consideration through your college applications , it matters whether you’ve followed due process! Make sure that you consider all of these aspects before you start working on your research project.
Feeling Inspired?
Interested in doing an exciting research project? Click below to get matched with one of our expert mentors!
- Research Portal
- Award Lifecycle
- Find Funding
- Develop and Submit Proposal
- Set Up Project
- Manage Project
- Project Closeout
- Research Compliance
- Fiscal Compliance
- Policies and Guidance
- Research Education
- Systems/Portals
- SPARCS Staff Contacts
- Contracts and Grants Staff Contacts
- College and Unit Research Contacts
Step 1: Determining if IRB Approval is Required
Do i need irb approval .
The NC State IRB Office asks three sequential questions to determine if IRB approval is necessary for a project and, if so, from where the IRB approval should be sought. If the answer is “yes” to the first question, we then proceed to the next question, and so on. If the answer to one of the first two questions is “no,” then the study does not need IRB approval.If the answer to question number three is “no,” the project does not require IRB approval through NC State University but may require it elsewhere. If a researcher needs an official determination regarding if IRB approval is required or not required, please contact [email protected] .
A research study is a very careful way of looking at something and collecting data in order to answer a specific question aiming to inform or solve a problem. Research can be done with or without the involvement of humans. Regulated research with living humans at NC State University is supervised by the IRB office and research with vertebrate animals that aren’t humans at NC State is supervised by the Institutional Animal Care and Use Committee ( IACUC ). Research is different from quality improvement and assessment/evaluation activities .
Is It Research?
Research is defined as “a systematic investigation, including research development, testing, and evaluation , designed to develop or contribute to generalizable knowledge.” There are two aspects of this definition that make a research project regulated, and both aspects must be present in a research project for it to be reviewed and approved by the IRB. These aspects include the practice of a systematic investigation and the contribution to generalizable knowledge. Note: if you are testing the safety or efficacy of a device, drug, or biologic, regardless of contributing to generalizable knowledge, you are considered to be completing research by the FDA.
- If the answer to the question “ Is It Research ?” is “no,” then IRB approval is not required. Please also review the pilot study and feasibility work IRB unit standard .
- If the answer to this question is “yes,” then IRB approval may be required. To determine the IRB’s jurisdiction, we ask “Is it Human Subjects Research?”
Is It Human Subjects Research?
Human subject is defined as a living individual about whom an investigator conducting research: 1) obtains information or biospecimens through intervention or interaction with the individual, and 2) uses, studies, or analyzes the information or biospecimens, including manipulation of the human’s environment. This also includes when an investigator conducting research obtains, uses, studies, analyzes, or generates identifiable private information or identifiable biospecimens. You can read more in our guidance “ Is is human subjects research? The definition of a human subject .”
Is IRB Approval Through NC State University Needed?
If you are affiliated with NC State University and acting as a researcher for NC State University — even if you are a student — you are considered an agent or an employee. As a result, your research project will need IRB approval through NC State University. This can only be done by submitting an application to the NC State IRB.
An institution’s employees or agents refers to individuals who either:
- Act on behalf of the institution in the scope of their role at NC State;
- Exercise institutional authority or responsibility in the scope of their role at NC State; or
- Perform institutionally designated activities in the scope of their role at NC State.
Employees and agents can include staff, students, contractors, and volunteers, among others, regardless of whether the individual is receiving compensation.
- If you are completing cooperative research with other institutions affiliated with an IRB or with individuals unaffiliated with an IRB, please review our guidance on cooperative research .
- If you are an NC State researcher and you think you qualify for the use of a private IRB due to the “more than minimal risk” nature of your biomedical study, please review our information about the use of the WCG IRB as an NC State Researcher.
- If an investigator is engaging in outside research for another entity and there is no NC State University involvement, the NC State University IRB cannot serve as the IRB of record (reviewing IRB). This includes when an NC State person is serving as an investigator and completing human subjects work as a part of their “Start Up,” or other External Professional Activities for Pay. For faculty innovators and NC State Start-Up companies, please refer to the NC State Office of Research Commercialization for additional information.
- If you are external to NC State University and want to implement a survey targeting NC State populations, please register your survey in accordance with the NC State University Survey regulation .
- If you are external to NC State University and you want to recruit in person on NC State’s campus, you must comply with the Use of University Space regulation 11.55.02 and contact [email protected] or review Student Centers Events for Non-University Groups for space reservations to get appropriate permission or permits.
What Type of Approval Do I Need?
There are three levels of review: convened full board, expedited, and administrative. The IRB determines which level of review is required, and consequently, whether approval — if granted — will result in an exemption determination, or result in either expedited or convened full board review. Related to these reviews, a researcher can also request a “ Not Human Subjects Research ” determination or, for studies about to be funded, a “.118 Determination” “Just-in-Time” request.
Why There are Different Types of Review Processes
Individual research studies range in type and complexity, and as a result, are governed by varying laws and regulations with different approval standards. The regulation governing research with human subjects ( 45 CFR 46.111 ) details the criteria for approval and the varying types of review/approval levels.
The criteria for IRB approval focus on balancing the risks and benefits of the individual research project, how the individual project transparently communicates the research and its risks and benefits to participants, and how the individual research project uses sound research design that does not expose people to unnecessary risk.
Each Level of Review/Approval Explained
Full board review and approval.
This type of review and approval is carried out by an IRB office staff member and the convened IRB full board. An IRB office staff member helps the researcher prepare their application for final review by the IRB-convened full board. Studies reviewed by the IRB convened full board are either more than minimal risk to participants or are minimal risk to participants but do not fit into any of the expedited categories . Studies reviewed at the convened full board level must have all informed consent requirements of 45 CFR 46.116 (unless appropriately waived) and meet the criteria for approval at 45 CFR 46.111 .
Occasionally, a convened full board study will warrant an additional layer of review. This can be due to the research design; the annual review requirements for an individual study; additional obligations from applicable regulations; sponsor requirements; special populations considerations; or in some cases, the study conducting international research.
The convened full board meets once a month , usually on the first Tuesday of the month (varies due to the academic calendar, holidays, or the need for an out-of-cycle meeting).
Expedited Review and Approval (Mid-level Review)
An IRB office staff member and an IRB full board member with appropriate expertise carry out this type of review and approval. An IRB office staff member helps you prepare your application for final review by the full board member. When the application is ready for final review, the IRB staff member will refer the study to the full board member for final review. Please note: expedited review does not equate to “rushed review” in this sense; it is simply the name of the “mid-level” review.
Expedited review is for studies that are both minimal risk and fit into one of the “ Expedited Review Categories .” Every expedited-level study is subject to the regulations outlined in 45 CFR 46.110 . Studies reviewed at the expedited level must have all informed consent requirements of 45 CFR 46.116 (unless appropriately waived) and meet the criteria for approval at 45 CFR 46.111 .
Administrative Review and Approval Leading to Exemption
This type of review is completed by IRB office staff. Determinations from this type of review can only lead to a determination of exemption. Though not all IRBs review and approve studies that can be exempted from 45 CFR 46.111, NC State University’s human subject regulation ( REG 10.10.03 ) requires that the IRB office make the exemption determination instead of the researcher.
An exemption determination means that your study is minimal risk and it fits into the categories of exemption outlined in 45 CFR 46.104 . Examples of exempt research include minimal risk surveys with adults, interviews and focus groups with adults, benign behavioral interventions, taste tests with commercially available or GRAS ingredients, or interventions that can be considered “normal educational practices.” Research studies determined to be exempt from the regulations are still considered research with human subjects. An exemption determination just means that not all of the regulations in 45 CFR 46 must be applied to the individual study, and the NC State IRB office may instead choose whether or not to apply some standards. Studies reviewed at this level may be required to undergo a limited IRB review. Limited IRB review considers data protection and, where appropriate, broad consent — and can be completed by IRB staff.
Not Human Subjects Research Determination
A “Not Human Subjects Research” determination means that the research activity is not regulated by the IRB in any way because the activity is not considered human subjects research. A study is not considered human subjects research if the answer to the questions “Is it research as defined by the federal regulations?” or “Is it human subjects research as defined by the federal regulations?” is no.
Answering “no” to either of those questions means the IRB does not have jurisdiction to review the project and no IRB approval is necessary. Note that this determination is different from a study reviewed and determined to be exempt. Exempt studies are considered human subjects research; they merely meet certain criteria that allow for flexibility of review. A “Not Human Subjects Research”(NHSR) determination means that the project is not considered human subjects research at all.
If you need a “Not Human Subjects Research Determination” please complete the “ NHSR Request Form .” Once the form is received by the IRB, the IRB staff will respond to your request within 2-3 business days. After the determination is issued, if you need an official letter with the determination, please request an official NHSR letter .
Just-in-Time Approval “JIT” and .118 Determinations
Some organizations that sponsor research require IRB approval or pending approval before accepting new grant proposals. Other sponsors, including the National Institute of Health (NIH) and the National Science Foundation (NSF), as well as some private, non-profit organizations, will accept new grant proposals with the understanding that the researcher will proceed with the IRB review process upon receiving notification of a score in the fundable range. This is called the Just-in-Time procedure. Please refer to the guidance “ NIH Grants and the IRB ” for Just-In-Time guidance for NIH grants.
In addition to the “Just-In-Time” approval process, the NC State IRB also can provide researchers with a “.118 Determination Letter.” A .118 letter states that a study will involve research with human subjects but the protocol cannot be fully developed yet and lacks immediate plans for the involvement of human subjects. Researchers do not need a complete IRB application to receive a .118 determination letter.
Applying for a .118 Determination or Just-In-Time Request
Researchers do not need to have a fully complete IRB application to be granted a .118 determination or Just-In-Time request; they do, however, need to complete the following steps:
- Open a new IRB application in the eIRB system.
- Write “[.118 Determination Request]:” in the protocol’s title box along with the protocol title if the grant proposal is for NSF or “[Just-In-Time Request]:” for NIH grant proposals. The protocol title should match your grant title.
- List the funding source as “NSF” for .118 determinations or, for Just-In-Time requests, list the NIH as the funding source.
- List the faculty point of contact for the protocol. This should be someone at NC State who is also listed on the grant application.
- For .118 determination requests, go to the description tab of the eIRB application. State in the first narrative box that “All procedures and supplemental documents will be submitted for IRB review and approval before it is implemented. No study procedures including recruitment, consent, data collection, or data analysis will occur until after the PI has complete and full IRB approval via an amendment process.” For Just-In-Time requests, all designed procedures, or as many designed procedures as are presently available, should be provided and the IRB application should be completed to the best of the researcher’s ability.
- Click the “Save” button above the narrative boxes you just pasted text into.
- Navigate to the routing and status tab of the eIRB application and click the “Submit to IRB office” button. Aside from the information mentioned above that you must include in the title and description tabs, the rest of an IRB application for a .118 determination should be blank. The IRB office cannot process a .118 determination request or Just-In-Time request without a submitted protocol.
- Complete the letter request form on the NC State IRB website for a .118 determination or Just-In-Time request letter. No requests submitted over email can be honored.
Please note, that a .118 determination and Just-In-Time request is staged research; you should first familiarize yourself with the NC State IRB guidance on phased and staged research protocols before requesting a .118 determination or a Just-In-Time request.
- Step 2: Preparing and Submitting an Application
Tips and Tricks for a Successful IRB Submission and Review Process
Here are some tips for completing the Research Protocol to ensure that the IRB has the information it needs to review the study. Keep in mind that the IRB is reviewing the study to determine that it meets the criteria for approval. The more information the IRB has, the easier it can be to make the required determinations.
1. When completing the IRB application, remember to describe the entirety of the study. Imagine when completing the Research Protocol that you will give this to a future investigator who has never heard about the study before, and will help you conduct the research. If they read your protocol, could they complete all the study procedures without needing copious amounts of additional information? Is it clear who, in terms of collaborating institutions, is responsible for each research activity?
What am I supposed to write in my research protocol?
2. Be realistic with your enrollment size, feasibility of study, resources (financial, staffing, physical space, materials needed, etc), amount of time study will take, use of vulnerable populations, etc.
3. Use lay language – The IRB application should be written in a way that all can understand. The IRB recommends writing in a narrative form, explaining the specifics of what the participant will experience if they take part in the study, from beginning to end. If the Research Protocol is written in too technical of a manner (i.e., it’s not clear what you are doing), it may unnecessarily go to the Convened IRB. TIP: Have a non-scientist friend or family member read the protocol and consent forms…do they understand?
Here is an example of an overly technical description of study procedures vs. how the IRB would recommend study procedures are written:
Speaking Different Languages
“ Pre-Operative Measurements”
- Prior to surgery, the labial keratinized tissue will be measured at the mid aspect of the tooth to be extracted as follow:
- -Gingival width; Distance from the Free Gingival Margin (FGM) to the Mucogingival Margin (MGJ) measured at the midbuccal aspect of the tooth
- -Vestibular depth (using a bilateral retractor and a UNC 15mm probe)
- -Alveolar ridge: width measured on the CT scan at 5mm apical to the bone crest in the mid-mesial-distal aspect of the tooth to be extracted
- -The vertical distance of the line connecting neighboring Cemento-Enamel Junctions (CEJ) to the buccal FGM (measured just prior to surgery)
IRB (for the same procedures):
Prior to the surgery measurements will be taken:
- Gums will be measured by placing a periodontal probe (a small ruler) on top of the tissue
- Gum thickness will be measured with a thin needle and rubber stopper
- -Intra-operative dimensional measurements will be taken during tooth extraction. The patient will still be numb.
- -Bone width will be measured with a caliper (an instrument that gently goes around the bone).
- -Teeth and bone will be measured by placing a periodontal probe (small ruler) on top of the structures.
4. Use pictures in your application documents, if possible (e.g. graph in Research Protocol of what surveys are given to which participant groups and when, picture in the consent form of the device used, etc.)
5. If you’re collecting prospective data from participants, you need to think about your recruitment process.
- Recruitment materials may be an email, letter, or script and must be included for IRB review. Providing participants with a recruitment document prior to consent gives them the opportunity to decide if they even want to participate in the type of research being conducted before having to go through the entire consent process.
- In most research studies, initial contact with a potential participant through an email or letter is customary. Cold calling or showing up at someone’s house to recruit them should be well-justified in the Research Protocol, if such methods are necessary for the study. In some international settings, this type of recruitment is the norm. The IRB will honor any local customs or procedures so these should be described in the protocol.
- If you are using an established organization, site, or group to assist you with recruitment please include the names of these organizations/groups and confirm that you have approval from the leaders/administrators of the groups to assist you with recruitment (e.g. the moderator of a list serve agrees to send your recruitment email out to their list; the administrator of a social media group agrees to post your recruitment message to the group board; the leader/administrator of a company agrees to give the PI a list of their employee emails to use for recruitment purposes, etc.).
6. Think about your consent process:
- If you are recruiting participants into the study, you must obtain consent. Consent is not just a signature, it is a process involving a discussion with the participant. The IRB wants to see a well thought out process described in the IRB application, not just a means to get a signature.
- Give potential participants time to review the consent, ask questions, talk over their decision to participate with family and friends, etc.
- For Exempt and NHSR studies that involve interactions with or data collection from participants, a signature on the consent form is not required per the regulations. However, if an investigator wants to collect a signature, they may. The Exempt consent template includes all the required elements of consent for this type of research. In this type of research, it is often typical for consent to be either a verbal yes/no or indicated by completion of an online survey, questionnaire, etc. The process of consent still applies but the signature requirements are not as stringent and the consent form is not as long.
- For Expedited and Convened IRB studies, a full consent form that includes Key Information must be used. There are few, but some in special cases, exceptions to this so if you have concerns about using the full form, talk to your IRB Review Specialist .
- Adapt the consent process to your study. Are you working with a vulnerable population, will you ever interact with participants, are you meeting with participants before the study? If it is not practicable to obtain a signature, explain why and write in your protocol that you’re requesting a waiver. Below are two such waivers that can be requested:
- Final thoughts on consent: remember that practicable is not synonymous with possible . Although it may be inconvenient to meet with participants to obtain consent (i.e. because their appointment schedule doesn’t match the PI’s work schedule, the consent process will lengthen their appointment, the consent is long, etc.), the investigator should be obtaining consent when they can. Being impracticable would be if the investigator never interacts with the participant (online survey/medical record review/secondary data analysis only) or if the participant is not in the same physical location as the investigator (interview via phone or Zoom).
7. Plan ahead if you plan to travel to conduct your research. You cannot begin human subjects research before obtaining Harvard and in-country IRB approval, even if you’ve booked your flight! Be sure to discuss your project with in-country collaborators to check on any special approvals or permissions needed before you arrive.
8. Report if things happen (which they do sometimes!). Reportable Events include unexpected events that may be out of your control or reports from local IRBs, sponsors, DSMB, etc. It is not intended to be punitive, the intention is to utilize the IRB’s resources to resolve or respond to the issue. You must adhere to the IRB’s Prompt Reporting Requirements . Keep in mind that reportable events may not be limited to huge problems. Reportable events could be a DSMB report or new information that has come to light because of routine study or data monitoring. Sometimes events reported are out of the study team’s control. The IRB will work with you to resolve any reports of new information; the report is not meant to penalize the researchers! Keep these things in mind when completing this section. (Link to RNI instructions)
9. Explore reliance agreements with collaborating institutions – Are you already on the personnel roster of an existing study? You may be able to join that study rather than submit a new application through the HLC IRB. See our website for more instruction on ceding review to another IRB . Note: Reliance agreements can only be executed if the study is considered non-Exempt research.
News from the School

Nurturing student entrepreneurs

40 years after Bhopal disaster, suffering continues

Meet the Dean

Making health care more affordable
The IRB Process
The IRB reviews protocols to ensure appropriate safeguards to protect the rights and welfare of research subjects are in place, according to 45 CFR 46.111 . Federal regulation and institutional operating procedure require that the IRB reviews all the research documents and activities that bear directly on the rights and welfare of the subjects of proposed research. The application or protocol, the consent/assent document(s), tests, surveys, questionnaires and similar measures, and recruitment documents are examples of documents that the IRB reviews.
The IRB process can be broken down into three sections:
Criteria of Approval
Estimated time of review, categories of review.
Before any human subject is involved in research in relationship to this institution, the IRB will give proper consideration to:
The risks to subject are minimized by using procedures which are consistent with sound research design and which do not unnecessarily expose subjects to risks and whenever appropriate, by using procedures already being performed on the subjects for diagnostic or treatment purposes 45 CFR 46.111(a).
It is necessary, therefore, that the IRB assess the validity of the study design in relation to the risks to human subjects. Research that is not scientifically valid exposes research subjects to risks of harm without any possibility of benefit. At BYU the validity of the study design can be accomplished in several ways as outlined in this guidance .
Other factors that contribute to sound study design: evidence that key personnel are qualified by virtue of training and experience to conduct the research; a clear, well-written protocol that adheres to established principles of the discipline help to establish qualifications of the principal and co-investigators and ensures that the operational aspects of the research have been thought through.
On the other hand, poorly written protocols that do not demonstrate principles of sound scientific design cannot be approved. Poorly written and designed protocols are the most common reasons for delay in IRB approval.
Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result...
The investigator should proceed by doing the following:
- Assessment of all of the risks associated with the research by identifying all potential harms that could befall a subject and the magnitude and the probability of those harms;
- Ensuring that the appropriate steps have been taken to minimize the risks that are identified; and
- Assessment of the possibility and importance of potential benefits to subjects (if any) and to science and society.
The research may be approved by the IRB provided that the benefits outweigh the risks to participants.
Factors that impact risk include:
- the procedure (possible harms);
- the person performing the procedure (training, experience, skill);
- the setting (privacy protections, availability of resuscitation equipment, etc.); and
- the characteristics of the research subject (age, health status).
It is the investigators obligation to explain what will be done, by whom and to whom and where it will be done. Protocols and applications that incompletely describe the study procedures frequently result in requests for more information.
The IRB takes into account the purposes of the research and the setting in which the research will be conducted and should be particularly cognizant of the special problems of research involving vulnerable populations, such as children, prisoners, pregnant women, mentally disabled persons, or economically or educationally disadvantaged persons.
This criterion comes from the ethical principle of justice. No group is unduly burdened or will unfairly benefit from the research. Equitable selection does not mean that all groups are represented in proportion to the population. It means that the selection criteria should be both fair and appropriate to the research question.
Researchers should be mindful of their study design and assess if it is feasible to include non-English speakers as subjects. The exclusion of non-English speakers must be scientifically supported and not used as a mere inconvenience to the researchers. In some cases, this exclusion is supported when surveys tests, questionnaires are not available in other languages. Some tests are not valid in any other language than English.
The protection of privacy applies to the human subjects in the study and confidentiality is the protection of their data.
Privacy focuses on the environment where the study will take place, how researchers contact prospective subjects, and how research activities will be realized. Privacy issues depend on the study design and the target population.
Confidentiality should consider the following :
- How will access to data be managed?
- Who should be permitted access to the study documents?
- Who should be allowed to know the identities of those participating as subjects?
- What security plan (password protections, locked cabinets, encryption methods, separate storage of Master Lists from study data) is sufficient to adequately protect the subjects given the inherent sensitivity of the data?
It is important to note that the degree of data security depends on the sensitivity of the data. However, it is good practice to limit identifiable data to research personnel that need to know only. It is also good practice to de-identify data as soon as possible. Investigators should consult with their area data security to create a plan that is up to date and appropriate for the sensitivity of data.
Informed consent will be sought from each prospective subject or the subject’s legally authorized representative, in accordance with, and to the extent required by federal regulations for the protection of human subjects.
Informed consent will be appropriately documented, in accordance with, and to the extent required by federal regulations for the protection of human subjects.
The consent process involves providing the information that a reasonable person would want to know in language that is understandable to the subject. Understandable means at a grade level that they can understand and in their native language.
The IRB has the authority to approve, require modifications to secure approval, and disapprove all research activities overseen and conducted by the organizations. The IRB has the authority to suspend, place restrictions, or terminate approval of research activities that fall within its jurisdiction that are not being conducted in accordance with IRB requirements or that have been associated with serious harm to subjects. The IRB has the authority to observe or have a third party observe the consent process and/or the research if the IRB determines it to be indicated.
The IRB processes the protocols when they are received. Once protocols are logged in iRIS, an email is sent to the PI indicating receipt of the protocol, the designated IRB number, and that it is in the queue for assignment to an IRB staff member. The IRB staff initially screens submissions to determine the completeness and the appropriate type of review. Submissions may be returned to the study team for changes before the review type is assigned. The review type may be reassessed at any time during the review process.
There are several factors that will determine the time frame for approval. Depending on risk level and subject populations, attention to appropriate detail, protocols will proceed for exempt and expedited on a continual basis. Full board reviews are due the 10th of the month to go to the following month’s meeting, typically, the first Thursday of each month. The processing time is dependent on when you are able to respond to the issues. Please see the below chart for a visual depiction of this process.

Exempt reviews: If your protocol qualifies for one of the exemption categories, once you address any issues we can provide approval. Please allow 2-3 weeks from submission to approval.
Expedited review : The protocol will be sent to one or two IRB members for their review. Once you have addressed any issues we can provide approval. Please allow 4-5 weeks from submission to approval.
Full Board review : The protocol will be placed on an IRB meeting agenda. Full board protocols must be ready (all pre-review issues addressed) before it may be placed on an agenda at least 2 weeks before the scheduled meeting.
Review time is dependent on the Board’s stipulations and the duration for these to be resolved. Please keep in mind that these timelines may be shorter or longer depending on how busy our office is and how quickly you respond to any issues/questions.
Getting Started

- SHSU Library
- Research Guides
- Scholarly Communication
Undergraduate's Guide to Creating & Communicating Research
- Getting Approval for Research
- What Do I Search For?
- Where Do I Search?
- Can I Get Help Searching?
- Now What? A Lit Review How-To
- Asking a Research Question
- Data Analysis and Visualization
- Research Posters
- Conference Presentations
- How to Write About Research
- Where Could I Publish a Paper?
- SHSU Campus Resources
- Upcoming Training & Events

IRB Application Walk-Through for Students
- Introduction
- Create a New Study or Re-Open a Draft
- Complete Application Information Page and Sections 1-2
- Complete Section 3
- Additional Sections
- Finalizing a Submission
- Modifications
What is the Institutional Review Board (IRB)?
IRB is a committee of faculty who review proposals for human subjects research to ensure that studies are safe, ethical, and in compliance with relevant laws. At SHSU, the IRB is a division of the Office of Research and Sponsored Programs (ORSP).
What research must be approved by the IRB?
Most research involving living humans cannot begin until the IRB approves it. Depending on how high-risk or low-risk the research is, more or less detail may be required, and IRB review may be more in-depth or more expedited.
If our research only involves compiling existing data, or studying inanimate objects (like literature), then IRB approval is not required.
If our research involves living animals, then we will instead need approval from Institutional Animal Care and Use Committee (IACUC).
What steps should I complete before I request IRB review?
Before you request IRB review of a research study, you must complete CITI training with scores of 80% or greater in each module. If multiple people will collaborate on a research project, every one of them must complete CITI training. The ORSP Compliance website provides a link to the training and instructions for registering on the CITI site.
You should discuss your project with your faculty advisor before you begin your IRB application. You may also want to consult them along the way if you are not sure of the best way to answer a certain question. Be aware that IRB applications take time to review—this may be faster or slower depending on the complexity of the project and the risks involved, as well as how many other applications are waiting for review—so start your application as early as you can. ORSP provides some additional information about student-led and class projects and things to take into account concerning application timing.
You should have some general notes about your planned project before you begin, but it’s ok if you aren’t ready to complete every part of the application in one sitting. You can begin an IRB application, save it, and come back to it at a future time to add or edit information.
How do I submit my research for IRB review?
IRB review processes are done online in a special software program, Cayuse Human Ethics. To access Cayuse Human Ethics:
- Start at the SHSU IRB homepage .
- Click the blue “Submit IRB” button at the top of the page.

The tabbed pages of information below will offer detailed guidance through a student IRB application.
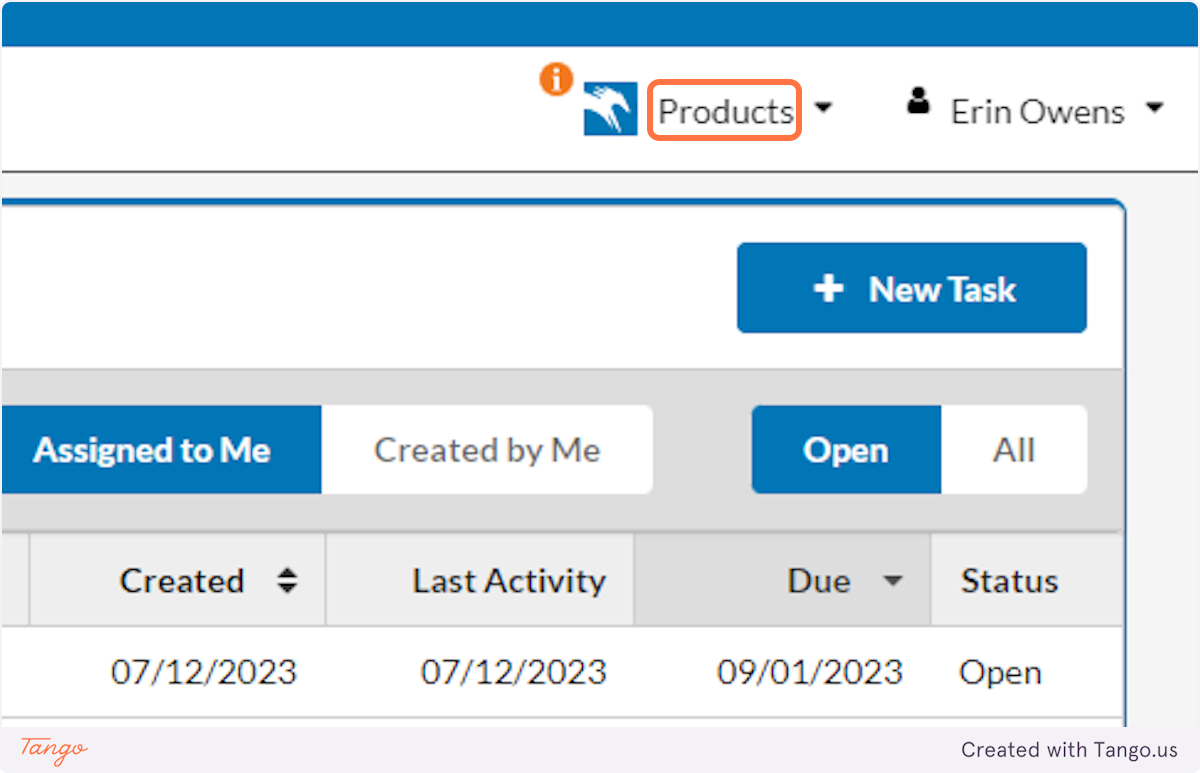
Start by logging in to SHSU's Cayuse Research Suite
1. click on products.
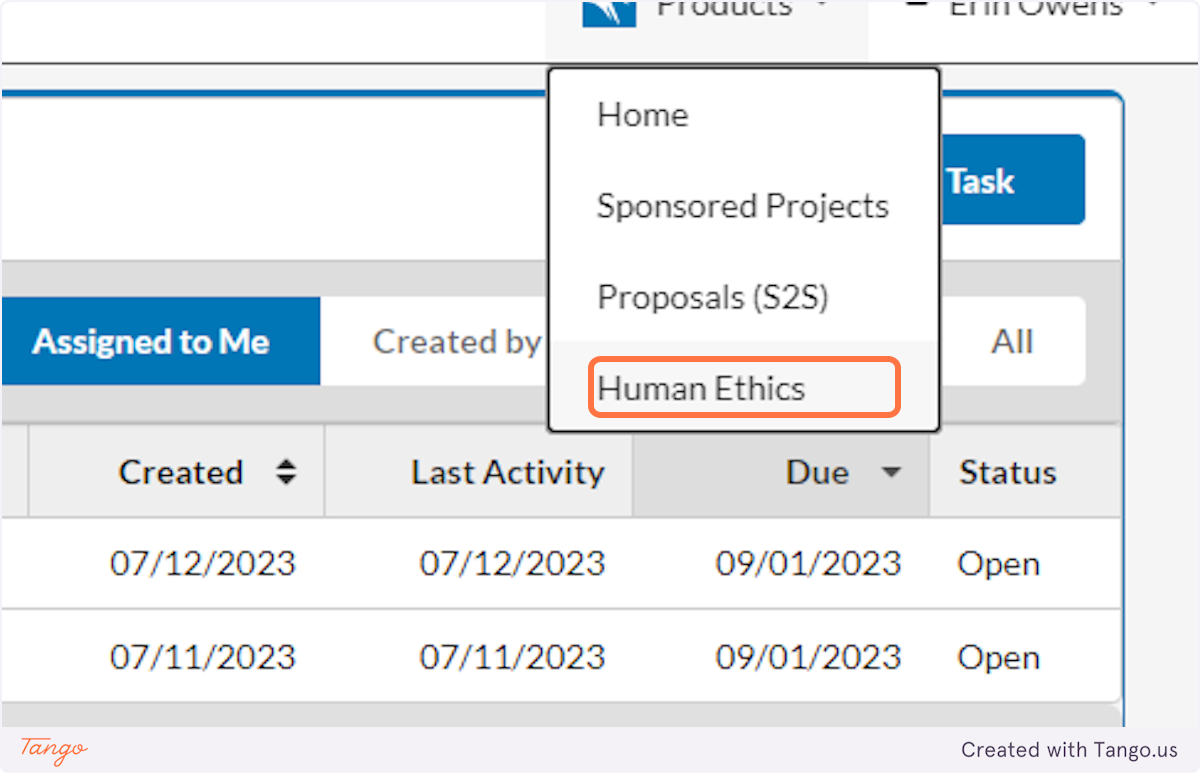
2. Click on Human Ethics
3. Click on the New Study button
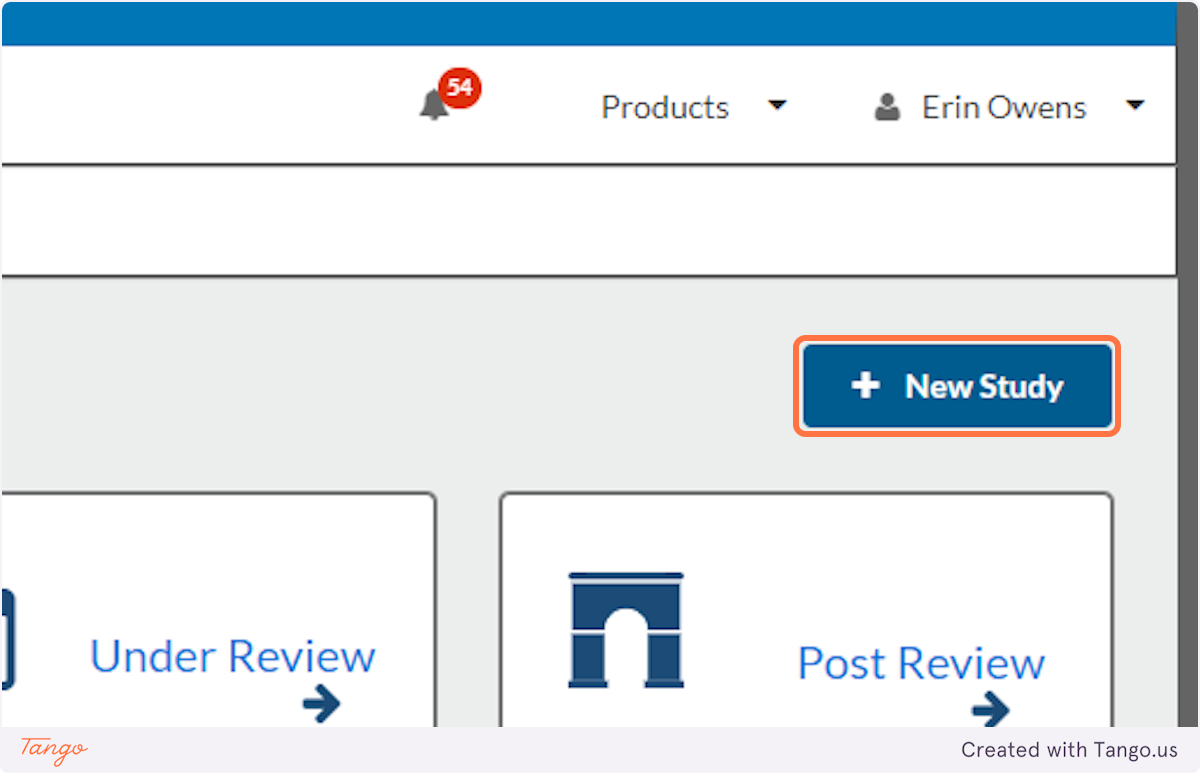
4. Enter a clear and concise title describing your project
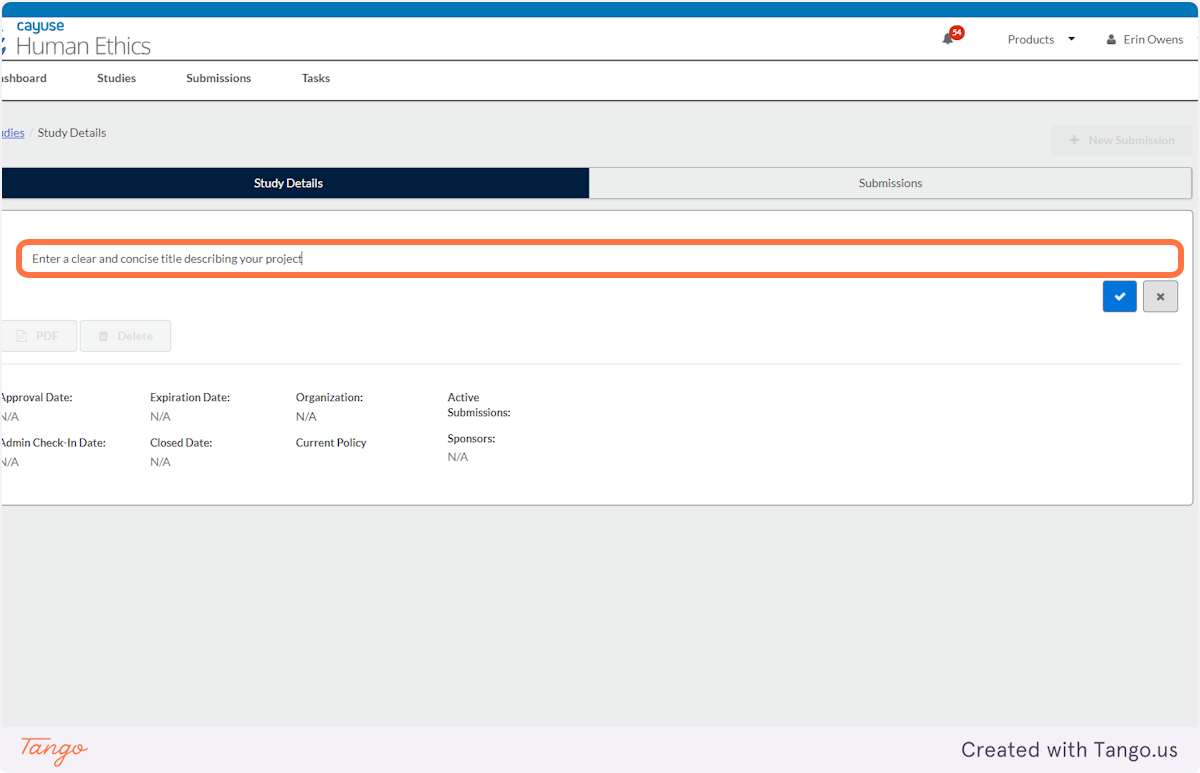
5. Click on the blue checkmark to confirm
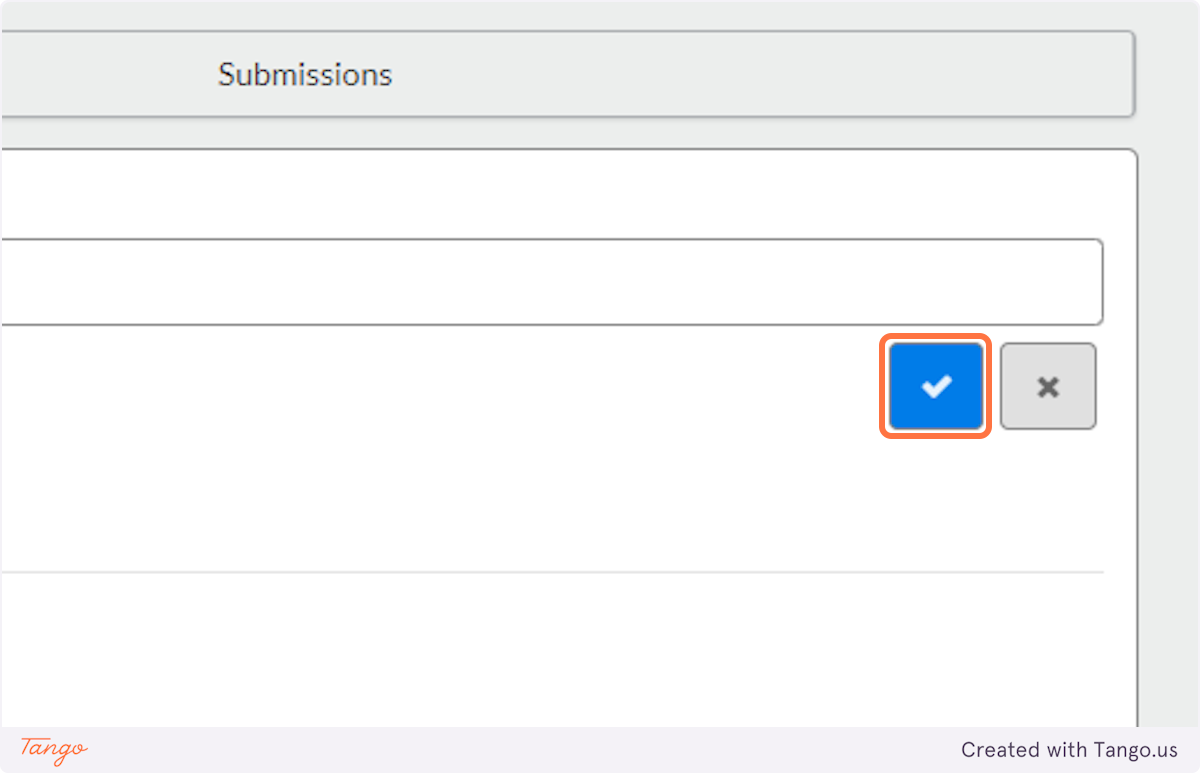
6. Click on the "New Submission" button
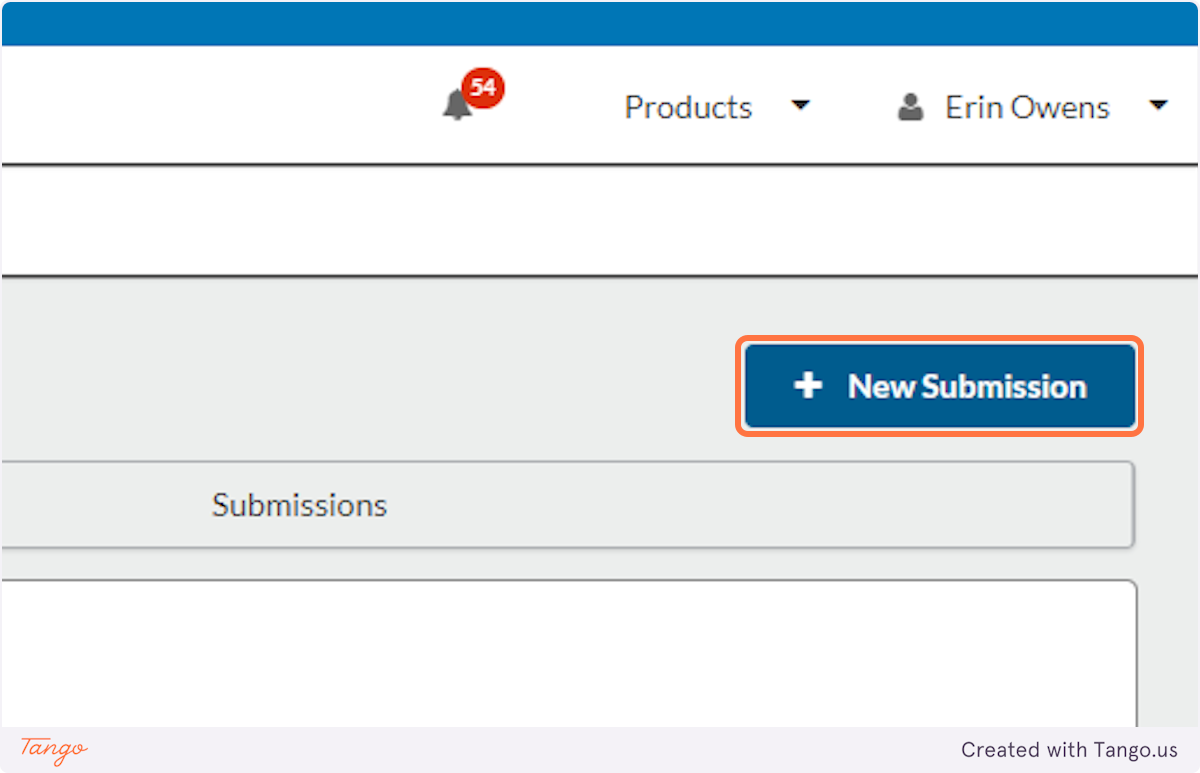
7. Click on Initial
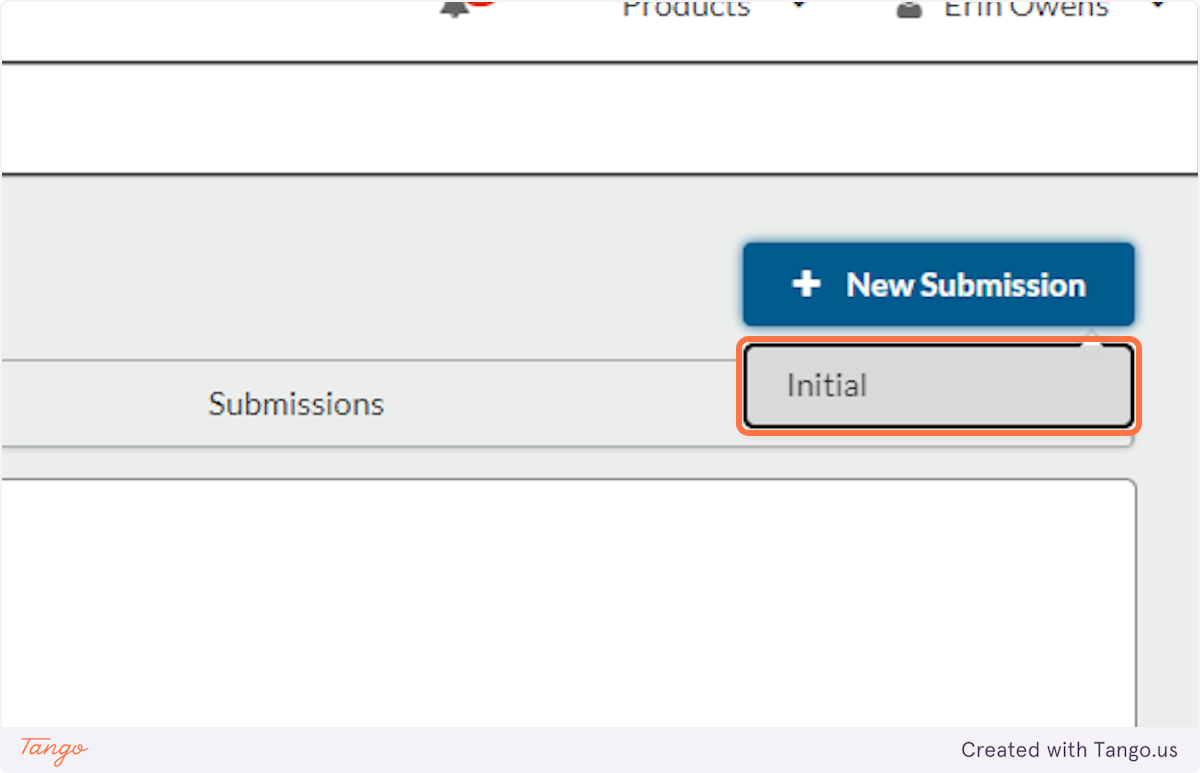
8. Click on Edit this submission
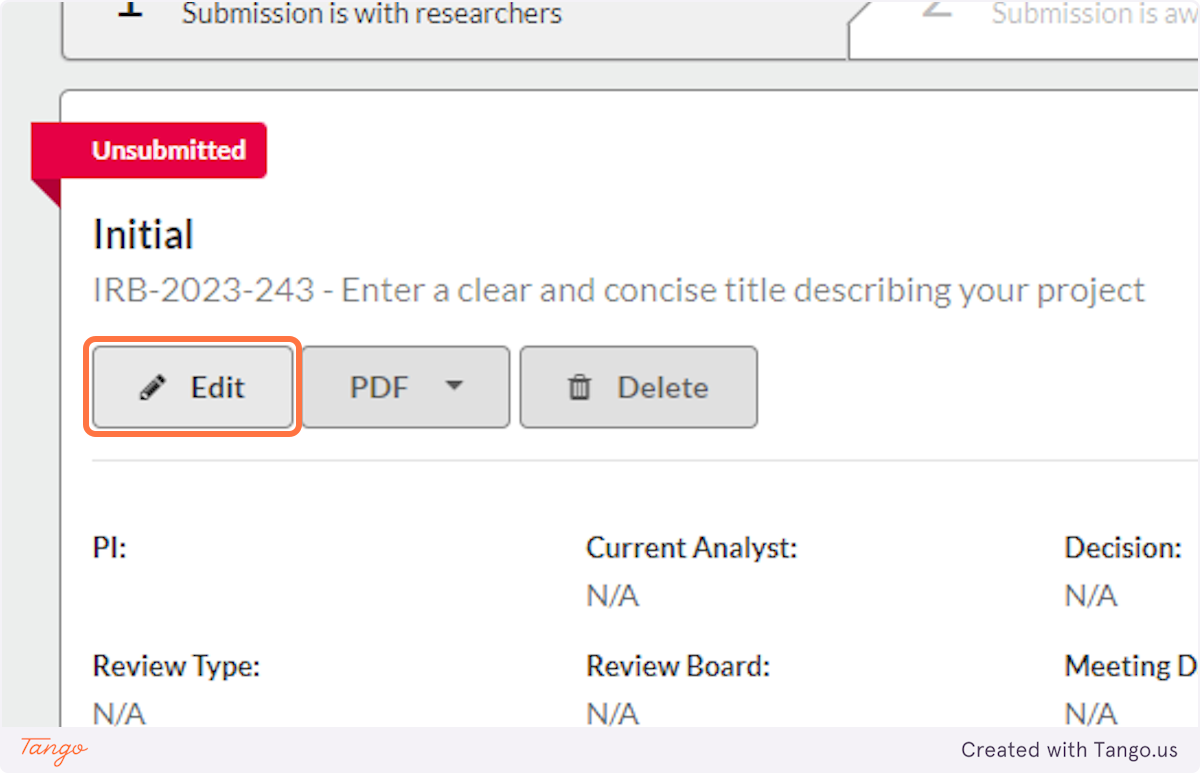
9. Here is where you will begin entering details about your study (see the walk-through on the next tab for details). You can always save a draft and come back to work on it another time.
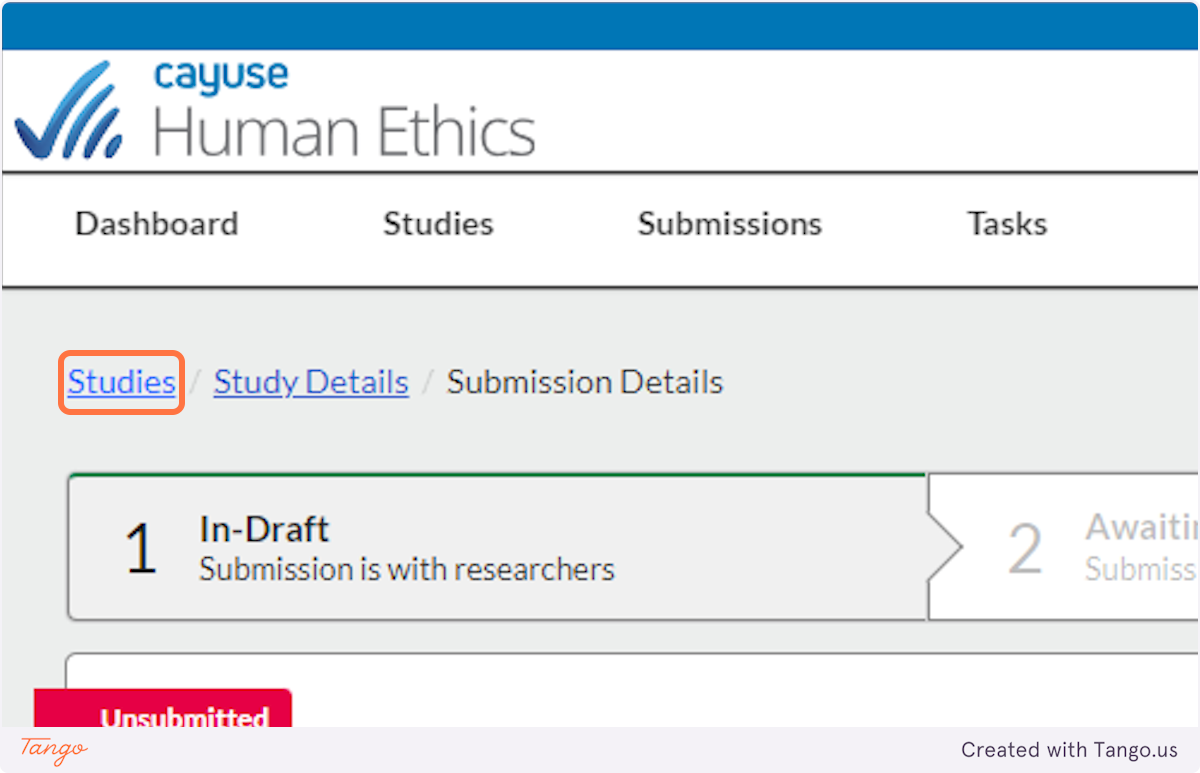
10. When you revisit Cayuse Human Ethics in the future, you will see a list of "My Studies" where you can click on the title to resume editing your draft study.
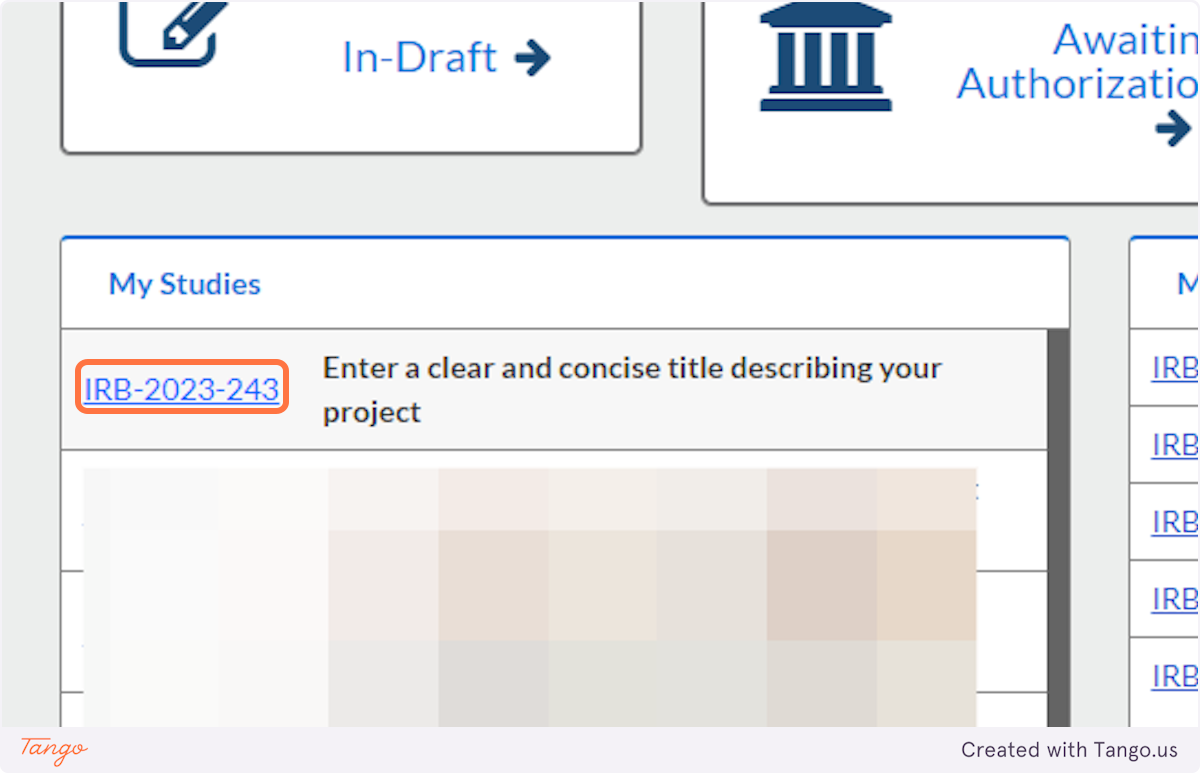
11. After you choose the study title, you will have to click on the Submissions tab...
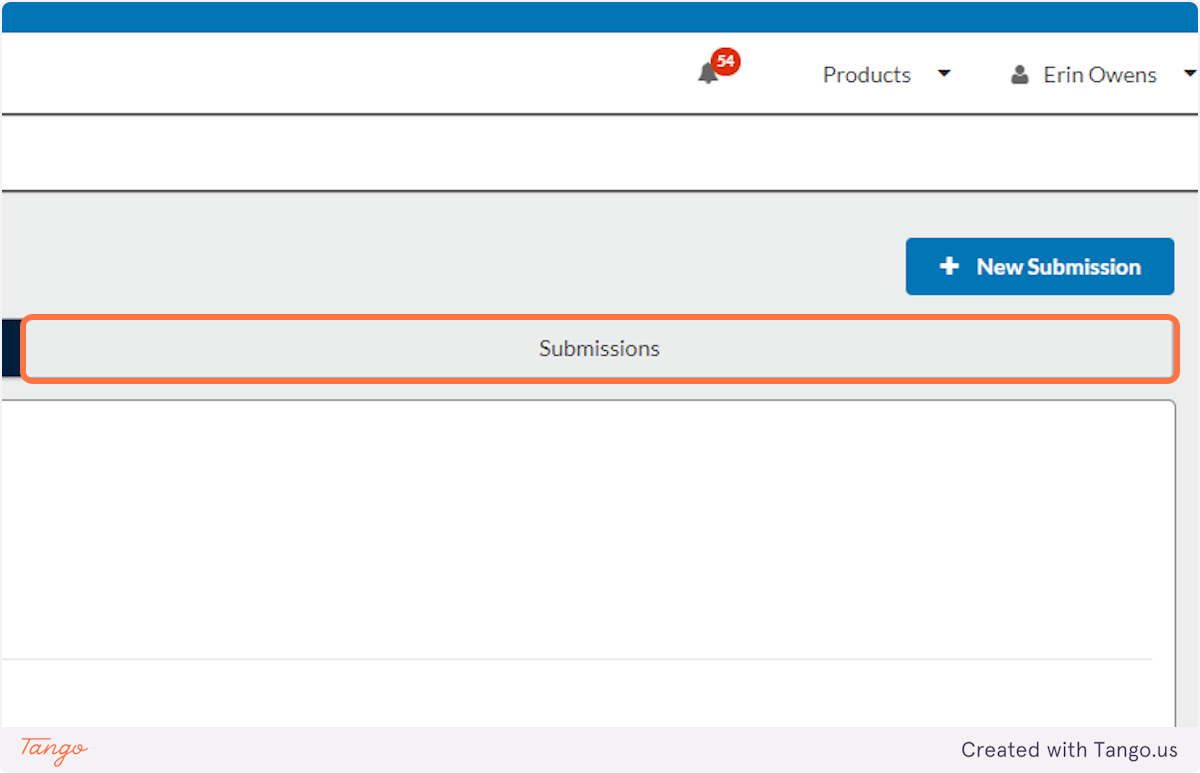
12. ...and click to open the "Initial" submission that you had begun drafting.
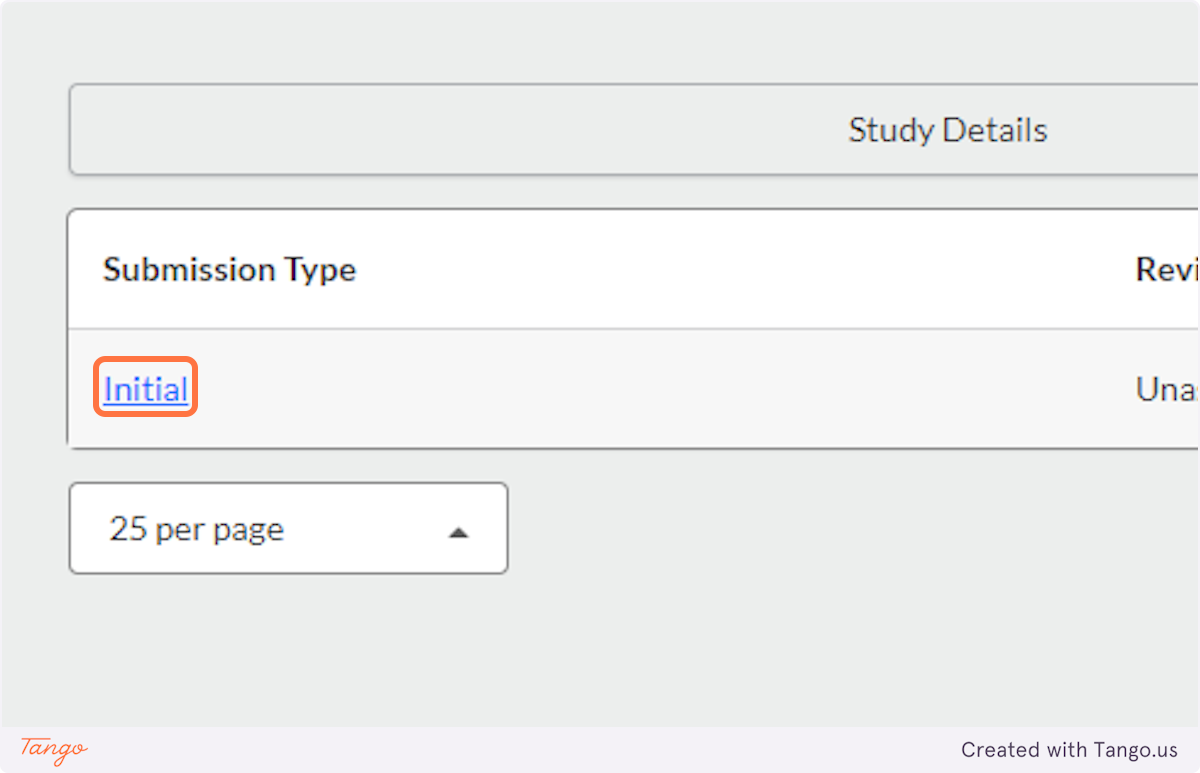
13. Click on Edit this submission to resume entering details about your study
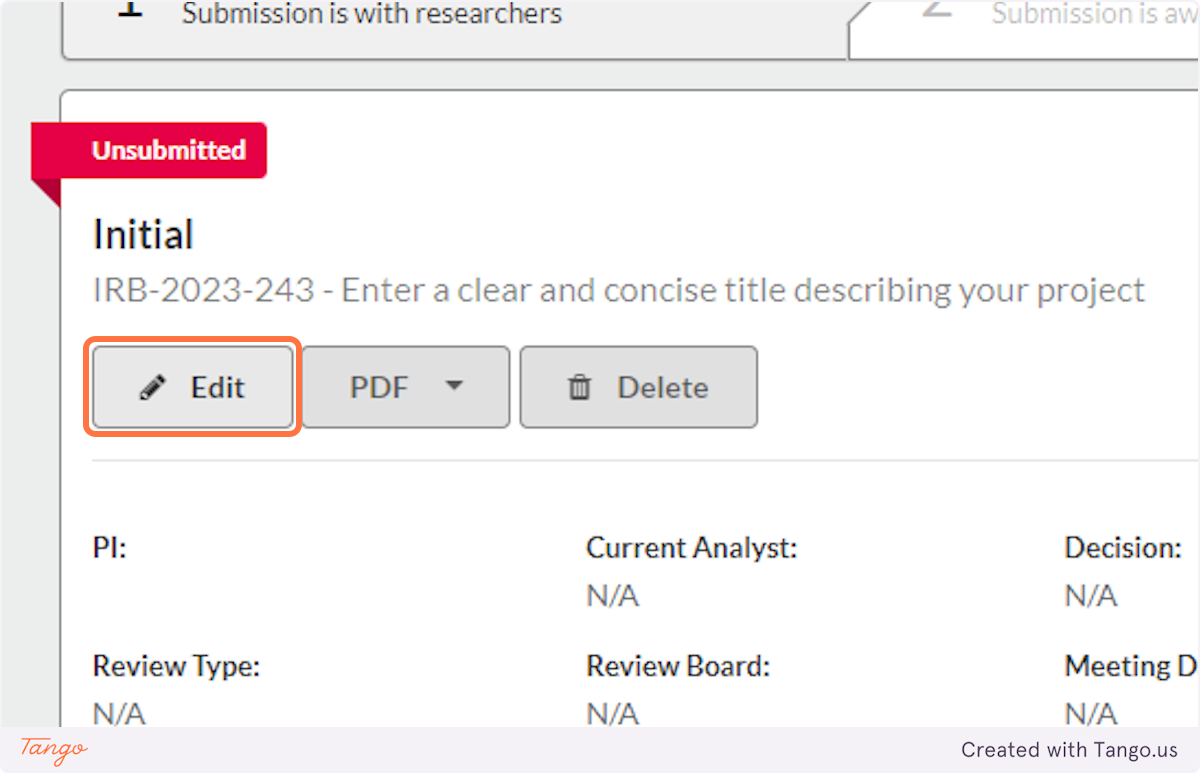
Creating a New Study or Accessing a Draft Study
Begin by opening the Initial Submission for your project in Cayuse Human Ethics
1. read all details on the application information page carefully, including (but not limited to) the fact that all project personnel must have completed the appropriate citi human ethics training..
If you (or any collaborators) have not completed CITI, you will need to do so BEFORE you complete this submission.
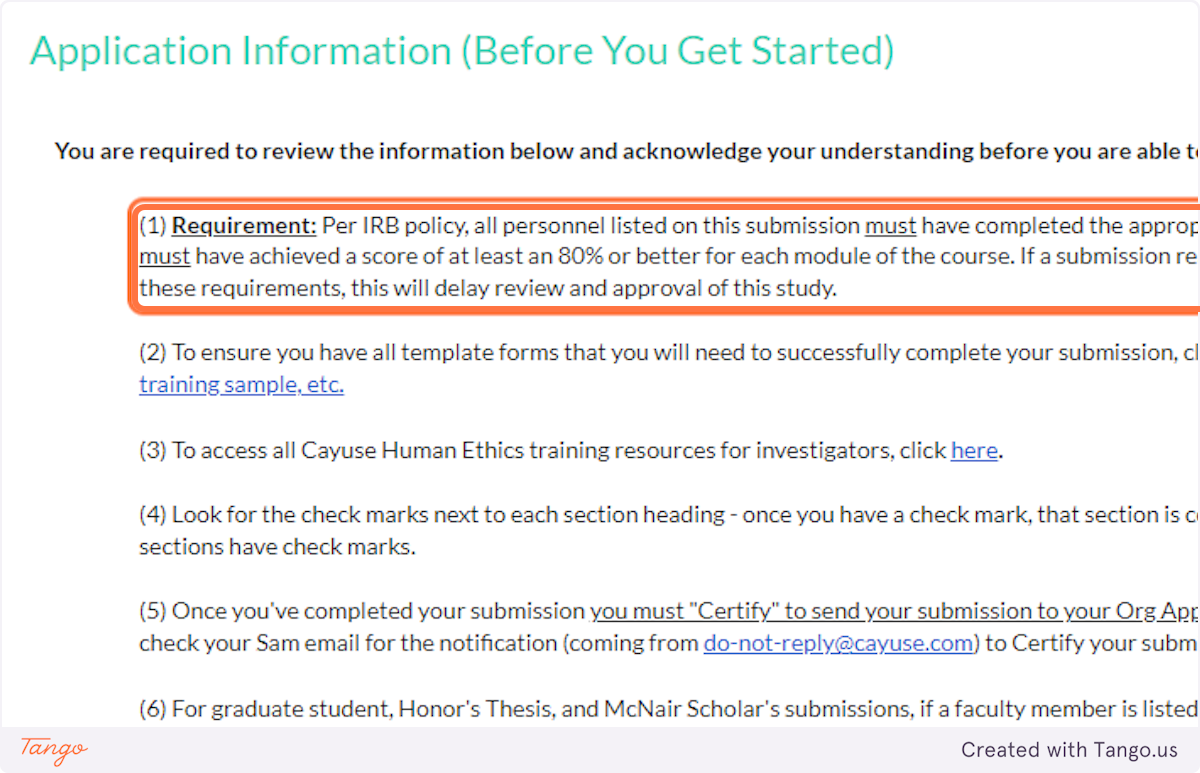
2. The application details explain to look for a white checkmark beside each application section in the blue menu.
If a section does not yet have a checkmark, you have an incomplete item somewhere in that section.
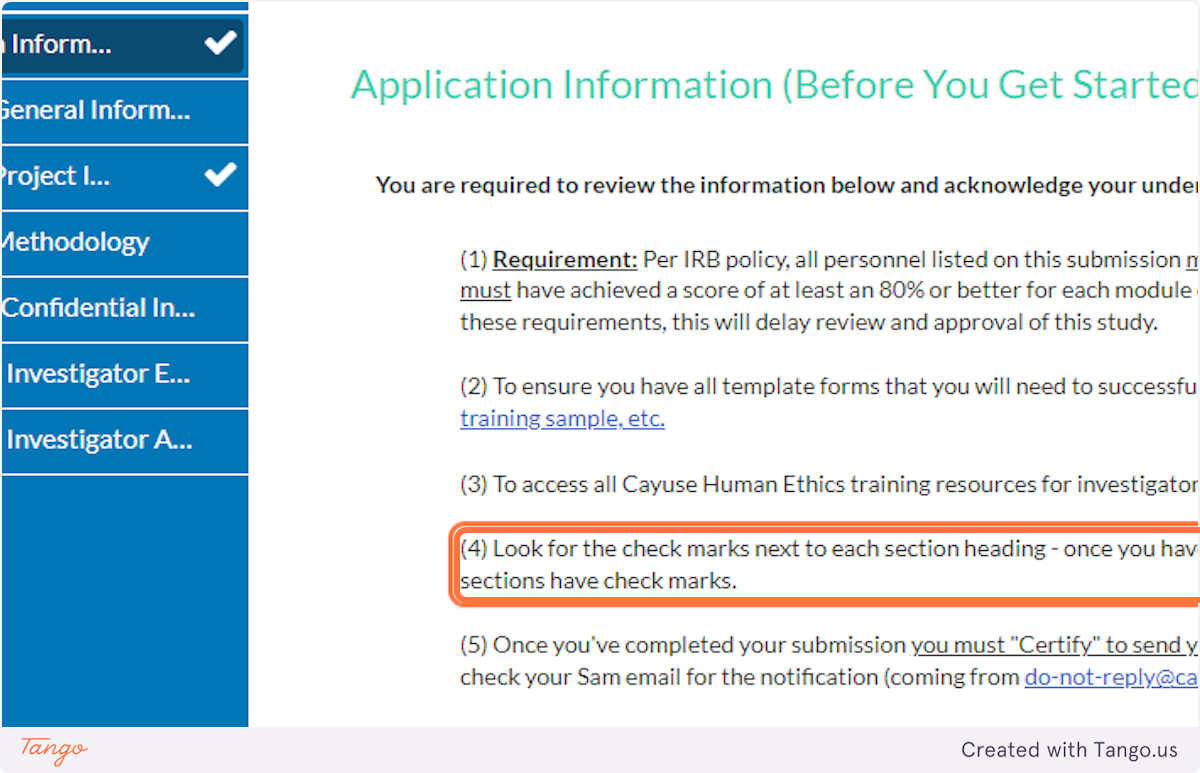
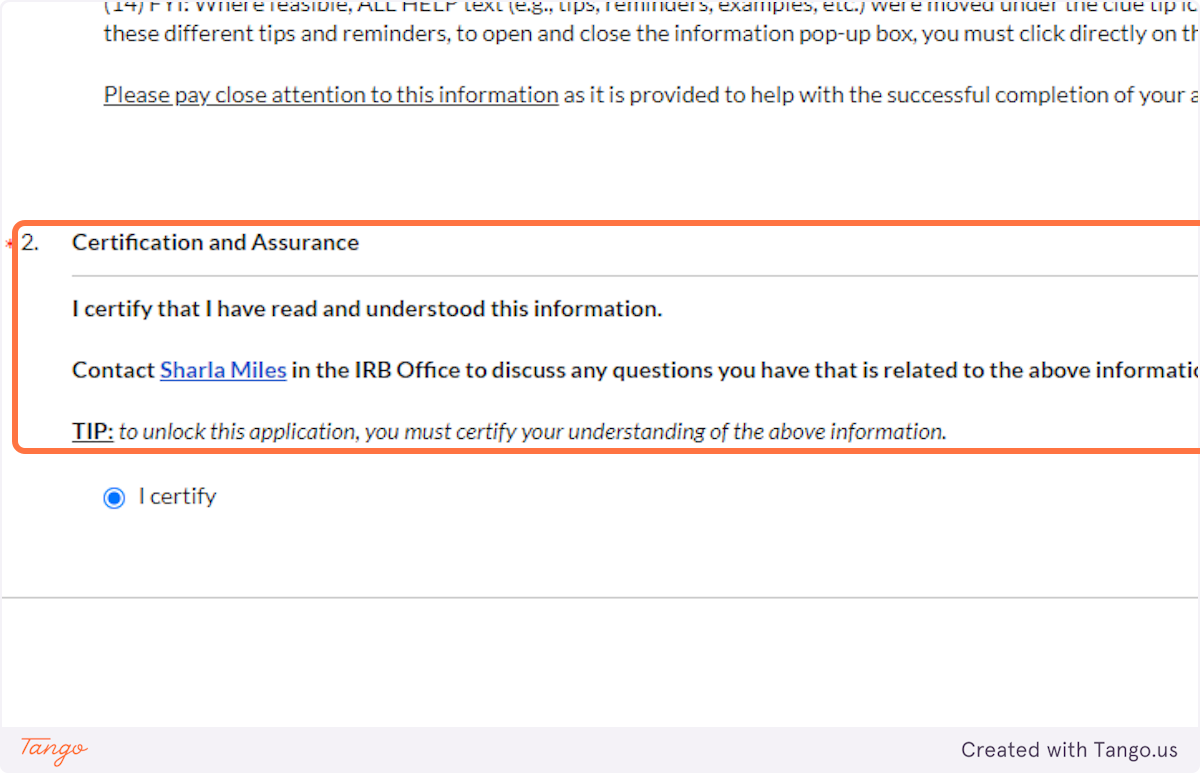
3. After you have read all details CAREFULLY, click the button to certify your understanding.
4. Click on the arrow at the lower right to go to Section 1.
5. The first field asks for the Primary Contact and likely already lists you as the creator of the submission. Verify that this is correct for this project -- that you want all IRB communication to come to you.
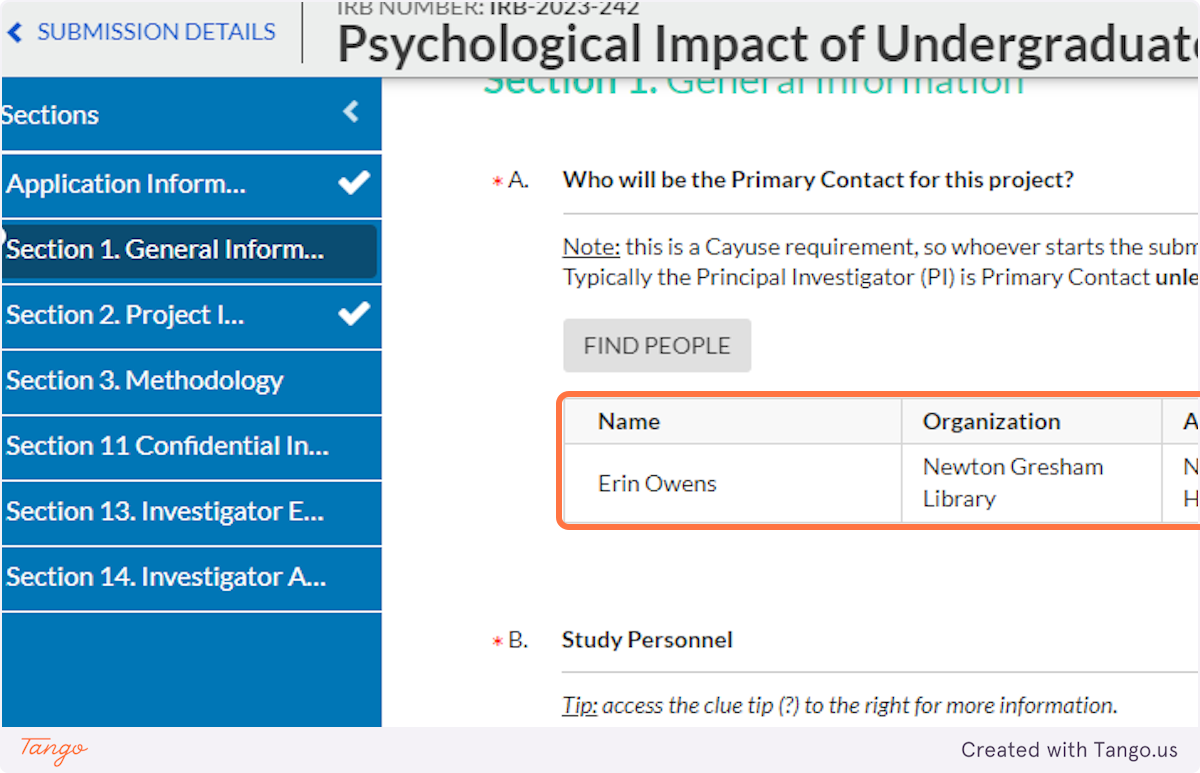
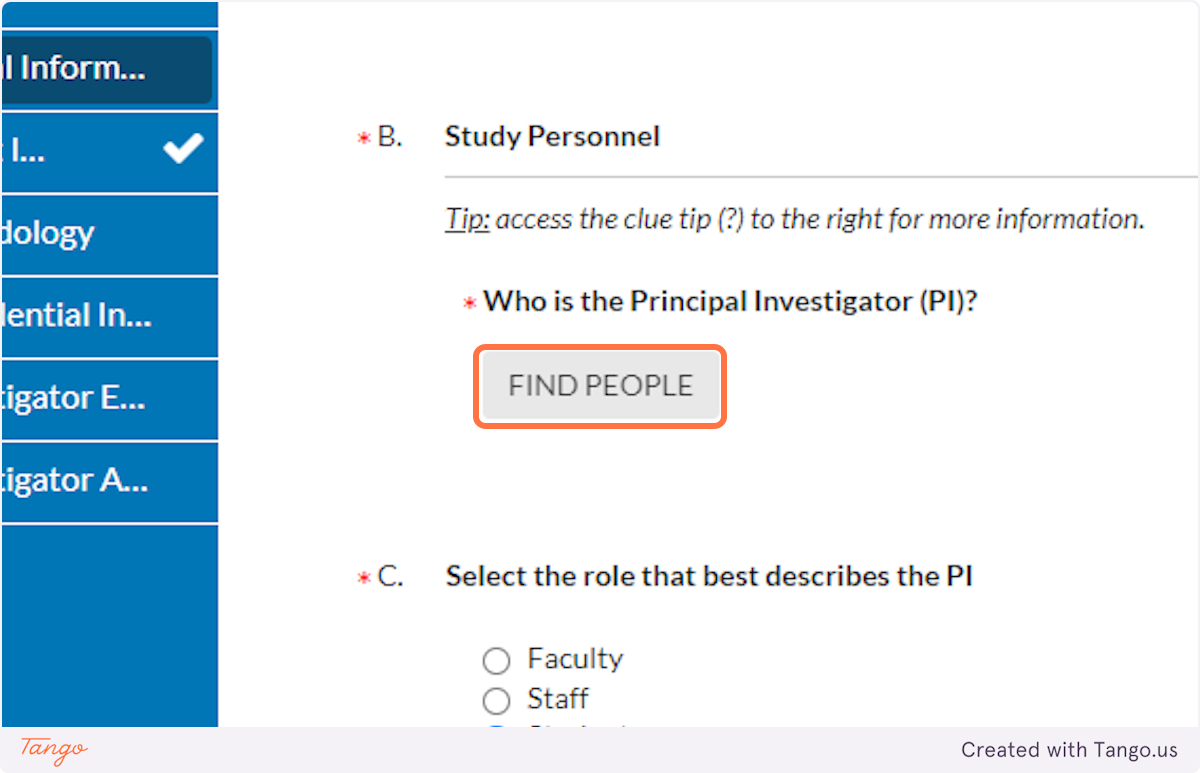
6. The next question asks who the actual Primary Investigator (PI, or head researcher) will be. Click the "Find People" button to select the appropriate person.
This might be you! Or it might be your faculty advisor, or even another student collaborator. Think carefully about your situation and " who is ultimately responsible for the conduct and oversight." If you are unsure who to enter, ask your faculty advisor.
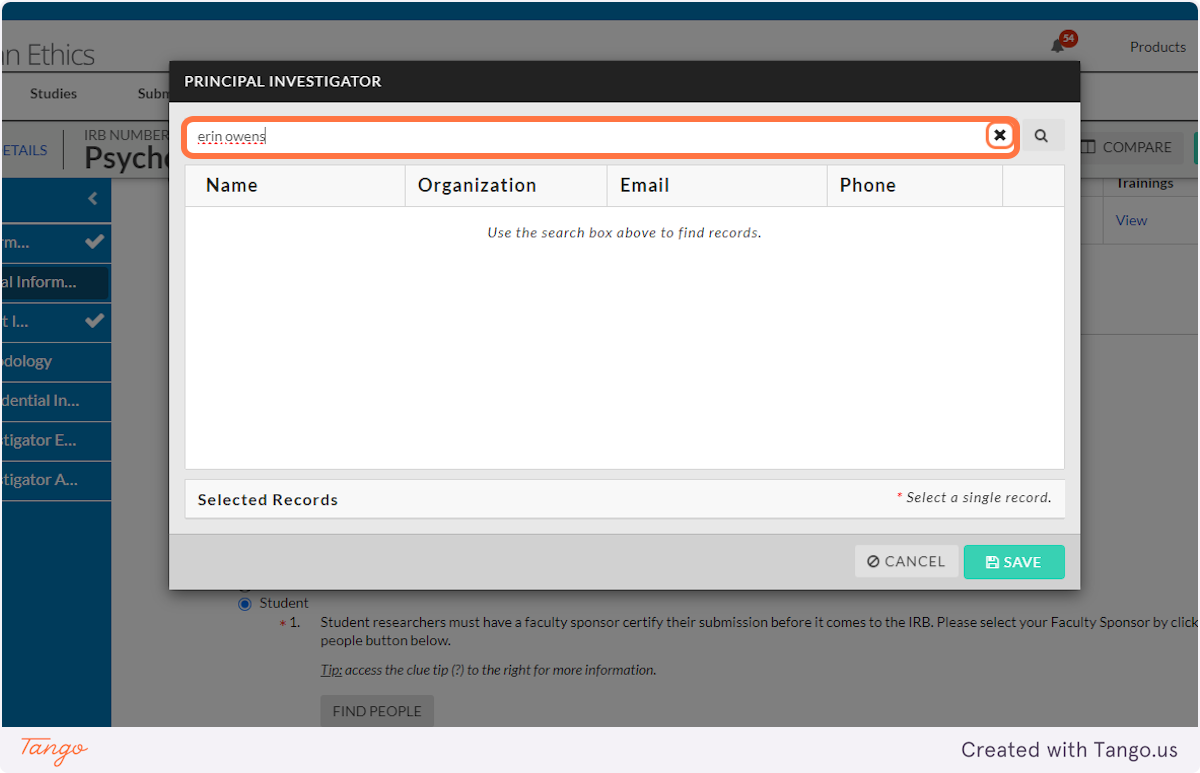
7. Enter a name (yours or another) to search for.
8. Press Enter or click the magnifying glass to search.
9. Click to select the correct name from those listed.
10. Click on SAVE
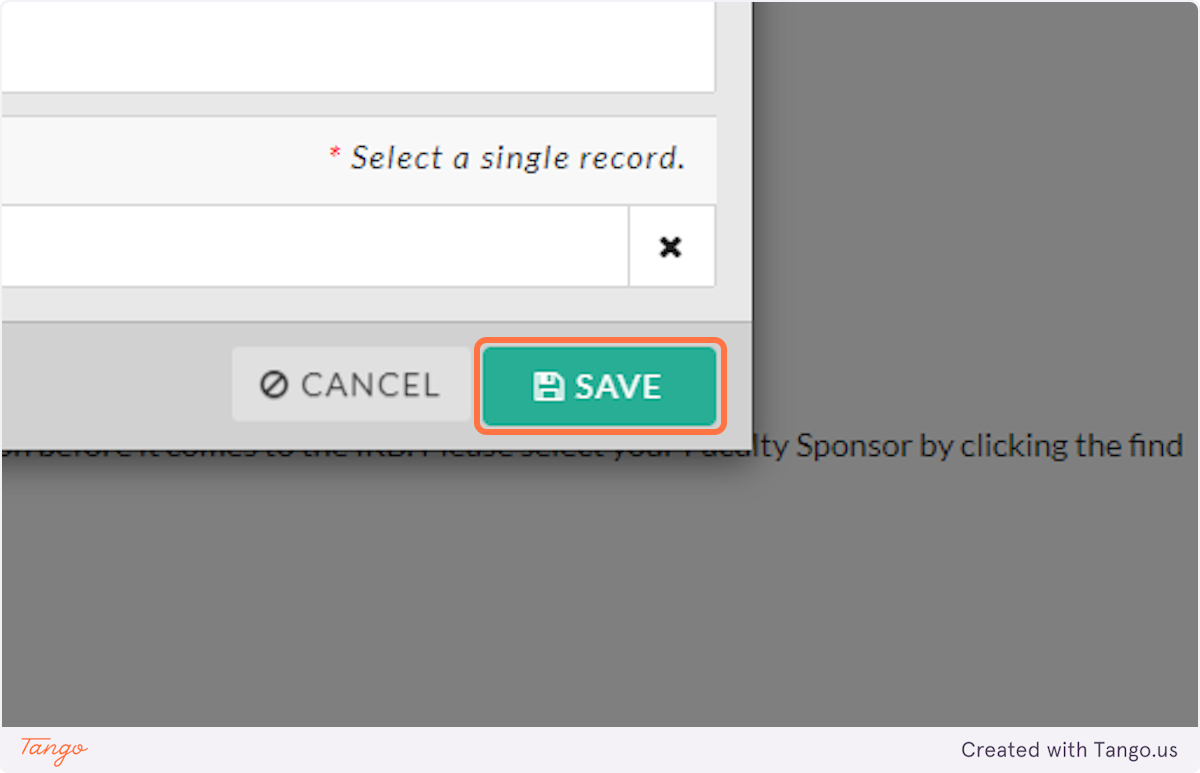
11. Based on who you selected above as the PI, click the correct button to describe that PI's role at SHSU. If you are the PI, you will choose Student.
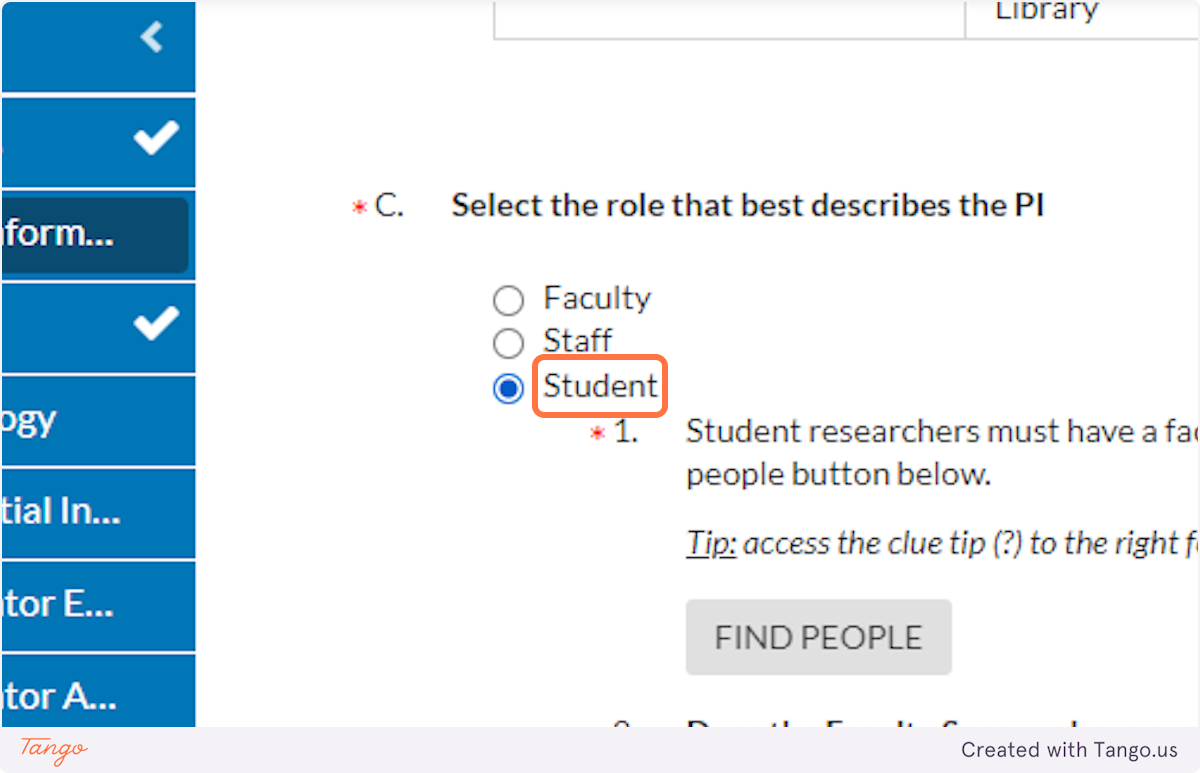
12. A note will inform you that student PIs must have a faculty sponsor, who must be identified in this IRB submission.
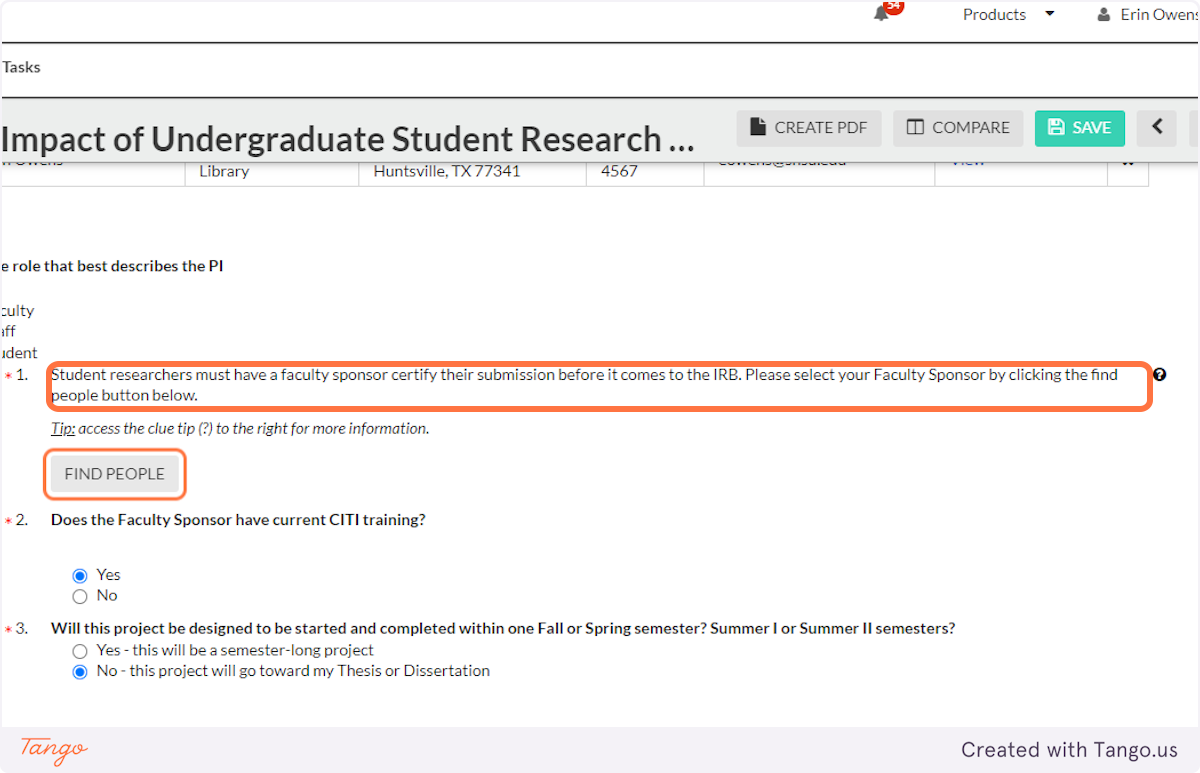
13. Click on FIND PEOPLE…
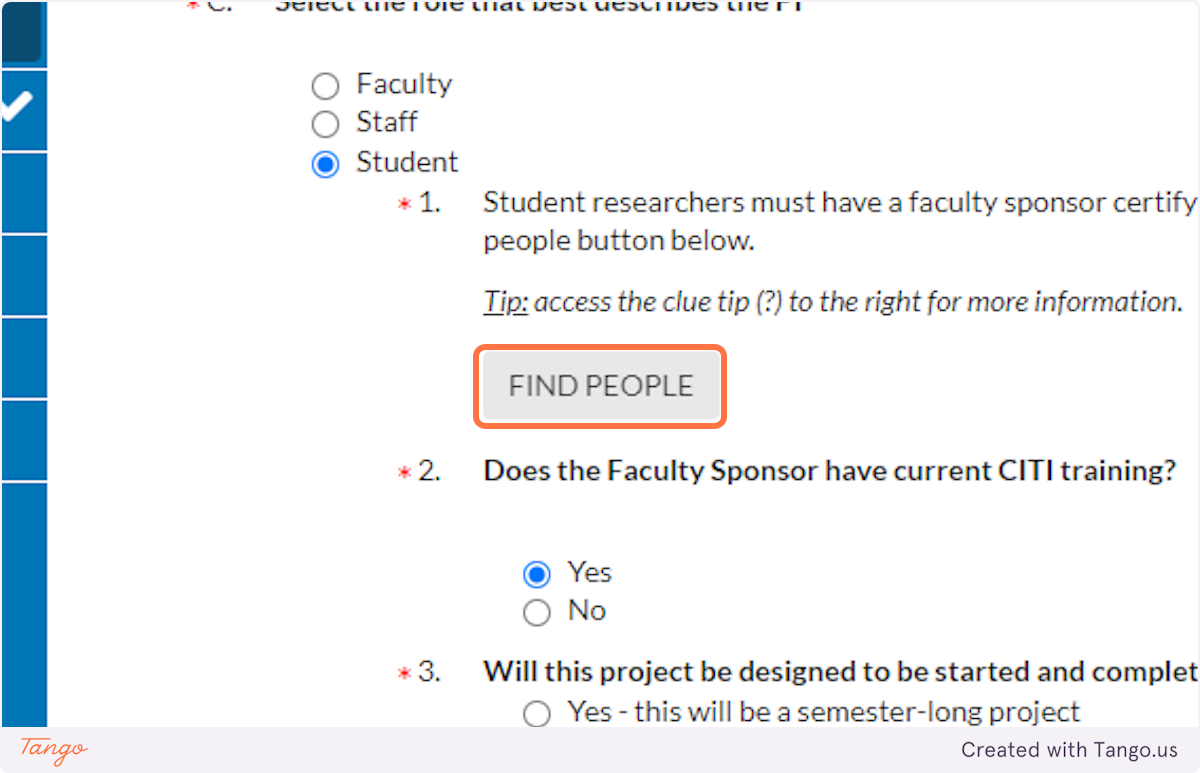
14. Enter a name to search for.
15. Press Enter or click the magnifying glass to search.
16. Click to select the correct name from those listed.
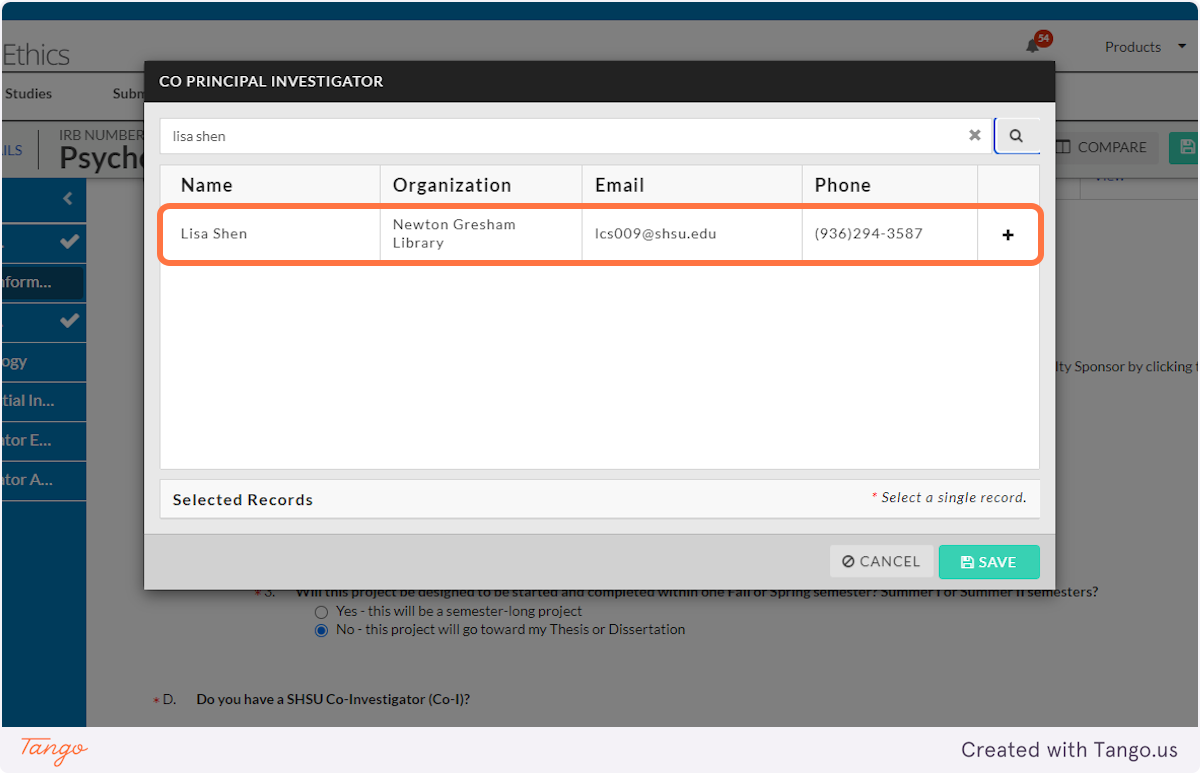
17. Click on SAVE
18. Indicate whether your faculty sponsor has up-to-date CITI training on record with SHSU's Office of Research. If you aren't sure, ask your sponsor!
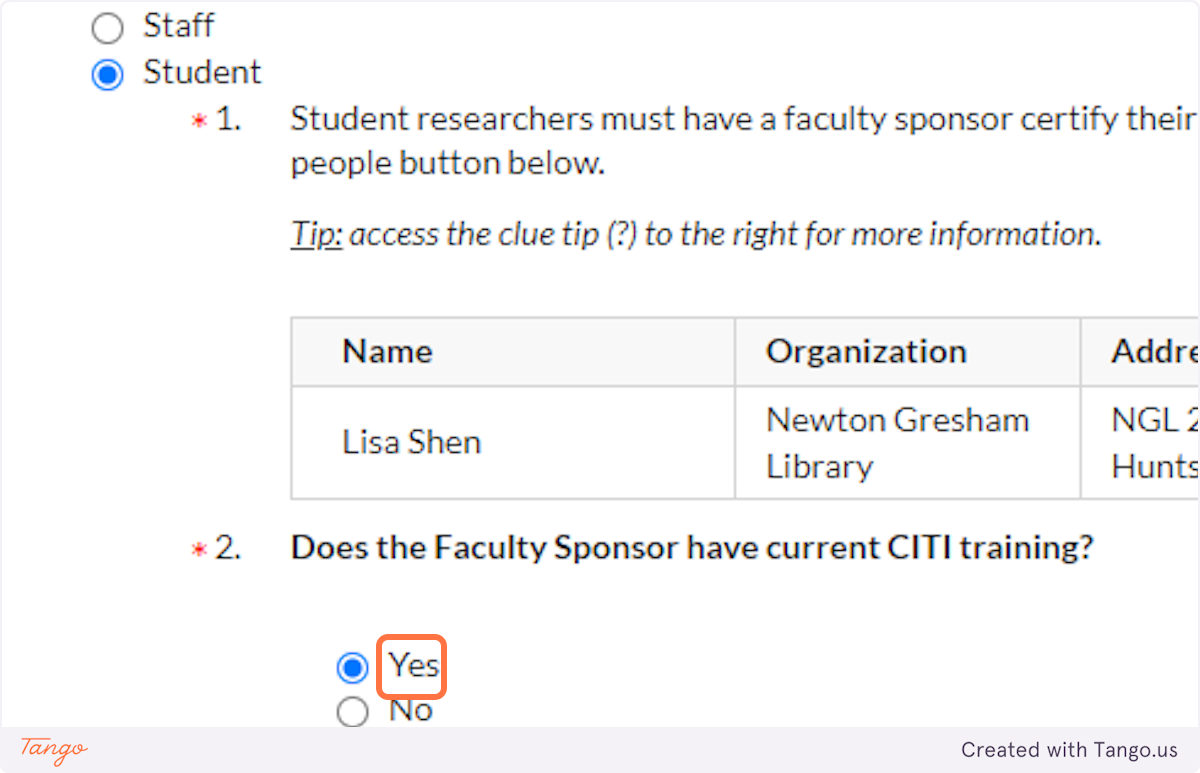
19. Select whether this is a class project that will only last for a semester...
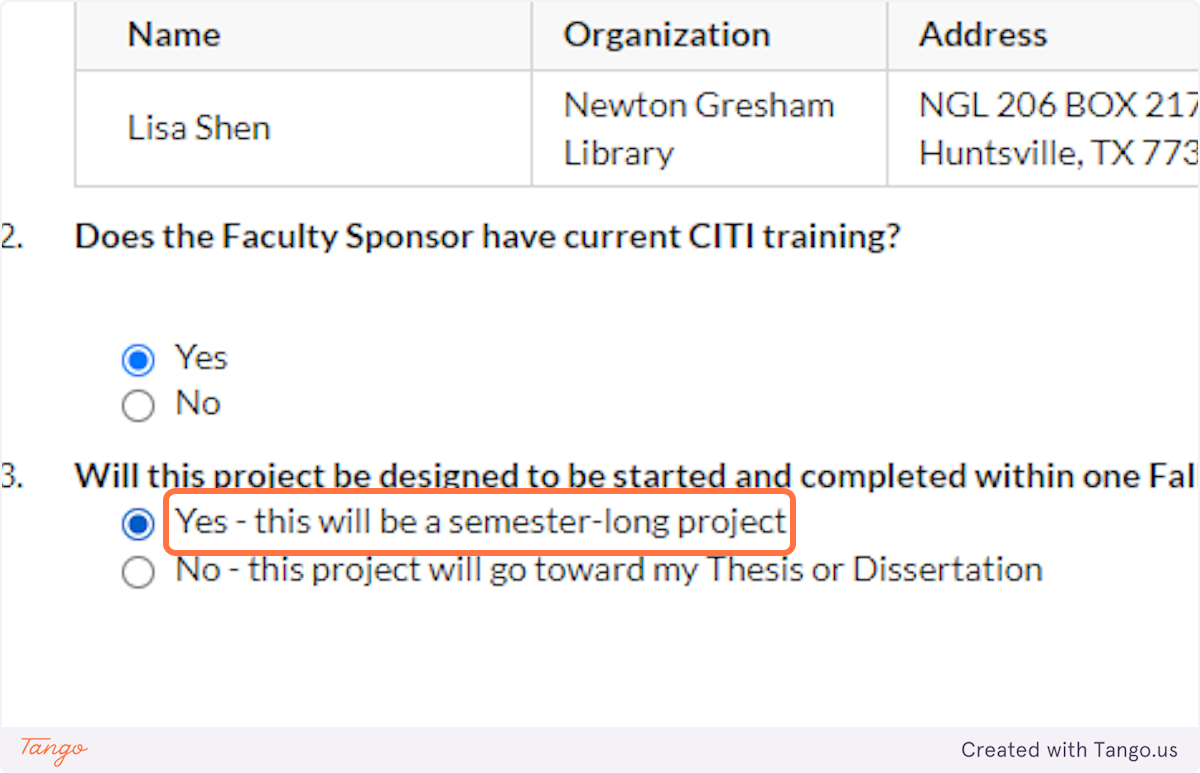
20. ...Or whether this project will be longer-term, towards your thesis or dissertation.
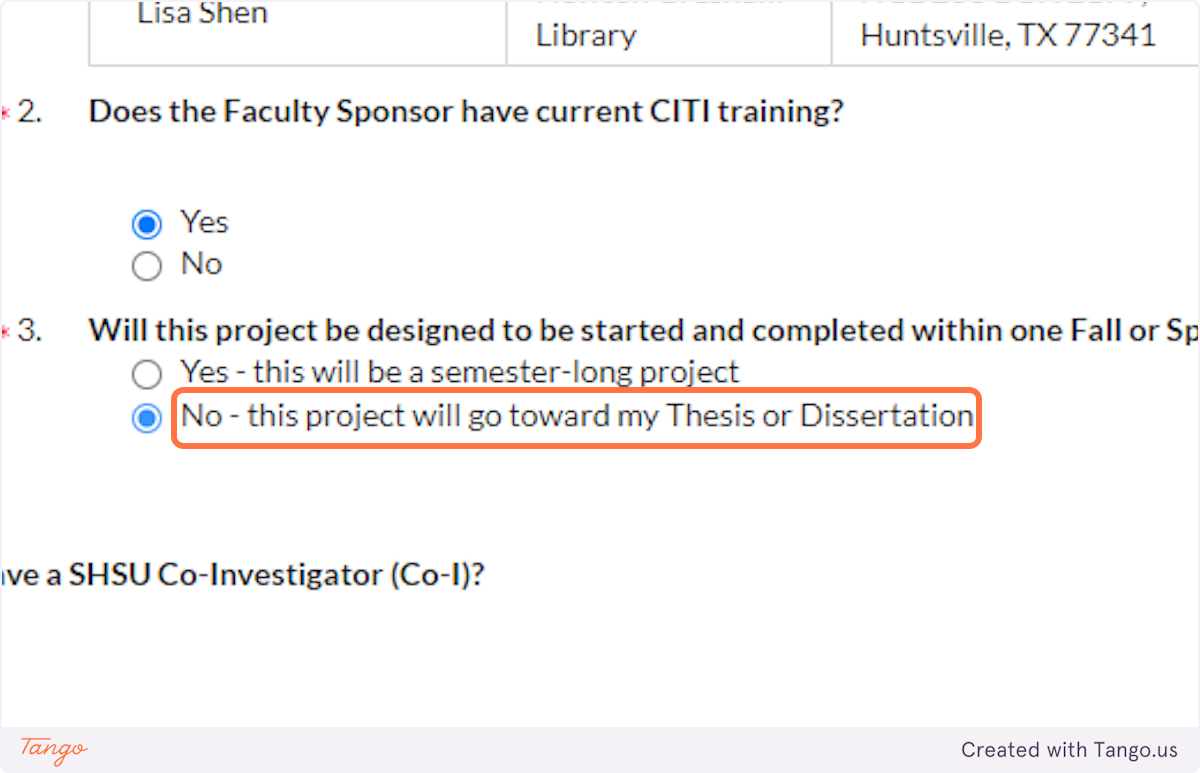
21. If you have other collaborators INSIDE of SHSU, such as other student researchers, click Yes and identify them here.
Anyone who was already identified in Section 1 does NOT need to be added again here.
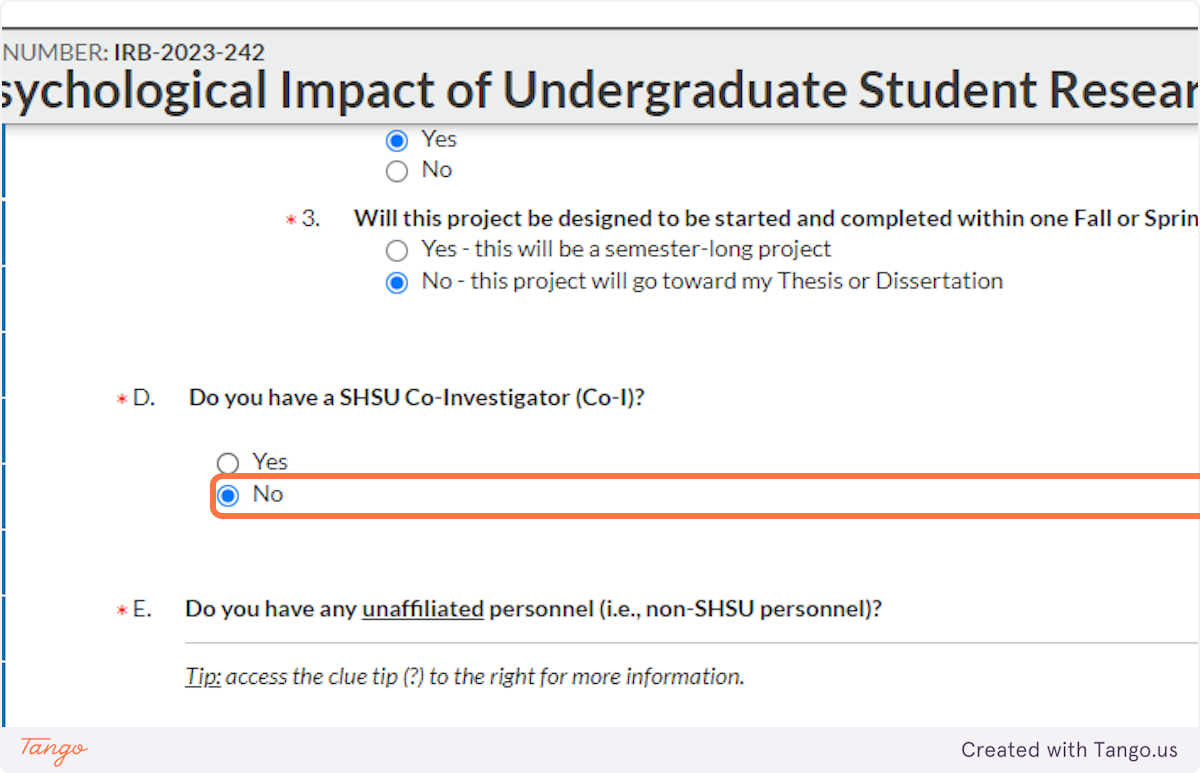
22. If you are collaborating with any other researchers OUTSIDE of SHSU, click Yes and identify them here. This will not be common for most student projects.
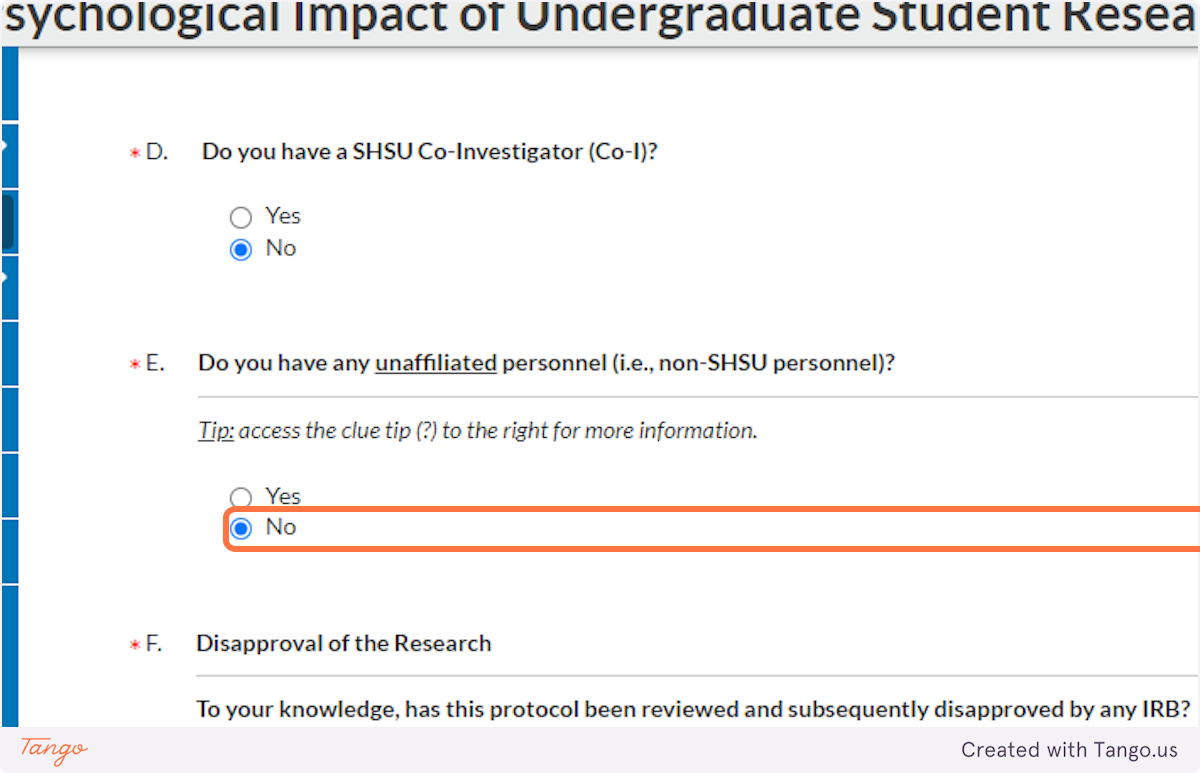
23. If this is the first time this project has been proposed to any IRB (at or outside of SHSU), click No. If this project has been proposed before AND BEEN REJECTED, select Yes.
For past disapprovals, you will need to provide details and documentation.
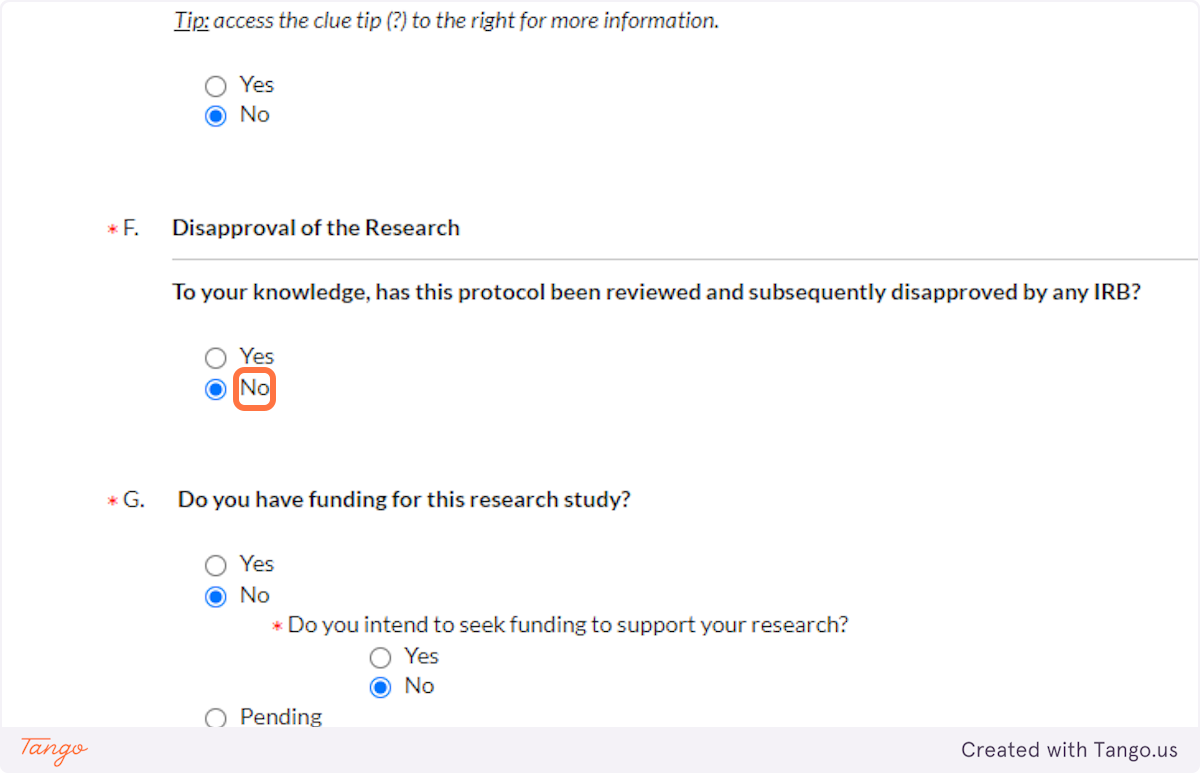
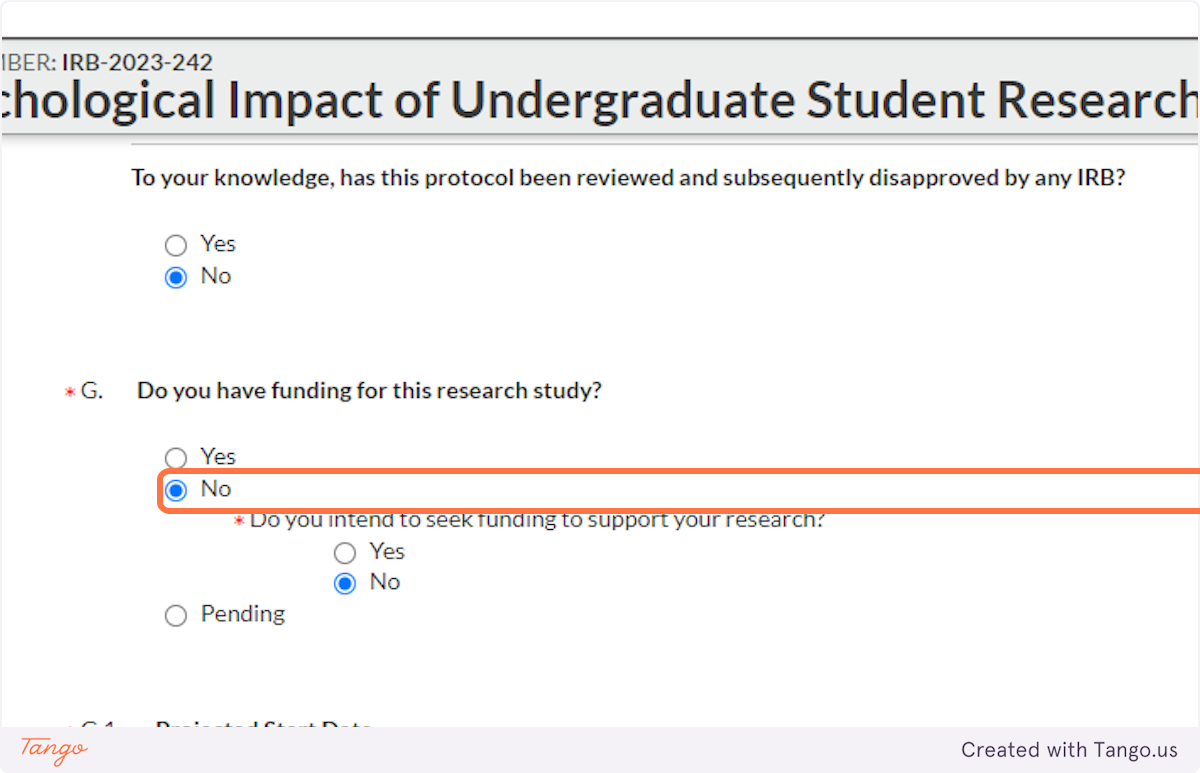
24. If you have secured grant funding, or intend to apply for funding, indicate that here. This DOES include grants from within SHSU, such as a EURECA or ORSP grant.
Note that if a funding application is pending but not yet decided, you will mark Pending, but then you will also eventually need to send the IRB an update when that funding application is either received or rejected.

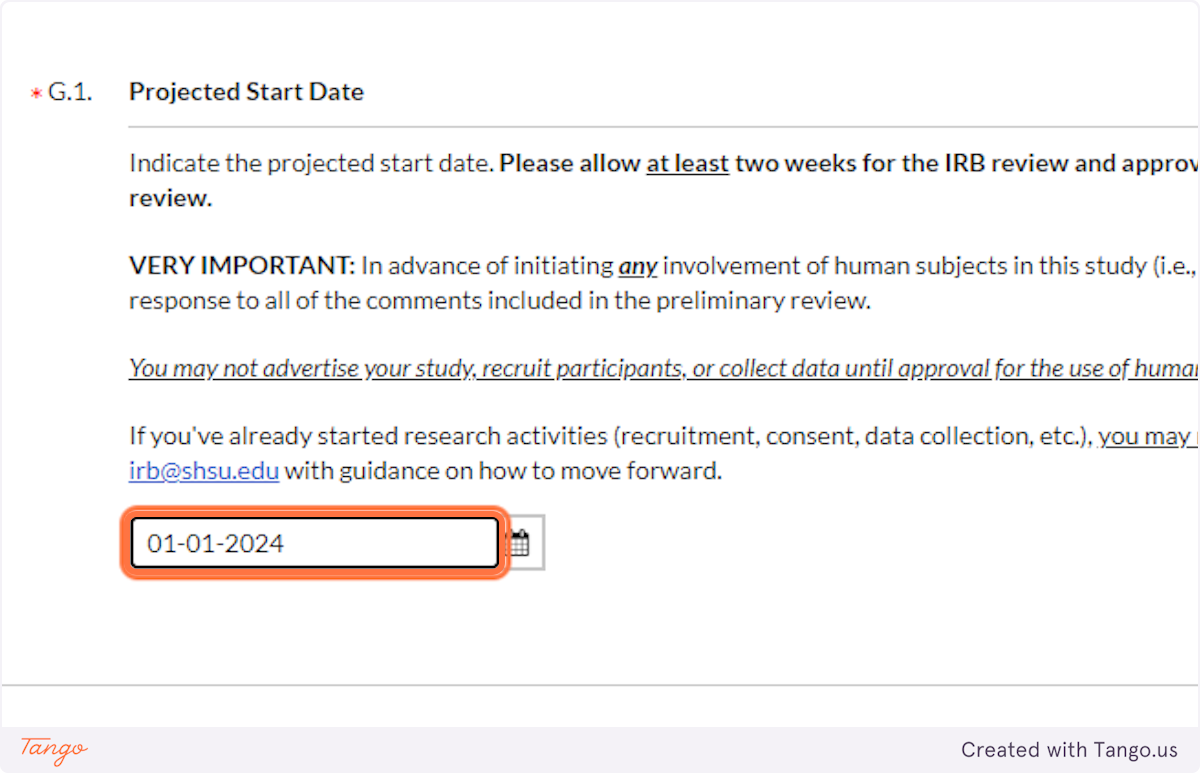
25. Enter the date AT LEAST two weeks in the future (or further) when you expect to start collecting data for this project, if it is approved.
26. Click on the arrow at the lower right to go to Section 2.
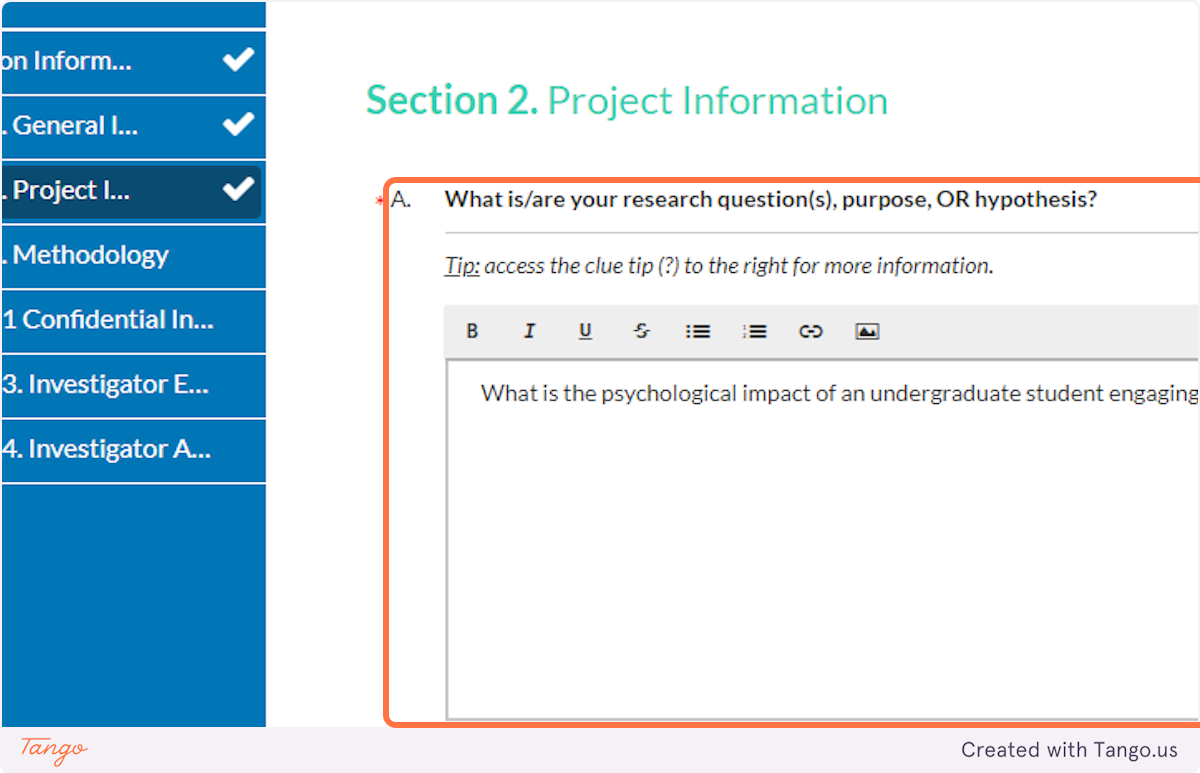
27. In this field, enter your research question(s), hypothesis, or a clear, concise statement of the research purpose. If you are completing a thesis or dissertation, the purpose/questions here should match those in your thesis or dissertation.
Note: Do NOT include explanatory background of the problem or literature in this field. Just state clearly and concisely the overall question or phenomenon you are studying and what you hope to learn about it.
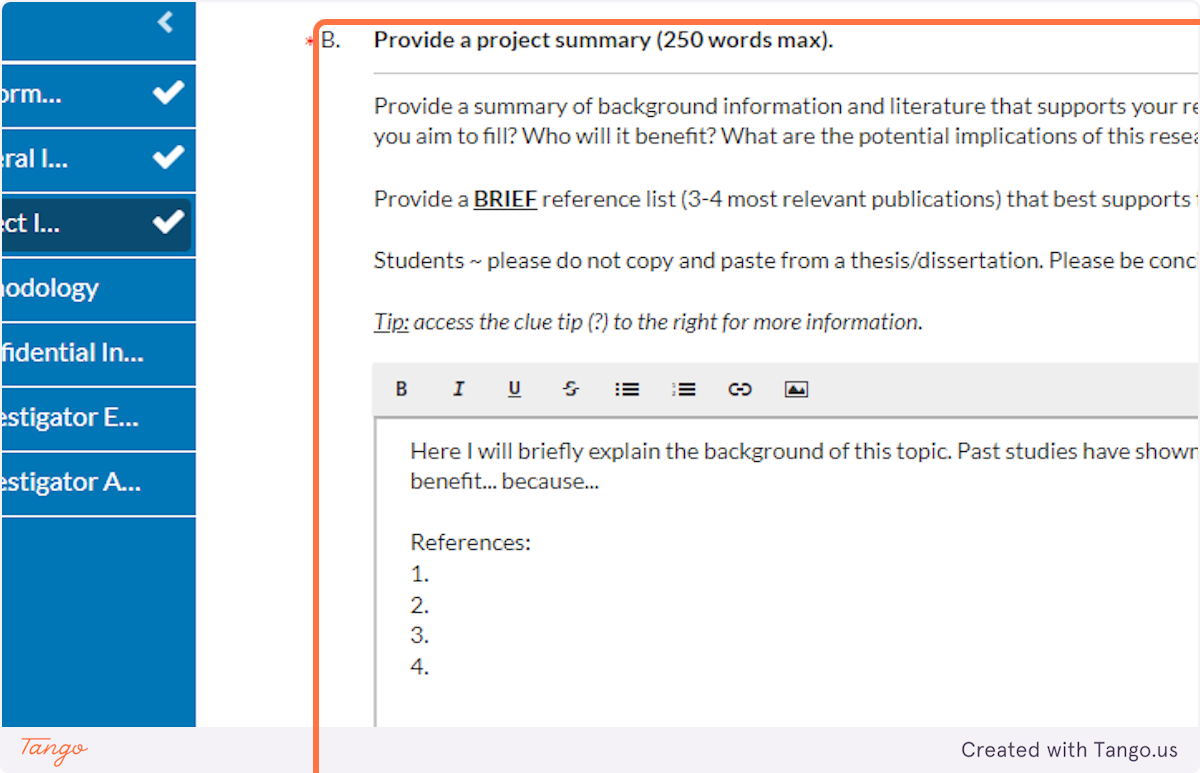
28. Your project summary in this field will SUMMARIZE what would be the introduction/background and literature review in your complete paper. This is where you should briefly explain the context and importance of your research question for someone who is not yet familiar with it.
Do not cut and paste full sections from your thesis or dissertation; this should be a very short summary. Cite only the 3-4 most crucial publications to support what you will be doing and why.
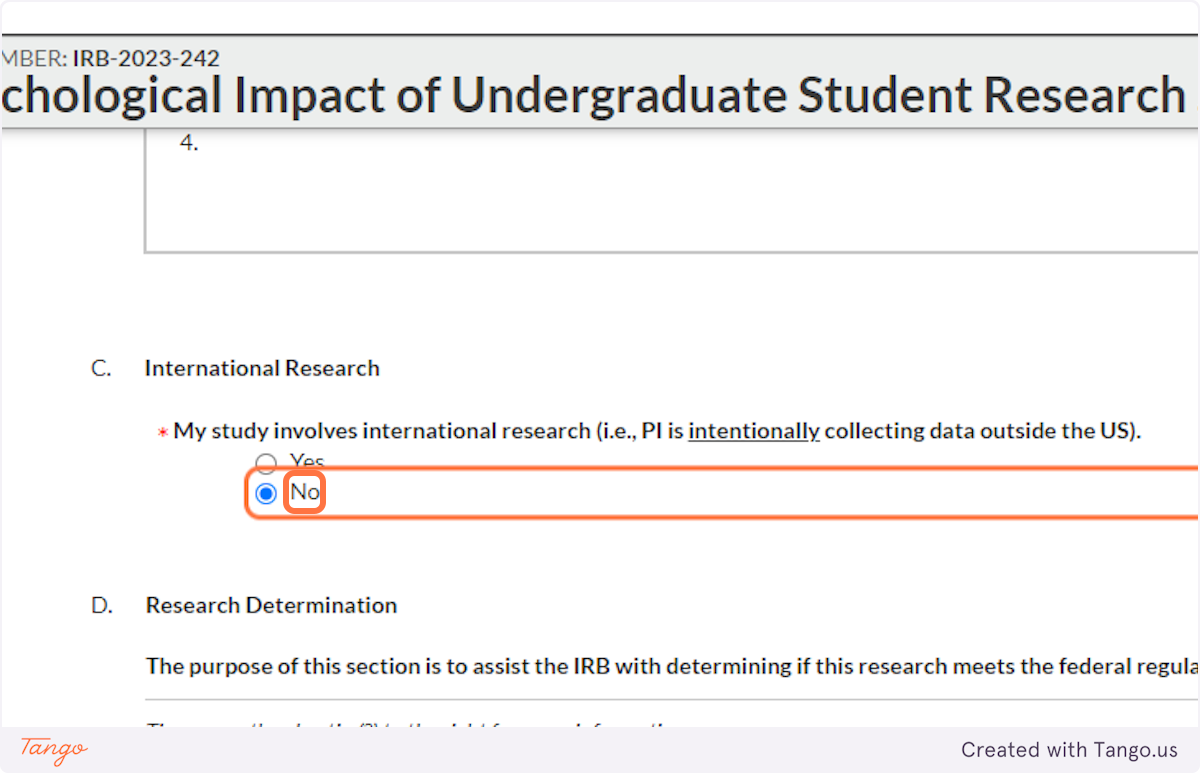
29. Are you deliberately seeking to collect data from outside the U.S.? If so, additional regulations may apply.
If the answer to this question is Yes, be prepared to provide additional details about what country or countries your data will come from and whether you have identified their equivalent of an IRB for approval of your research.
30. If this research will be applied to support a thesis, dissertation, presentation, publication, or similar, select YES for this question.
If your project will not be applied in ANY way that contributes to generalizable knowledge, then it may not qualify as "research" as defined by federal regulations, and you may not need IRB approval. You are encouraged to call the SHSU IRB office and ask for clarification or guidance.
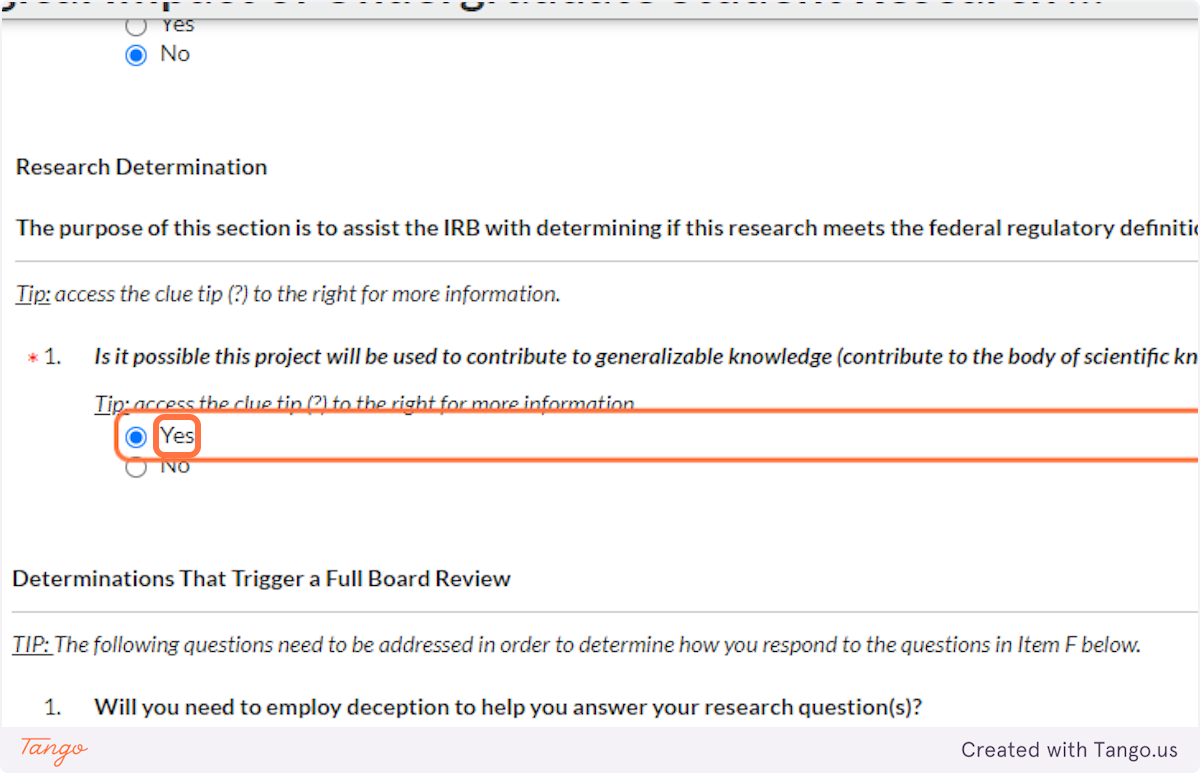
31. In most disciplines, student projects are unlikely to involve deception -- not being truthful with your research participants -- but if that applies to your study, you must indicate it here. This will trigger a more rigorous review of your proposal.
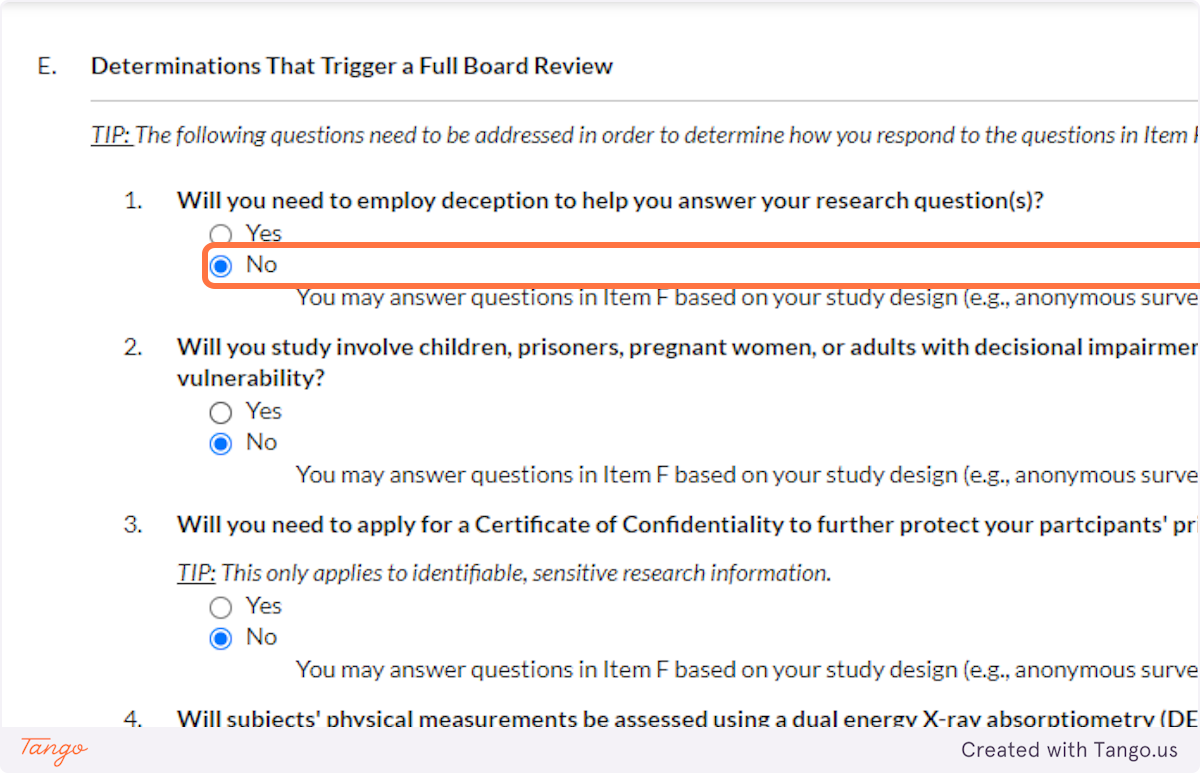
32. Indicate whether you will be working with vulnerable populations such as those listed. Again, answering Yes to this question will trigger a more rigorous review to ensure participant safety.
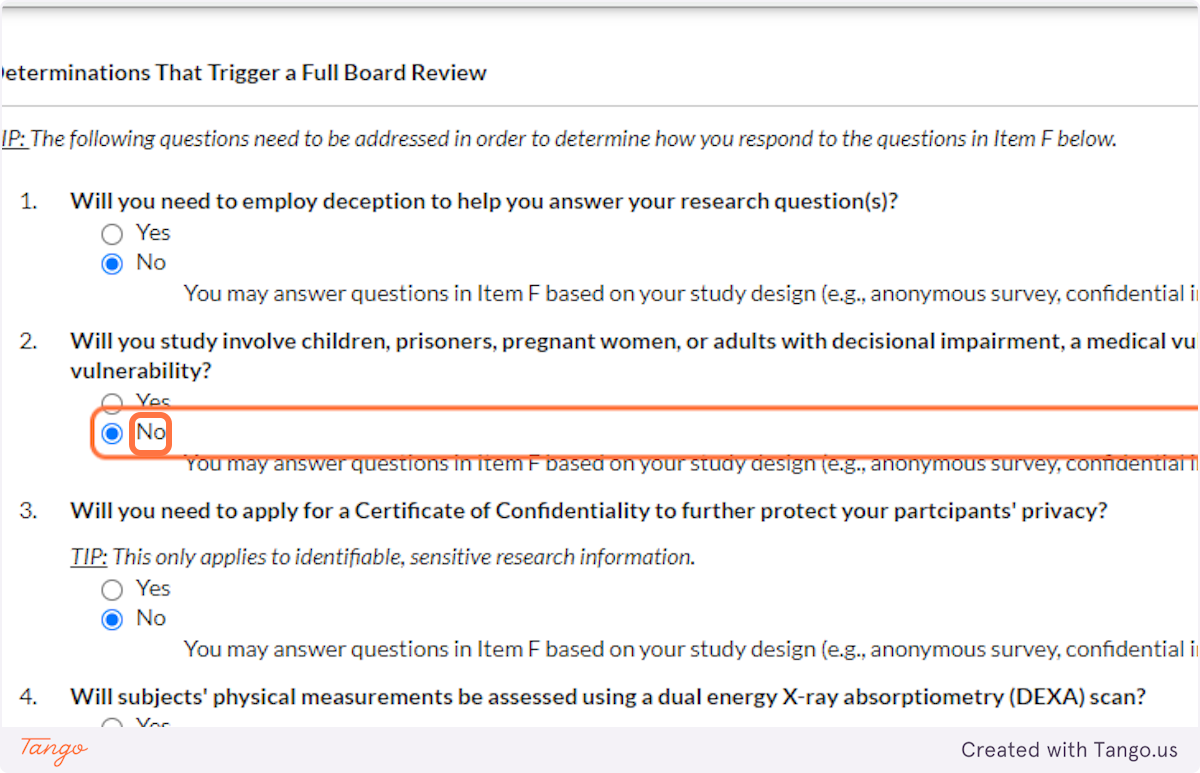
33. A Certificate of Confidentiality will most often NOT apply to student research projects. If this applies to you, it is likely something that you and your faculty advisor have already discussed.
If you are uncertain, speak with your advisor or the SHSU IRB for clarification. Answering yes here will trigger a more rigorous review of your proposal.
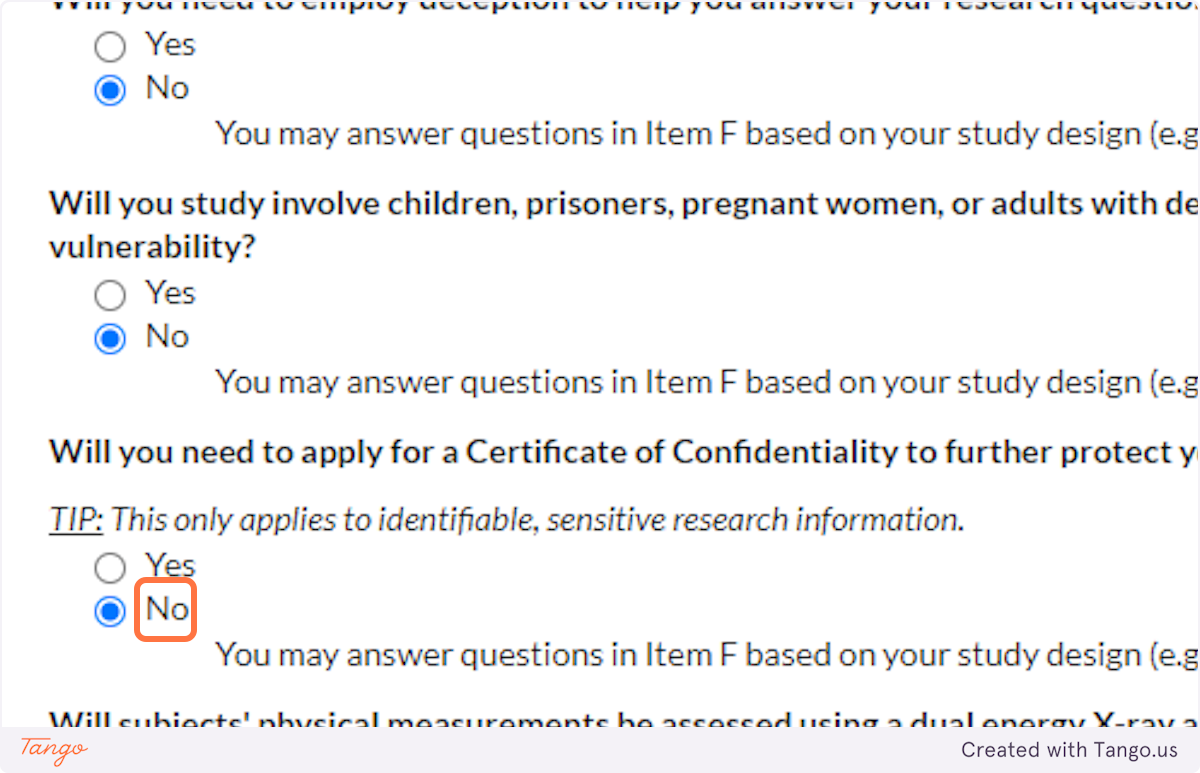
34. Unless your methods involve a DEXA scan, select NO for this question. Answering yes here will trigger a more rigorous review of your proposal.
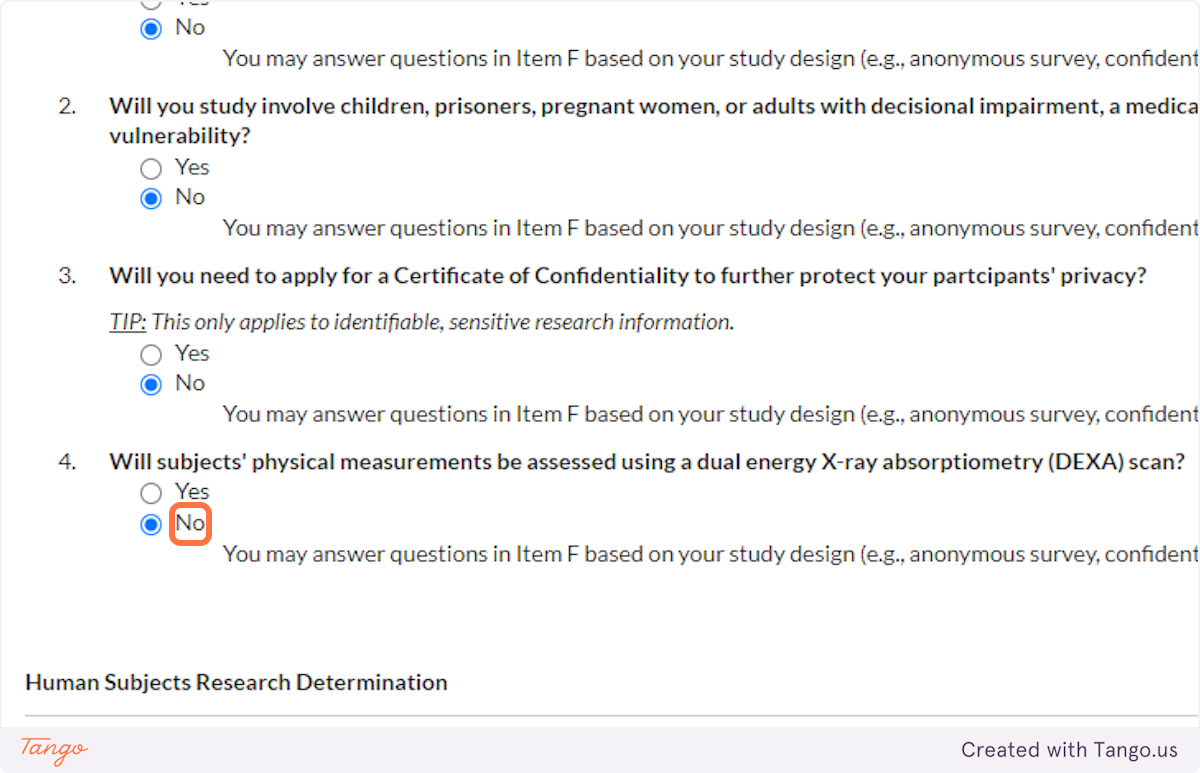
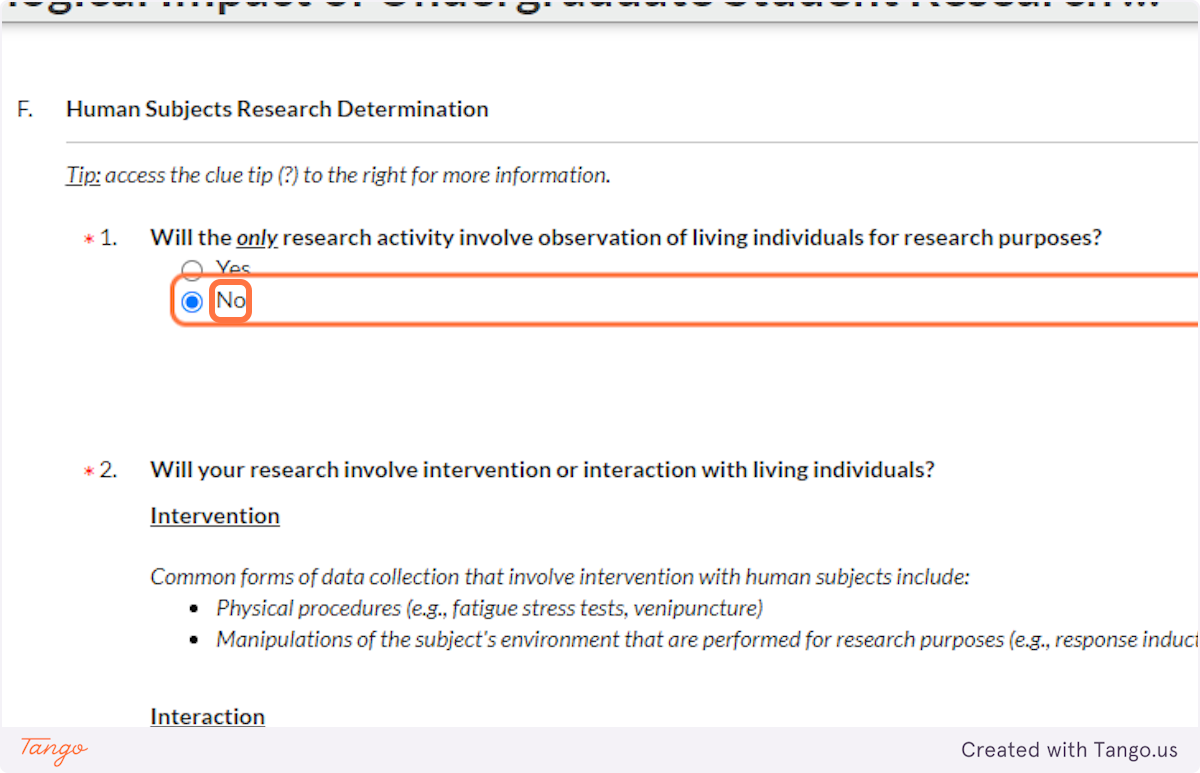
35. If you will be interacting with your human participants in some way -- whether through face to face conversation, email, online survey, syringe, blood pressure cuff, etc. -- you will answer NO to this question.
You should also answer NO if you will be observing participants, with or without interaction, in a PRIVATE place. Note that a "public school" is a "private place" for the purposes of this question.
If you will be sitting in a PUBLIC place like a park and watching people without interaction, you may need to choose YES here. You will likely not need to enter any more information about your project or receive IRB approval, if you are not actually interacting with human participants.
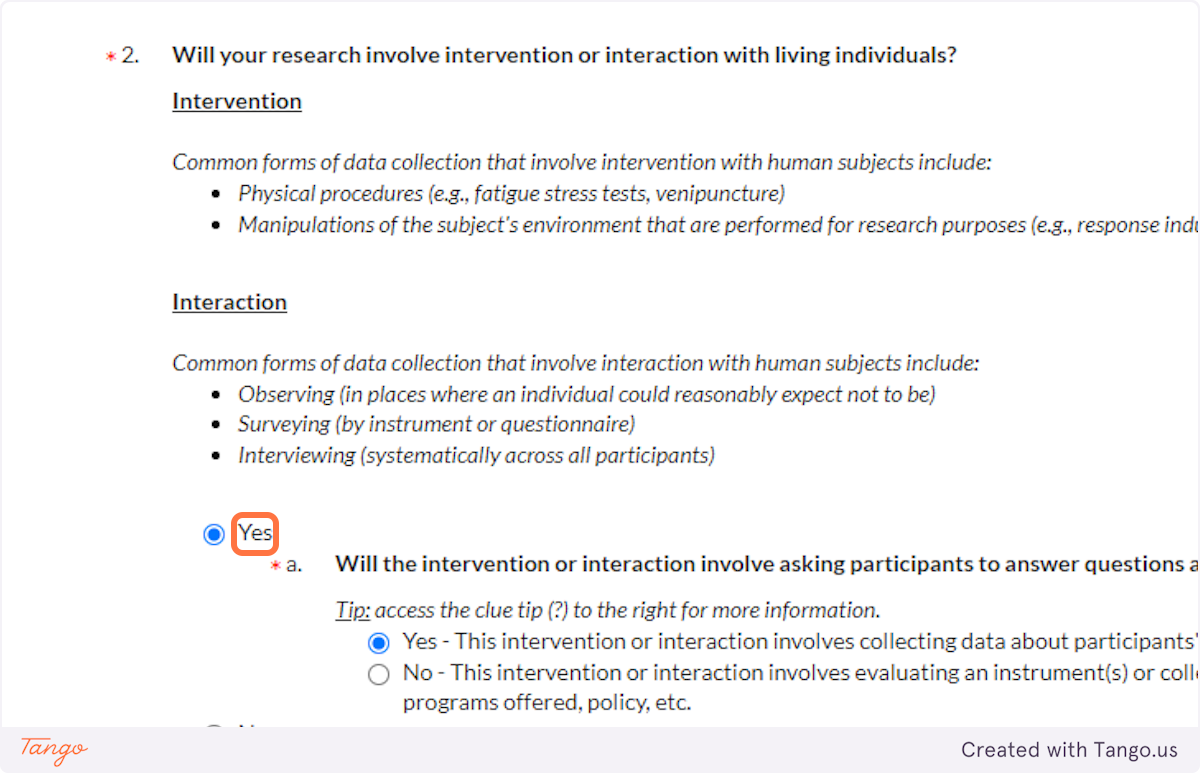
36. Most research projects that require IRB approval involve interacting with human participants in some way -- whether through face to face conversation, email, online survey, syringe, blood pressure cuff, etc. If that's your case, choose YES here.
There are some exceptions to this, for example if you are conducting secondary research with identifiable private information or biospecimens whose collection was unrelated to this project. If your research falls under one of these exceptions, and you are truly NOT interacting with human participants, then choose NO here. You will then answer accordingly to the question 4 regarding secondary research, and you will need to complete an additional application section detailing the secondary data source and the original purpose for its collection.
37. If your research only involves analyzing a public dataset in which participants are unidentifiable, you will not require IRB approval; select Yes here. Otherwise, select NO to continue pursuing IRB approval.
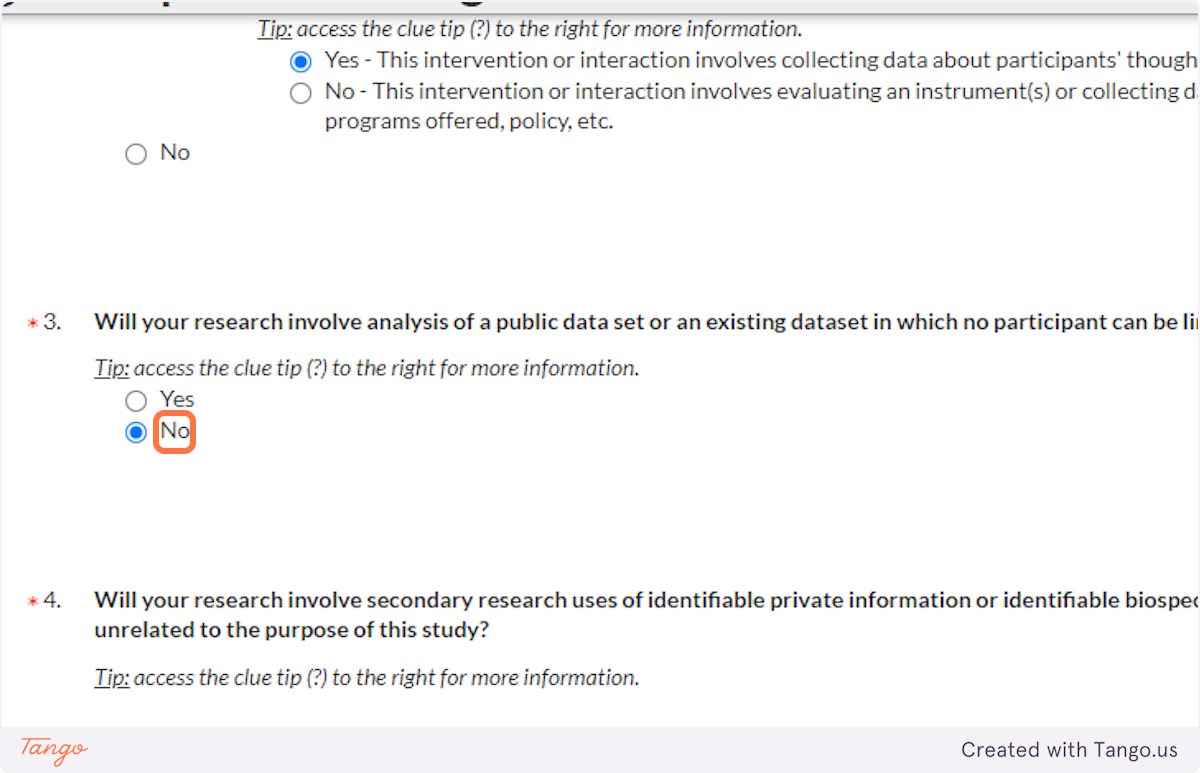
38. As discussed above, if you are doing secondary research with identifiable information or specimens, select Yes here. Otherwise, select NO.
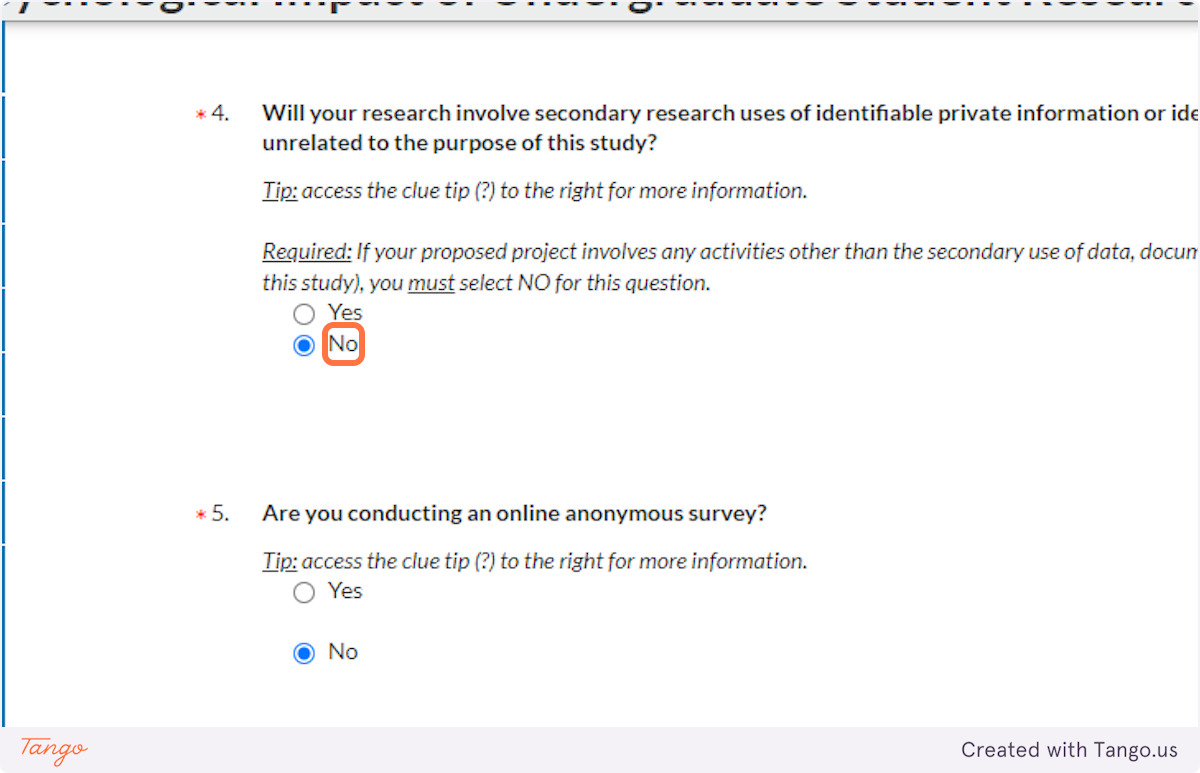
39. If you are conducting ONLY an anonymous online survey, choose Yes here. This will trigger creation of a new section in the application that will ask you more detailed questions to ensure anonymity of the survey.
If you are NOT conducting an online survey, or it is not online and anonymous, select No instead.
Note: For logical consistency, if you select Yes here, you also should have selected Yes above for "interaction or intervention."
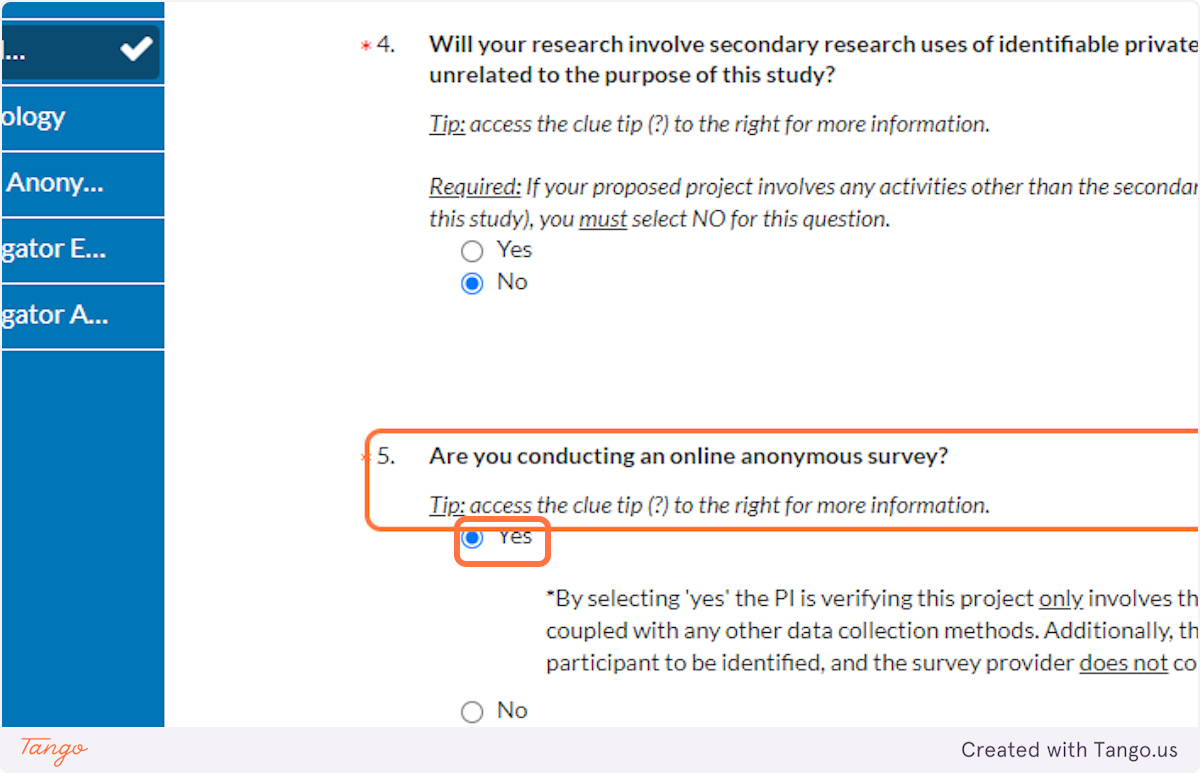
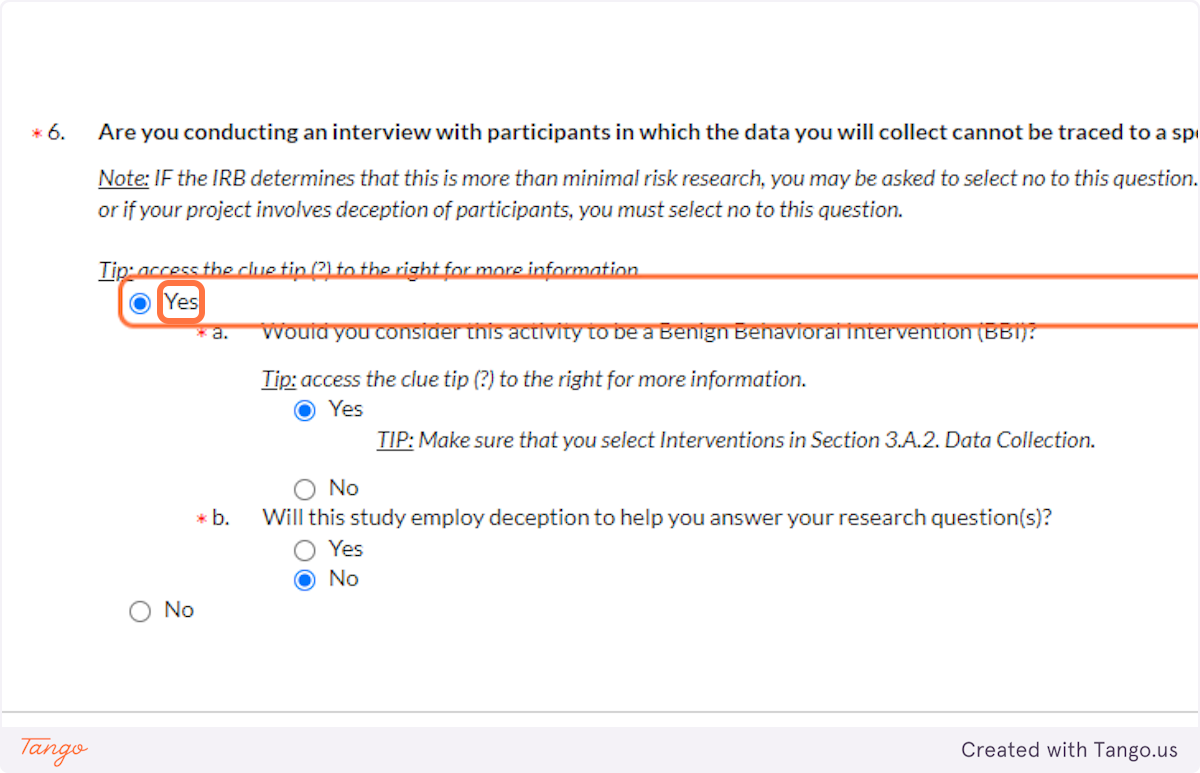
40. If you are not conducting interviews at all, your answer to question 6 will be NO. If you are: Ask yourself whether the data could be traced to a participant, and answer Yes or No accordingly.
If you answer Yes:
You will be asked further whether or not you consider this activity "benign" (brief, painless, not invasive or embarrassing). If you are unsure, definitely click on the question mark icon to read more details about benign interventions. Check with your faculty advisor if you find you still need clarification.
You will also be asked again whether your study involves deception, or being untruthful with participants.
41. Click the arrow at the lower right to on go to Section 3.
Completing the Application Information Page and Application Sections 1 and 2
- << Previous: Asking a Research Question
- Next: Data & Research Methods >>
- Last Updated: Oct 21, 2024 5:38 PM
- URL: https://shsulibraryguides.org/undergradresearch
Newton Gresham Library | (936) 294-1614 | (866) NGL-INFO | Ask a Question | Share a Suggestion Sam Houston State University | Huntsville, Texas 77341 | (936) 294-1111 | (866) BEARKAT © Copyright Sam Houston State University | All rights reserved. | A Member of The Texas State University System

- Find My GCO
- IACUC applications (Cayuse Animal Management System)
- IBC Applications (eMUA)
- IRB Applications (RASS-IRB) External
- Institutional Profile & DUNS
- Rates and budgets
- Report external interests (COI)
- Join List Servs
- Ask EHS External
- Research Development Services
- Cornell Data Services External
- Find Your Next Funding Opportunity
- Travel Registry External
- RASS (Formerly Form 10 and NFA) External
- International research activities External
- Register for Federal and Non-Federal Systems
- Disclose Foreign Collaborations and Support
- Web Financials (WebFin2) External
- PI Dashboard External
- Research metrics & executive dashboards
- Research Financials (formerly RA Dashboard) External
- Subawards in a Proposal
- Proposal Development, Review, and Submission
- Planning for Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Budgets, Costs, and Rates
- Collaborate with Weill Cornell Medicine
- Award Negotiation and Finalization
- Travel and International Activities
- Project Finances
- Project Modifications
- Research Project Staffing
- Get Confidential Info, Data, Equipment, or Materials
- Managing Subawards
- Animals, Human Participants, r/sNA, Hazardous Materials, Radiation
- Project Closeout Financials
- Project Closeout
- End a Project Early
- Protecting an Invention, Creation, Discovery
- Entrepreneurial and Startup Company Resources
- Gateway to Partnership Program
- Engaging with Industry
- Responsible Conduct of Research (RCR)
- Export Controls
Research with Human Participants
- Research Security
- Work with Live Vertebrate Animals
- Research Safety
- Regulated Biological Materials in Research
- Financial Management
- Conflicts of Interest
- Search
Submit or Manage Your IRB Protocol
Submission requirements for irb protocol approval.
UPDATED February 1, 2023
NIH Data Management and Sharing Policy Update: Learn more here . TRAINING UPDATE: Effective October 1, 2022, all Cornell study personnel involved in Exempt research protocols are now required to complete CITI training in human participant research ethics. See details here . For more information about Cornell IRB training requirements, visit the IRB Training webpage .
Avoid delays by understanding the IRB process from protocol submission to review, revision, and approval.
All research involving human participants conducted by Cornell faculty, staff, and students must be reviewed in advance by the Cornell IRB, or determined to be exempt from IRB review by IRB staff. Read on for more information about the IRB submission process, including links to important forms and templates.
Not Human Participant Research
Not all interactions with humans or data collected from humans meets the definition of "human participant research" that is subject to the federal regulations.
If your project is considered research under IRB rules, you must submit an application and receive either confirmation of exemption, or a letter of approval from the IRB before research can begin. Unsure about whether your project is regulated human participant research? Use our decision tree , contact a member of the IRB staff , or review our guidance document, Determining Whether a Research Activity Needs IRB Review .
Get your Submission Right the First Time
The single most significant cause of delays in protocol approval is submitting an incomplete application to the IRB. We urge you to take the time to send in a complete application the very first time, use the consent templates , check out the FAQs and guidance documents, and complete required training before you submit your application.
Common omissions that cause delay include:
- Including research personnel who have not completed or renewed required IRB training
- For students, sending in an application without a faculty advisor's support. (Note: in the new IRB system, RASS-IRB, upon the submission of your protocol, your advisor will receive an email prompting them to provide their attestation)
- Not including a needed consent document or script, or failing to submit survey instruments or interview guides when these procedures will be used in the research
In addition to missing items or information, these common mistakes can delay the IRB approval:
- Leaving relevant questions blank on the application form
- An insufficient consent document – Consent scripts/documents that are missing fundamental elements of consent will result in the need to revise and resubmit
- Using unclear, academic or “jargon”-laden language in the application or consent document, overstating potential benefits to participants (it is fine if there is no benefit to participants!), insufficient attention to issues of confidentiality and data protection, and missing or out of date contact information for the IRB.
What Happens After I Submit My Protocol?
The process your application takes from here depends upon the completeness and quality of your submission (see above) and the level of review that the IRB needs to apply, as governed by the regulations and Cornell IRB policy:
- Exempt means that your protocol is regulated research, but that it poses no real risks to participants and fits into certain categories of research that can be reviewed and approved by a member of the IRB office staff. The term "exempt", which comes from the federal regulations, is confusing because does NOT mean that your study is ‘exempt’ from any review by the IRB office. Rather, an exemption review is a more minimal one, and can done by staff as opposed to a voting member of the IRB committee.
- Expedited means that your protocol poses no more than minimal risk to participants, but must be reviewed and approved by a voting member of the IRB committee according to the federal regulations. "Expedited" does NOT mean that you should expect the IRB to process your application on an accelerated schedule.
- Full Board means that your protocol poses more than minimal risk to participants and must therefore be reviewed by the full IRB committee at one of its monthly meetings.
How Long Will it Take to Receive IRB Approval?
It depends. How long you will need to wait for approval depends on a number of factors outside your control -- including what the federal regulations require given the type of research you want to do, and the volume of other IRB submissions making their way through the IRB process at the same time -- and many that are within your control -- how complete and clear your application is, and how thoughtfully and quickly you respond to any feedback or requests for edits that you receive from the IRB.
If you submit a complete application to the IRB, you can generally expect the following turnaround times:
- If your project is exempt: 2-3 weeks (rolling review)
- If your project receives expedited review: 3-4 weeks for initial approval; 1-2 weeks for an amendment (rolling review)
- If your project needs full board review: a minimum of 4-6 weeks (for initial, continuing, or amendment approval at a convened committee meeting). See the IRB Committee page for meeting dates and information about submission deadlines.
If your application is incomplete, please expect to add 5-7 days to the ranges listed above.
NOTE: During heavy protocol submission periods (e.g., mid-semester), reviews will take longer.
Prepare Your Protocol for Submission
To ensure that your submission is reviewed in a timely manner, please submit a complete application and all supplemental documents via RASS-IRB . You can find help documentation for how to use the system on the RASS Guide Site .
- First, complete the online IRB training and ensure all research personnel on your project have done so as well. The IRB will not be able to approve an expedited or full board protocol until all persons named on the IRB protocol are current in their IRB training.
- Create a new protocol for initial approval.
- Request an amendment to make changes to your protocol . Note that if your protocol received an exemption determination prior to the implementation of RASS-IRB , you can continue to submit requests to amend that exempt protocol by sending an email to [email protected], identifying the protocol (protocol number, title, PI), describing the proposed changes, and attaching any documents that need to be changed, such as consent forms. Otherwise, all other amendment requests must be submitted through RASS-IRB .
- Request continuing review. Annual continuing review (sometimes called renewal) is generally required for protocols that received Full Board (Convened Committee) initial review. Rarely, a continuing review may also be required for protocols that received Expedited review (e.g., upon a sponsor's request). You can amend your protocol and submit a continuing review at the same time.
- Prescreening (Program Development) Approval: If your study procedures or materials are not fully developed, but a sponsor is requesting proof of IRB approval for your project, the IRB office can grant an administrative Prescreening (sometimes called Program Development) approval for a very limited purpose (usually to enable the release of grant funds). This type of administrative approval does not permit any interaction with human participants, including recruiting. Once the project and study instruments are developed and finalized, you must amend the protocol in RASS-IRB to receive a complete IRB review and approval (or determination of exemption). Contact the IRB office to determine whether this type of review may be appropriate for your circumstances.
- IRB Reliance (or Authorization) Agreement: If you are involved in collaborative or multi-site research and want (or need) to have one institution's IRB review the entire research study, Cornell will consider entering into an IRB Reliance Agreement (sometimes called an IRB Authorization Agreement). In this case, one IRB is considered the IRB of Record or single IRB (sIRB). These agreements are negotiated by the IRB staff from each participating institution on a case-by-case basis. Cornell will consider entering into a Reliance Agreement when doing so makes sense from an administrative and research perspective, or is required by a sponsor; however, Cornell will not enter into a Reliance Agreement when the research is exempt from IRB review. You can request the use of a Reliance Agreement in RASS-IRB .
- Protocol Closure: To document completion of your research project and close the protocol, submit a closure request in RASS-IRB (one of the options at the top of the protocol record).
- Fill out all relevant sections of the application and ensure information is complete and accurate.
- informed consent/assent documents (form or oral consent script) . Note: Use our templates .
- debriefing documents (for studies involving incomplete consent disclosure or deception)
- data collection instruments (surveys, interviews, focus groups and/or interview guides)
- study procedures (narrative description of the steps involved in an experiment)
- standard operating procedures (related to biospecimen collection or device use)
- letter/email of support or permission (if applicable)
- recruitment materials (oral script/flyer/social media content, etc.)
- any other study-related documents
- Submit your application and all study materials through RASS-IRB .
What Happens Next?
After the IRB office receives your submission...
- Protocol submissions will be assigned to an IRB Administrator for initial review
- verify that the research activity constitutes human participant research
- verify that training is complete (for expedited and full board studies)
- verify completeness of study materials and/or coordinate with the PI to obtain missing or inadequate materials
- analyze the submission to determine the review type (exempt, expedited, full board, prescreening, or reliance agreement)
- determine whether the study is a clinical trial
- Based on their administrative review, IRB staff will either issue an administrative determination (exempt, prescreening, reliance), or send the submission to a committee member for their review.
- If IRB staff or the designated committee member(s) determine that the protocol requires revision, IRB staff will contact the PI via RASS-IRB (and email) with specific feedback and requests.
- Once all outstanding concerns have been addressed, IRB staff will issue a formal approval or exemption notice to the PI by email.
Maintaining Compliance After Approval
Once a protocol has been approved, the PI is charged with conducting the research consistent with the approved protocol, and otherwise maintaining compliance. This requires that:
- Any changes to procedures, documents, research personnel, etc., must be approved in advance through the amendment process in RASS-IRB .
- For full board protocols only, annual renewal requests must be filed six weeks before the expiration date, to ensure that there is no interruption of IRB approval. Request continuing approval in RASS-IRB .
- Ensure the safety and confidentiality of your research data, as described in your approved protocol.
- Promptly report unexpected events (including complaints or breaches of confidentiality) and protocol deviations in RASS-IRB .
- Ensure that all members of your research team renew their IRB training , when needed.
You can check the status and other key information about your protocols information about your protocols in RASS-IRB .
RASS-IRB System
About the irb committee, the life cycle of an irb protocol, irb announcements & newsletters, irb informed consent, irb training, irb considerations: human participant data, data sets and internet research, irb biomedical research, irb considerations for international research, irb considerations for clinical trials.

How to Get Approved on Your First Submission!
Information from truman’s irb to help you get your application approved the first time it’s submitted.
Applications & Sample Consent Forms
Introduction
The purpose of this information is to assist faculty, staff and students who are planning to conduct projects that involve human subjects. You are urged to read this information carefully in order to avoid unnecessary delay in obtaining Institutional Review Board (IRB) or Peer Review approval. If you have a question that is not answered below, or if you need to determine whether or not your project needs to be reviewed by the IRB, visit the IRB website at https://irb.truman.edu and/or contact the Grants Office, IRB Administrator, McClain Hall 203, 660-785-7245, or talk to the IRB member in your academic department.
What Is the IRB?
The IRB is a campus-wide committee established under the authority of the Vice President for Academic Affairs to ensure that the rights and welfare of human subjects are adequately protected in all research projects. The IRB is charged with the responsibility of reviewing, prior to its initiation, all research (whether funded or not) involving human participants. The IRB is charged with ensuring the rights of humans in research and protecting their welfare, and privacy. The IRB has the authority to approve, require modifications in, or disapprove all research activities that fall within its jurisdiction as specified by both federal regulations and local institutional policy.
What Projects Must Be Reviewed?
All research projects involving human subjects must be reviewed. Projects must be reviewed whether they are funded or unfunded, sponsored or unsponsored, or whether they are carried out by students, faculty, or other University employees, on campus or off campus. Investigators may consult the Grants Office or an academic department’s IRB member for advice about whether or not a project must be reviewed. Final authority for making this determination rests with the IRB.
If I’m not really doing research, I’m just talking to people and asking questions…it’s not like I’m taking blood or anything…do I have to submit an IRB application? YES! If you are collecting information from an individual (whether it is blood or information about how a person feels about 8 am classes) you are conducting research and your project must be reviewed and approved by the IRB before you begin the project.
How Is A Project Submitted For Review?
Projects are submitted by completing either the Exempt Research IRB Application Form or the Full Review IRB Application Form. You MUST choose the correct form according to the guidelines given on the IRB website. If you submit the wrong application it will be returned to you without review. Submit your application, along with all supporting documentation to the Grants Office in McClain Hall 203. You must submit the original and 5 copies.
Complete the application carefully, answering each question thoroughly. Remember that the reviewers don’t know anything about your project, so be sure to include information that may seem obvious to you. Don’t “blow off” the application! This is the primary document IRB members use to examine your project. If the application is unclear, if information is not included, or if sections are not completed your application will be returned to you for additional refinement.
NOTE: Once you’ve filled in all the blanks, re-read your application, make sure it makes sense, check for misspellings and missing words. Make sure your consent form is well-formatted, includes all the sections described in the Consent Template on the IRB website, and does NOT include language that a lay person cannot understand.
When Does the IRB Meet?
The IRB meets every other week throughout the academic year and as needed during the summer. Specific meeting dates and deadlines for submission are listed on the IRB website.
What Types of Review Are Conducted at Truman?
There are three types of review at Truman State University. These are: (1) Full Application IRB review conducted by Truman’s IRB (2) Exempt Application IRB review conducted by Truman’s IRB, and (3) Exempt Application review conducted by an IRB-approved Peer Review Committee.
What Kinds Of Projects Are Reviewed By the Truman IRB?
The IRB regularly reviews both full review projects and exempt category projects. Any project requiring review may be submitted to Truman’s IRB for review. A project that includes any of the following criteria MUST be reviewed by the IRB:
1. It is externally funded.
2. It places subjects at more than minimal risk (physical, emotional, psychological or social risk, etc.) Minimal risk is defined as those risks experienced in everyday life.
3. It involves minors or other vulnerable populations (prisoners, pregnant women and fetuses, mentally disabled individuals and other special populations).
4. It investigates behaviors and/or experiences related to sensitive topics.
5. It is carried out to partially fulfill a Master’s Degree requirement.
6. The IRB requests full review of the application.
What Kinds of Projects Can Be Reviewed By An IRB-Approved Peer Review Committee?
Peer Review Committees must be approved by Truman’s IRB and function under the guidelines established by the IRB. Peer Review Committees are not available in all academic disciplines. The IRB allows approved Peer Review Committees to review projects submitted within their academic area when the project falls within one of the six Exempt Research Categories. The six Exempt Research categories are listed below.
Category One: Research conducted in established educational settings that involves normal educational practices. Examples include: research involving educational strategies; comparisons of effectiveness of instructional techniques, curricula, or classroom management methods.
Category Two : Research involving the use of educational tests(cognitive, diagnostic, aptitude, achievement), IF information taken from sources is recorded in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.
Category Three: Research involving survey or interview procedures. Full review must be conducted when responses are recorded in such a manner that human subjects can be identified, directly or through identifiers linked to the subject; or the subject’s responses could reasonably place the subject at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation if they became known; or the research deals with sensitive aspects of the subject’s own behavior, such as illegal conduct, drug use, sexual behavior, or use of alcohol.
Category Four: Research involving the observation (including observation by participants) of public behavior. Full review must be conducted when observations are recorded in such a manner that the human subject can be identified, directly or through identifiers linked to the subject; or the subject’s responses could reasonably place the subject at risk of criminal or civil liability or be damaging to the subjects’ financial standing, employability, or reputation if they became known; or the research deals with sensitive aspects of the subject’s own behavior, such as illegal conduct, drug use, sexual behavior, or use of alcohol.
Category Five: Research involving the collection or study of existing data, documents, records, pathological specimens, or diagnostic specimens. Sources must be publicly available and the information collected must be recorded by the investigator in such a manner that subjects cannot be identified, directly or through identifiers linked to the subjects.
Category Six: Taste and food quality evaluation and consumer acceptance studies, IF (1) wholesome foods without additives are consumed, or (2) a food is consumed that contains a food ingredient at or below the level and for a use found to be safe, or (3) a food contains an agricultural chemical or environmental contaminant at or below the level found to be safe by the Food and Drug Administration, or (4) the food is approved by the Environmental Protection Agency or the Food Safety and Inspection Service of the U.S. Department of Agriculture.
Doesn’t “Exempt Category Research” mean that the project is excused from review? No! The federal guidelines, which set the minimum standard, requires that exempt category research be reviewed to determine that it is, in fact, minimal risk, provides adequate protection to subjects that are involved, and falls within one of the six categories listed above. In addition, Truman’s IRB policies require that every Exempt project be reviewed. Exempt level projects go through a similar review process to that of Full Application projects.
What Kind of Criteria Does the IRB Use to Review A Project? The overall criterion for IRB reviewers is that your research plan provides adequate protection for the rights and welfare of the human subjects involved. In particular, IRB reviewers focus on the following areas: **The purpose of the project. **The methodology as it relates to the protection of human subjects. **The risks and benefits of the project. **Adequate handling of informed consent **Whether the research deals with high risk or sensitive issues and if so,whether they are handled in a way that minimizes risk to subjects. ** The degree to which confidentiality is both assured (commitment made to the participant) and protected (steps taken to fulfill that commitment).
Do Student Research Projects Have To Be Reviewed?
Yes, student investigations that involve human subjects must be reviewed. Student investigators work under the direction of their faculty supervisor who is responsible for ensuring that the research is carried out according to the procedures that were approved. The faculty supervisor should thoroughly review the student’s project before it is submitted for review. After reviewing the project the faculty supervisor must sign the student’s application certifying that the project will be carried out under his/her supervision.
Student projects most commonly include class assignments or questionnaires and surveys, and educational research conducted in school settings. Projects must be reviewed whether they are initiated by the student or assigned during a class. Student organizations that are conducting surveys or questionnaires, or initiating an activity that involves human subjects may also need to have the project reviewed by the IRB. If uncertain, a representative of the organization should contact the IRB Administrator in the Grants Office.
What Mistakes Are Most Likely to Keep My IRB Application from Being Approved?
The project is submitted on the wrong application form. Do not automatically submit your project on the Exempt Research Application Form! If your project does not fall within one of the six exempt criteria areas it will be returned to you for resubmission on the correct form. Read the criteria carefully.
The Project start date is earlier than the date the project will be reviewed. Your start date must reflect the date that your project will begin and CANNOT be a date prior to approval by the IRB.
The Faculty Advisor did not sign the Certification and Approval section . For student projects, the faculty must sign the cover page of the application on the line designated for him or her. This indicates that the faculty member is aware of the project, supports it, and is making a commitment to supervise the work of the student researcher.
The informed consent document does not include all of the sections required, or is inconsistent with the information in the application. This is one of the most frequent reasons that student applications are not approved. The informed consent document must clearly provide all of the information that is described in the Consent Template available on the IRB website. The consent document will be given to a participant to sign or to read, and should reflect high standards of neatness, organization, and accuracy. It should be written in language for an eighth-grade reading level, and it should not include jargon or technical terms. Your consent form is a reflection of your research project, your faculty sponsor, and of Truman State University.
Issues of confidentiality and anonymity are misstated. A participant’s responses or participation is Anonymous only if he/she cannot be identified by name or by any other descriptor that might indicate his/her identity. This means that you, the researcher, cannot identify which person completed which survey once the surveys are collected. If the project does not require identifying individual subjects, then names should not be recorded, (i.e. subjects will be anonymous). Confidentiality means that only the investigator(s) can identify the responses of the individual subjects. If identification of individual subjects is important, then every effort should be made to prevent anyone outside of the project from connecting individual subjects with their responses. One common method to maintain confidentiality is to assign codes to subjects, record codes only on the data sheet, and keep a copy of subjects’ codes only until no longer needed. Neither anonymity or confidentiality may be stated when subjects’ names are going to be included in the research. Oral history projects sometimes may have reason to include the subjects name with statements or reflections he/she made. In this case, the consent document must clearly make this request to participants and a signed consent document must be secured by the investigator.
The risks of the project are not described, even though they may be minimal. In the informed consent document and the application, under the section on Risks simple saying “Risks are minimal,” or “there are no risks” is not sufficient. You must explain what the possible risks might be or state that there is no risk more than encountered in normal daily activity.
Confidentiality of data is not adequately addressed Under the Confidentiality section, it is not sufficient to say that all records will be kept confidential. You must explain your plan to keep the records confidential. How will data be stored, with or without identifiers? If identifiers are used describe the type: name, job titles, number code, etc. How long are identifiers kept? If coding system is used, is there a link back to the subject’s ID? If yes, where is the code list stored in relation to the data and when is the code list destroyed? How will reports be written, in aggregate terms, or will individual responses be described? Will subjects be identified in reports? Describe disposition of tapes/films at the end of the study. If tapes are to be kept, indicate for how long and describe future uses of tapes.
Surveys, questionnaires, questions and other instruments are not included. If you are using questionnaires, survey, or instruments of any kind you must include a copy of the instrument or a script of the questions that will be asked.
The way you will recruit or select participants is not adequately described. When you inform individuals about your study (through posters/fliers, verbal announcements, e-mails, letters, etc.) you are recruiting an individual to be a part of your study. Copies of all recruitment materials must be submitted to the IRB with the application to ensure that appropriate information is shared with these individuals. If you are verbally asking other students or individuals to participate in your project you must describe in detail exactly how, where, and when you will do this. You should submit a script of what you will say to potential subjects. Individuals must understand exactly what you are asking of them and that their participation is completely voluntary and that if they choose to participate, they can withdraw at any time without any consequences. Recruitment may not begin until full approval has been granted by the IRB.
Audio or video taping is included in the project but is not explained. You must explain why the taping is being done and you must get permission from your subjects to tape them. You must clearly explain how the tape is to be used, who will have access to the tapes, who will listen to or view the tapes, how the tapes will be stored, who will transcribe the tapes, and when the tapes will be erased or destroyed. This information should appear in both the application and in the informed consent document. Projects that include audio or videotaping cannot be reviewed as Exempt Category Research.
The documents are sloppy and have grammatical errors in them. Taking time to proofread your documents before submitting them can go a long way toward getting your application approved.All submitted materials must be written using clear, easy to understand language this includes proper grammar, spelling and punctuation. Your application may be returned to you by the IRB.
Statement of Ethical Principles
Truman State University is committed to excellence in teaching, research, and public service. Concomitantly, the University is committed to the conduct of these activities with the highest possible ethical standards. For projects involving humans as subjects of research and research-related projects, Truman State University is guided by the ethical principles regarding all research involving humans as subjects as set forth in the Declaration of Helsinki , the Nuremburg Code , and the National Commission for the protection of Human Subjects of Biomedical and Behavioral Research entitled Ethical Principles and Guidelines for the Protection of Human Subjects of Research: The Belmont Report . In addition, the requirements set forth in Title 45, Part 46 of the Code of Federal Regulations will be followed for all applicable federally funded research.
Thus, the following broad principles are the basis for development of Truman State University’s policies concerning review of research involving humans:
A. Whereas, the participation of humans in research and training projects may raise fundamental ethical and civil rights questions, no distinctions in the monitoring of projects will be drawn between funded and unfunded projects, sponsored and unsponsored projects, or between projects carried out by students, faculty, or other University employees, on campus or off campus.
B. All activities involving humans as subjects must provide for the safety, health, and welfare of every individual. Rights, including the right of privacy, must not be unduly infringed upon.
C. The direct or potential benefits to the subject, and/or the importance of the knowledge gained, must outweigh the inherent risks to the individual.
D. Participation in projects must be voluntary and informed consent must be obtained from all subjects, unless this requirement is waived by the Institutional Review Board.
E. An individual does not abdicate any rights by consenting to be a research subject. A subject has the right to withdraw from a research project at any time or may refuse to participate without loss of benefits to which the subject would otherwise be entitled.
F. Safeguarding information about an individual that has been obtained in the course of an investigation is a primary obligation of the investigator.
- Skip to Content
- Online Services
Institutional Review Board
- Apply for IRB Review
The following pages and links detail the Institutional Review Board for Protection of Human Subjects in Research (IRB). The guidance within applies only to students, faculty, and staff of Bellevue College, Bellevue, WA as researchers or research subjects. IRB proposals that do not include Bellevue College faculty, staff, or students as either personnel or participants will not be reviewed.
Step 1: Determine if your project requires IRB approval
- Does my project need to be reviewed by the IRB?
Step 2: Complete the Mandatory Online Certification for Researchers
Mandatory online certification is required for all researchers, investigators, and faculty advisors (if applicable for student conducted research) who submit a proposal to the IRB. The certification must be renewed every 3 years. We accept CITI or PHRP certificates, or free training is available through the Office for Human Research Protections (we only require certificates acknowledging completion of lessons 1, 2 and 4).
Save a copy of the certifications as a pdf to submit as part of the research proposal.
Human Research Protection Foundational Training
Step 3: Complete the IRB Research Project Application
Complete the online Research Project Application , including attachments of study instruments, recruitment materials, informed consent, and other documents as necessary.
You can review the PDF version of the application to prepare your materials and responses before submitting the form; you are welcome to email [email protected] if you have any questions.
Step 4: Make adjustments as necessitated by IRB Review until approved
The IRB coordinator and/or reviewing members of the IRB may request revisions of the submitted form or materials before recommending the study for approval.
The full board of the IRB is scheduled to meet once a month if a study requires a full board review.
Step 5: Report Changes and Annually Renew Authorization (if needed)
If there are changes to your proposal after it has been approved, you need to notify the IRB using the Research Project Modification Form. For more information, refer to FAQ’s Research Modification.
If in the course of the study something negative happens to the subject then you must notify the IRB using the Adverse Incident Reporting Form . The IRB will then notify the Federal Office of Human Protection as required by law. Please note that this process must be followed regardless of the perceived seriousness of the incident, or whether or not you agree with the subject that something negative has occurred. If the subject reports an incident, then you must follow up within 48 hours of learning of the incident.
If your research duration is long than one year, you will need to submit an Annual Review Form prior to the anniversary of your research project application’s approval.
Step 6: Submit a Close-Out Form
Once you have finished gathering data, you will need to submit a Research Project Closure Form to the IRB. This notifies the IRB that its oversight responsibilities are over. Studies classified as “Exempt” do not require submission of this form.
Last Updated January 17, 2023
- Human Subjects Certification (Lessons 1, 2, and 4 Required)
- Definitions Related to IRB
- Frequently Asked Questions
Reporting Problems
- Accessibility
- Emergency alerts
- Privacy notice
- Public disclosure
- Website info
- We are an equal opportunity institution
Applying for ethical approval for research: the main issues
Affiliation.
- 1 Faculty of Health, Social Care and Education, Anglia Ruskin University, Cambridge, England.
- PMID: 26758167
- DOI: 10.7748/ns.30.20.40.s46
The need to obtain research ethical approval is common to all research involving human participants. This approval must be obtained before research participants can be approached and before data collection can begin. The process of ethical review is one way that research participants can be confident that possible risks have been considered, minimised and deemed acceptable. This article outlines some of the main issues researchers should consider when planning an application for research ethical approval by answering the following six questions: 'Do I need research ethical approval?', 'How many applications will I need to make?', 'Where should I apply for research ethical approval?', 'What do I need to include in my application?', 'What do research ethics committees look for?' and 'What other approvals might I need?' Answering these questions will enable researchers to navigate the ethical review process.
Keywords: ethical approval; ethics; governance; informed consent; research; research ethics.
- Biomedical Research / ethics*
- Ethical Review / standards*
- Ethics Committees, Research*
- Informed Consent / ethics
- Research Design
- United Kingdom
- Resources
- Prospective Students
- Current Students
- Faculty & Staff
- Alumni & Friends
- News & Media
Procedures and Criteria for Approval
- Initial Approval The principal investigator may be asked to meet with the IRB should it be apparent that clarification or modification of statements in the application are required. No individual involved in the conduct and/or supervision of the research project shall participate in its review, except to provide information to the IRB. The IRB may approve the project as is or may approve it with certain modifications required to meet federal standards. In the latter instance, the investigator will be informed by letter. The IRB approval letter will be sent upon written receipt of the modification. The faculty advisor will be copied on any correspondence sent to student investigators. If the IRB action is to disapprove the application, reasons for this negative decision will be provided in writing to the principal investigator or project director. If the researcher decides to modify the proposed research in such a way as to meet the objections of the IRB, the investigator may resubmit the modifications of the proposal for consideration at the next IRB meeting. If desired, the investigator may request a personal hearing at the next scheduled IRB meeting.
- Continuing Review and Submission of Annual Update Complex and potentially dangerous projects will be reviewed at a frequency commensurate with the related risks. When initial approval of such a protocol is given, the IRB may indicate the need for re-evaluation of the project after a specified interval so that continued acceptance of the protocol is assured. Projects that are determined to be exempt will not require additional review. Non-exempt proposals are approved for a maximum period of one year only. For projects which continue beyond one year, it is the responsibility of the principal investigator to submit to the IRB an annual update (Appendix B). The first annual update is due twelve months following the date the proposal was approved and released. Upon receipt of the annual update the IRB will review and approve, if appropriate, continuation of the project for the next twelve month period. Investigators will receive one notification regarding the due date of an annual update. Failure to submit an update within 30 days following the due date will result in termination of IRB approval. Projects can be updated annually for a maximum approval period of five years. Continuation of projects beyond five years requires resubmission. If the IRB determines that a project requires review more often than annually, the investigator will be so notified. The IRB has the authority to directly observe ongoing research projects and the consent process as well as audit research records. When a project that has required continuing review is terminated/completed, the investigator must immediately notify the IRB in writing. Ongoing projects modified to include humans as subjects also must be submitted to the IRB for review and approval prior to the use of human subjects. In the case of an externally funded project, the granting agency would be notified of the IRB action prior to the appropriation cycle for a budget period during which human subject involvement is proposed.
- Reporting Proposed Changes in a Research Protocol Any proposed change in a protocol which affects the human subjects must be reviewed and approved by the IRB prior to the implementation except where an immediate change is necessary to eliminate a hazard to the subjects. Investigators should submit a Request for Change in Protocol (Appendix C) and a revised consent form as required. Minor changes during the period for which approval is in force will be reviewed by an expedited review procedure. If a change in protocol is relatively minor (e.g., change in investigator, change in the sequence of follow-up visits) it is not necessary to have the subject sign a revised consent form or an addendum to the consent form. If, however, the change is not minor (e.g., addition of an intervention not addressed in the original consent form, disclosure of a previously unidentified risk) the investigator should have all new subjects sign a revised consent form and all currently enrolled subjects who are actively participating in the protocol sign an addendum to the consent form. If the change is considered significant (e.g., disclosure of a serious risk) the use of a witness is required.
- Submission of a Report of Injury If a subject suffers an injury, the investigator must submit a Report of Injury (Appendix D) to the IRB within 48 hours.
- Reporting Non-Compliance with IRB Guidelines Any incident of non-compliance with IRB Guidelines should be reported immediately to the IRB. Non-compliance with IRB requirements is a violation of federal regulations for the protection of human subjects.
- School of Graduate Studies and Research
- Associate Dean for Research Stright Hall, Room 113 210 South Tenth Street Indiana, PA 15705-1081
- Phone: 724-357-7730
- Fax: 724-357-2715
- [email protected]
Redirect Notice
Grants process.
Take time to learn about each step in the grants process from planning to apply through developing and submitting your application to award and post-award reporting.

An official website of the United States government
Here’s how you know
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock A locked padlock ) or https:// means you’ve safely connected to the .gov website. Share sensitive information only on official, secure websites.
Approval of Research with Conditions: OHRP Guidance (2010)
This guidance represents OHRP’s current thinking on this topic and should be viewed as recommendations unless specific regulatory requirements are cited. The use of the word must in OHRP guidance means that something is required under HHS regulations at 45 CFR part 46. The use of the word should in OHRP guidance means that something is recommended or suggested, but not required. An institution may use an alternative approach if the approach satisfies the requirements of the HHS regulations at 45 CFR part 46. OHRP is available to discuss alternative approaches by telephone at 240-453-6900 or 866-447-4777, or by email at [email protected] .
Date: November 10, 2010
Scope: This document applies to non-exempt human subjects research conducted or supported by HHS. It provides guidance on the authority of institutional review boards (IRBs) to approve research with conditions. In particular, OHRP offers guidance on the following topics:
- What actions can an IRB take when reviewing research?
- What does IRB approval with conditions mean?
- What circumstances preclude the IRB from approving research?
- What circumstances permit the IRB to approve research with conditions ?
- How should the IRB handle changes to research that are proposed after the IRB has approved the research with conditions?
- How do conditions on IRB approval at the time of initial review affect the initiation of research?
- May an IRB approve some components of a proposed research study and defer taking action on other components at the time of initial review?
- How do conditions on IRB approval at the time of continuing review, or at the time of review of proposed changes in previously approved research, affect ongoing research?
- What must the IRB records include regarding the documentation of conditions of IRB approval of research?
Target Audience: IRBs, investigators, HHS funding agencies, and others that may be responsible for the review, conduct, or oversight of human subjects research conducted or supported by HHS.
Regulatory Background:
An IRB must review proposed research, including proposed changes to previously approved research, at convened meetings at which a majority of the members of the IRB are present, including at least one member whose primary concerns are in nonscientific areas, except when expedited review is authorized (45 CFR 46.108(b) and 46.103(b)(4)). In order for research to be approved, it must receive the approval of a majority of those members present at the meeting (45 CFR 46.108(b)).
IRBs reviewing research have the authority to approve, require modifications in (to secure approval), or disapprove the research (45 CFR 46.109(a)).
An IRB may use the expedited review procedure to review either or both of the following:
- Some or all of the research appearing on the list of categories of research that may be reviewed by the IRB through an expedited review procedure (see );
- Minor changes in previously approved research during the period (of one year or less) for which approval is authorized.
Under an expedited review procedure, the review may be carried out by the IRB chairperson or by one or more experienced reviewers designated by the chairperson from among the members of the IRB. In reviewing the research, the reviewers may exercise all of the authorities of the IRB except that the reviewers may not disapprove the research. (45 CFR 46.110).
HHS regulations at 45 CFR 46.102(h) define IRB approval as the determination of the IRB that the research has been reviewed and may be conducted at an institution within the constraints set forth by the IRB and by other institutional and federal requirements.
In order to approve research, IRBs must determine that all of the following requirements are satisfied in accordance with HHS regulations at 45 CFR 46.111:
- Risks to subjects are minimized (i) by using procedures which are consistent with sound research design and which do not unnecessarily expose subjects to risk, and (ii) whenever appropriate, by using procedures already being performed on the subjects for diagnostic or treatment purposes;
- Risks to subjects are reasonable in relation to anticipated benefits, if any, to subjects, and the importance of the knowledge that may reasonably be expected to result. In evaluating risks and benefits, the IRB should consider only those risks and benefits that may result from the research (as distinguished from risks and benefits of therapies subjects would receive even if not participating in the research). The IRB should not consider possible long-range effects of applying knowledge gained in the research (for example, the possible effects of the research on public policy) as among those research risks that fall within the purview of its responsibility.
- Selection of subjects is equitable. In making this assessment the IRB should take into account the purposes of the research and the setting in which the research will be conducted and should be particularly cognizant of the special problems of research involving vulnerable populations, such as children, prisoners, pregnant women, mentally disabled persons, or economically or educationally disadvantaged persons.
- Informed consent will be sought from each prospective subject or the subject’s legally authorized representative, in accordance with, and to the extent required by 45 CFR 46.116.
- Informed consent will be appropriately documented, in accordance with, and to the extent required by 45 CFR 46.117.
- When appropriate, the research plan makes adequate provision for monitoring the data collected to ensure the safety of subjects.
- When appropriate, there are adequate provisions to protect the privacy of subjects and to maintain the confidentiality of data.
- When some or all of the subjects are likely to be vulnerable to coercion or undue influence, such as children, prisoners, pregnant women, mentally disabled persons, or economically or educationally disadvantaged persons, additional safeguards have been included in the study to protect the rights and welfare of these subjects
When applicable, IRBs must determine that the additional protections of subpart B (Additional Protections for Pregnant Women, Human Fetuses and Neonates Involved in Research), subpart C (Additional Protections Pertaining to Biomedical and Behavioral Research Involving Prisoners as Subjects), or subpart D (Additional Protections for Children Involved as Subjects in Research) of 45 CFR part 46 have been met.
A. What actions can an IRB take when reviewing research?
Given the authorities that IRBs have under HHS regulations at 45 CFR 46.109(a), when conducting an initial or continuing review of a research study, or a review of proposed changes to a previously approved research study, an IRB can take any of the following actions:
- Approve the research study or proposed changes either (a) as submitted without any conditions, or (b) with conditions (note that, as explained in section B below, when research is approved by the IRB with conditions at a convened meeting, further review by IRB at a subsequent convened meeting is not necessary);
- Require modifications to secure approval and defer or table the research study or proposed changes for further review at a future date after the required modifications are submitted by the investigator; or
- Disapprove the research study or proposed changes.
B. What does IRB approval with conditions mean?
In the course of initial or continuing review of research, or review of proposed changes to previously approved research, IRBs often request that investigators (a) make specified changes to the research protocols or informed consent documents; or (b) submit clarifications or additional documents. When doing this, depending on the circumstances, the IRB is either:
- precluded from approving the research, as described in section C below; or
- permitted to approve the research with conditions, as described in section D below.
By IRB approval with c onditions (sometimes referred to as “conditional approval” or “contingent approval”), OHRP means that at the time when the IRB reviews and approves a research study (or proposed changes to a previously approved research study), the IRB requires as a condition of approval that the investigator (a) make specified changes to the research protocol or informed consent document(s), (b) confirm specific assumptions or understandings on the part of the IRB regarding how the research will be conducted, or (c) submit additional documents, such that, based on the assumption that the conditions are satisfied, the IRB is able to make all of the determinations required for approval under the HHS regulations at 45 CFR 46.111 and, if applicable, subparts B, C, or D of 45 CFR part 46. With respect to research reviewed and approved with conditions by the IRB at a convened meeting, note that because the IRB is able to make all these determinations, the IRB may designate the IRB chairperson (and/or other individual(s) with appropriate expertise or qualifications) to review responsive materials from the investigator and determine that the conditions have been satisfied, and further review by the IRB at a subsequent convened meeting would not be necessary.
C. What circumstances preclude the IRB from approving research?
Any time the IRB reviewing a research project cannot make one or more of the determinations required for approval by the HHS regulations at 45 CFR 46.111 and, if applicable, subparts B, C, or D of 45 CFR part 46, the IRB must not approve the research project. This applies to both initial and continuing review of research, and review of proposed changes to previously approved research.
For example, the IRB must not approve a proposed research project undergoing initial review when the IRB (a) is unable to make the required determinations about research risks and benefits, the adequacy of privacy and confidentiality protections, or the adequacy of the informed consent process because the research protocol provides insufficient information related to these aspects of the research, and (b) is unable to specify changes to the research protocol that if made would allow the IRB to make these required determinations.
When an IRB reviewing a research project at a convened meeting is unable to approve research because it cannot make the determinations required for approval, the IRB can either disapprove the project, or defer or table the project for further review at a future date. When deferring or tabling the project, the IRB, under its authority to require modifications in order for an investigator to secure approval, may require that the investigator (a) make changes to the protocol or informed consent documents, or (b) submit clarifications or additional documents prior to the next review. If the IRB defers or tables a research project, the research may not proceed until the IRB reviews the revised research project and approves it at a subsequent convened meeting.
When an IRB reviewing a research project under an expedited review procedure is unable to approve the project because the chairperson (or designated reviewer(s)) cannot make the determinations required for approval, the IRB chairperson (or designated reviewer(s)) can either refer the project to the IRB for further review and action at a convened meeting, or defer approval of the research project and require that the investigator (a) make changes to the protocol or informed consent documents, or (b) submit clarifications or additional documents prior to further review by the IRB chairperson (or designated reviewer(s)). Research may not be disapproved under an expedited review procedure (45 CFR 46.110(a)). Examples of required changes or clarifications that generally would preclude the IRB from approving the research include the following:
- Providing a justification for using a placebo and withholding currently available treatment for a serious medical condition for subjects assigned to a control group (OHRP notes that in this example the IRB would need the investigator’s response in order to make the determinations under 45 CFR 46.111(a)(1) and (2));
- Providing a justification for enrolling children in the research and an explanation of how the research would satisfy the requirements of subpart D of 45 CFR part 46 (OHRP notes that in this example the IRB would need the investigator’s response in order to make the determinations under subpart D of 45 CFR part 46);
- Revising the study hypothesis and, accordingly, the study design (OHRP notes that in this example the IRB would need the investigator’s response in order to make the determinations under 45 CFR 46.111(a)(1), (2), and (4));
- Providing a description of procedures that the control group will undergo (OHRP notes that in this example the IRB would need the investigator’s response in order to make the determinations under 45 CFR 46.111(a)(1), (2), and (4));
- Providing clarifying information needed to assess the risks to subjects, such as clarifying whether individuals who have taken aspirin within 14 days prior to enrollment will be excluded from the study because of concerns about the risks of bleeding (OHRP notes that in this example the IRB would need the investigator’s response in order to make the determinations under 45 CFR 46.111(a)(1) and (2); see example (5) in section D below for an alternative approach that would allow the IRB to approve the research with conditions);
- Clarifying the timing and circumstances under which the informed consent of prospective subjects will be sought (OHRP notes that in this example the IRB would need the investigator’s response in order to make the determinations under 45 CFR 46.111(a)(4); see example (6) in section D below for an alternative approach that would allow the IRB to approve the research with conditions); or
- providing a plan to implement additional subject monitoring in order to reduce risks to subjects, given the number of serious adverse events that have occurred in study subjects since the prior IRB review (OHRP notes that in this example the IRB would need the investigator’s response in order to make the determinations under 45 CFR 46.111(a)(1), (2), and (4)).
D. What circumstances permit the IRB to approve research with conditions? The IRB may approve research with conditions if, given the scope and nature of the conditions, the IRB is able, based on the assumption that the conditions are satisfied, to make all of the determinations required for approval under the HHS regulations at 45 CFR 46.111 and, if applicable, subparts B, C, or D of 45 CFR part 46. The authority to approve research with conditions extends to the IRB’s initial review of research, continuing review of research, and review of proposed changes to previously approved research. This authority also applies to IRB review of research at a convened meeting or under an expedited review procedure.
The IRB may require the following as conditions of approval of research:
- Confirmation of specific assumptions or understandings on the part of the IRB regarding how the research will be conducted (e.g., confirmation that the research excludes children);
- Submission of additional documentation (e.g., certificate of ethics training);
- Precise language changes to protocol or informed consent documents; or
- Substantive changes to protocol or informed consent documents along with clearly stated parameters that the changes must satisfy.
When the IRB approves research with conditions, verification procedures must be included as part of the IRB approval process, under which the IRB chairperson (and/or other individual(s) designated by the IRB) will review responsive materials from the investigator required by the IRB, and determine whether the conditions of approval have been satisfied (45 CFR 46.102(h)). The IRB’s verification that the investigator has satisfied all conditions of approval stipulated by the IRB helps to ensure that the investigator does not initiate any research that is different from what was approved by the IRB (45 CFR 46.102(h)).
Note that OHRP does not consider this verification process by the IRB chairperson or any other individual designated by the IRB to represent the review and approval of minor changes under an expedited review procedure. As a result, IRBs have significant flexibility regarding who may be designated to verify that conditions have been satisfied, including designation of someone other than an IRB member.
Individuals designated by the IRB to review responsive materials from the investigator and determine whether the IRB’s conditions for approval have been satisfied should have appropriate expertise or qualifications. Depending upon the nature of the required conditions, the IRB could designate any of the following individuals or groups of individuals to determine that the conditions of approval have been satisfied:
- The IRB chairperson;
- Another IRB member or group of IRB members with particular subject matter expertise or experience;
- A consultant with particular subject matter expertise who is not an IRB member; and/or
- An IRB administrator or other qualified IRB administrative staff person, who need not be an IRB member.
For some conditions, the review of responsive materials from investigators will require medical, scientific, or other technical expertise. In such cases, the IRB should designate an individual having the appropriate expertise to review the responsive materials from the investigator; typically, this would be the IRB chairperson, another IRB member, or an expert consultant. For others conditions for which the investigator simply needs to make verbatim changes to the protocol or informed consent document or to submit a specific document, review of the responsive materials from investigators typically will not require any special expertise. In these cases, the IRB could designate an IRB administrator or other IRB administrative staff person to review the responsive materials from the investigator.
The following examples illustrate the types of conditions IRBs could stipulate when approving research, as well as the type of individual who might be designated by the IRB to determine that the conditions of approval have been satisfied; these examples are not intended to be all-inclusive, nor are they intended to suggest that the type of individual designated in the example is either appropriate or necessary in all such circumstances:
- Requiring submission of documentation of an endorsement letter from a department chair, as required by institutional policy, and designating an IRB administrator or other qualified IRB staff member to confirm receipt of the required documentation;
- Requiring correction of minor grammatical and typographical errors in the informed consent document, and designating an IRB administrator or other qualified IRB staff member to review the revised informed consent document and confirm that the required corrections were made;
- Requiring that a listed investigator provide a copy of his approved clinical privileges/hospital staff appointment document in order to confirm that he has approval to perform the procedures (e.g., percutaneous liver biopsies) proposed in the research protocol at the institution where the research is to be conducted, and designating an IRB administrator or other qualified IRB staff member to review this document and confirm that the clinical privileges of the listed investigator include authorization to perform such procedures.
- Requiring that the investigator re-locate in the informed consent document the statement “You will receive $500 for participating in this study” from the “Benefits” section of the form to a separate section under the heading “Compensation,” and designating an IRB administrator or other qualified IRB staff member to review the revised informed consent document and verify the re-location;
- Requiring that the investigator – in order to ensure that risks to subjects are minimized – add “a history of aspirin use in the past 14 days” to the exclusion criteria for subject enrollment in the research protocol, and designating an IRB administrator or other qualified IRB staff member to review the revised protocol and verify that the stipulated language was added to the exclusion criteria;
- For a randomized clinical trial comparing two types of surgical procedures, requiring that the investigator – in order to ensure that informed consent will be obtained under circumstances that provide prospective subjects with sufficient opportunity to consider whether or not to participate – revise the protocol to indicate that informed consent of the prospective subjects will be sought by the investigator during an outpatient clinic visit at least one week before the surgery, and designating an IRB administrator or other qualified IRB staff member to review the revised protocol and verify that the requested language regarding the process for soliciting informed consent of the prospective subjects was added to the protocol.
- Requiring the investigator to (a) confirm that any standard contrast material used in radiological procedures dictated by the research protocol will be limited to agents and dose levels specified in precise detail by the IRB, and (b) submit a revised protocol which includes the precise agents and dose levels, and designating an IRB administrator or other qualified IRB staff member to review the revised protocol and verify that the changes made by the investigator match those specified by the IRB;
- Requiring that the investigator modify the informed consent document to include standard template language used for research involving college psychology students, stating that comparable non-research alternatives for earning extra credit will be offered to students who choose not to participate in the research, and designating an IRB administrator or other qualified IRB staff member to review the revised informed consent document and verify the addition;
- Requiring the addition to the informed consent document of a description of the risks of a standard chemotherapy drug, where the risks are well-described in the research protocol, and designating an IRB member or consultant who is knowledgeable about those risks to review the revised informed consent document and confirm that the description of the risks is satisfactory;
- Requiring revision of the research protocol to include a description of the type and amount of standard contrast material to be used in the radiological procedures dictated by the research protocol, and designating an IRB member or consultant who is a radiologist to review the revised protocol and ensure that the use of standard contrast material is medically appropriate;
- Requiring simplification of the description of the study risks in the informed consent document to be at an 8th grade comprehension level, and designating the IRB chairperson to review the revised informed consent document and ensure that risks are accurately described and understandable at an 8th grade comprehension level;
- Requiring that the research protocol be revised to include a plan for (a) informing subjects about the results of standard clinical tests performed as part of the research protocol (e.g., cardiac function tests), and (b) referring subjects for appropriate clinical follow-up, and designating an IRB member or a consultant with appropriate clinical expertise (e.g., a cardiologist) to review the revised protocol and confirm that the plan is medically appropriate.
E. How should the IRB handle changes to research that are proposed after the IRB has approved the research with conditions?
After research has been approved with conditions by the IRB, additional changes are sometimes proposed by the investigator or recommended by designated reviewers before all conditions have been satisfied and the protocol documents have been finalized. The process for handling such changes is the same as for any change that is proposed during the period for which IRB approval has already been given (see 45 CFR 46.103(b)(4)(iii)).
Protocol corrections that are only administrative in nature (e.g., correction of typographical and spelling errors in the protocol) would not need additional IRB review because OHRP does not consider such corrections to be changes to the research.
Changes to the research that are “minor” may be reviewed by the IRB chairperson or by another experienced reviewer designated by the chairperson from among the members of the IRB under an expedited review procedure in accordance with 45 CFR 46.110(b)(2). OHRP notes that under 45 CFR 46.110(c), all members of the IRB must be advised of any such minor changes that are approved under an expedited review procedure.
Changes to the research that are more than minor would require further review by the IRB at a convened meeting.
OHRP recommends that institutions adopt policies for determining the types of changes in previously approved research that constitute “minor” changes which can be approved under an expedited review procedure, in contrast to greater than minor changes which require review by the IRB at a convened meeting.
F. How do conditions on IRB approval at the time of initial review affect the initiation of the research?
Whenever the IRB approves a research study with one or more conditions at the time of initial review, the effective date of the initial approval is the date on which the IRB chairperson (or any other individual(s) designated by the IRB) has reviewed and accepted as satisfactory any revised protocol or informed consent documents or any other responsive materials required by the IRB from the investigator. (For additional guidance on determining the effective dates of IRB approval and continuing review dates, see OHRP’s Guidance on IRB Continuing Review of Research at http://www.hhs.gov/ohrp/regulations-and-policy/guidance/guidance-on-continuing-review-2010/index.html .) In these circumstances, no research study activities involving human subjects may be initiated until the conditions have been satisfied in the manner set forth by the IRB and the approval becomes effective.
Once the investigator has responded to the IRB’s conditions, if the designated reviewer(s) determines that the responsive materials do not satisfy the conditions of approval stipulated by the IRB, then the IRB approval has not become effective, and the investigator may not proceed with the research. The investigator may submit additional revisions or material to the IRB for review by the designated reviewer(s) in an attempt to satisfy the IRB’s conditions, or may choose to submit a modified research proposal to the IRB. If the investigator chooses not to submit any additional revisions or materials to the IRB for review by the designated reviewer(s), then the approval for the research activity would not become effective, and the investigator may not conduct the research study.
When someone other than the IRB chairperson is the designated reviewer and the designated reviewer and investigator are unable to agree on whether the responsive material provided to the IRB by the investigator satisfies the conditions of approval, OHRP recommends that the designated reviewer and investigator consult with the IRB chairperson or that the matter be referred to the convened IRB.
G. May an IRB approve some components of a proposed research study and defer taking action on other components at the time of initial review?
Yes, at the time of initial review an IRB may approve some components of a proposed research study and allow an investigator to initiate research activities only related to those approved components, while deferring taking action on other components of the proposed study. In such circumstances, the IRB must ensure that the approved components of the research study are scientifically valid and satisfy all criteria required for IRB approval, even if the other components are never approved and conducted. The IRB may require that the investigator, in order for the investigator to secure approval for the unapproved components of the initially proposed research study, submit to the IRB for review (a) changes to the protocol or informed consent documents, or (b) clarifications or additional documents. The following example further illustrates this scenario:
- The investigator proposes a research study involving the enrollment of subjects ages 12-65 years, including pregnant women.
- Because the investigator did not provide sufficient information regarding the involvement of children and pregnant women, the IRB is unable to make the findings required for approval under subparts B and D of 45 CFR part 46. As a result, the IRB approves the research study for one year only for involvement of non-pregnant adult subjects, and the research may not involve pregnant women or children. Note that the IRB must ensure that the study as initially approved without inclusion of children or pregnant women is scientifically valid and satisfies all criteria for IRB approval under 45 CFR 46.111.
- The IRB requires that the investigator, in order to secure approval for inclusion of pregnant women and children in the study, submit additional information necessary for the IRB to make the findings required under subparts B and subpart D of 45 CFR part 46.
- The investigator subsequently submits sufficient information necessary for the IRB to make the determinations required under subparts B and D. The IRB reviews this information, makes the required determinations, and approves the involvement of children and pregnant women in the study. At this point, the investigator can begin enrolling pregnant women and children.
H. How do conditions on IRB approval at the time of continuing review, or at the time of review of proposed changes in previously approved research, affect ongoing research?
When approving research with conditions at the time of continuing review, or at the time of review of proposed changes to previously approved research, the IRB should be careful to specify whether any conditions need to be satisfied before an investigator can continue particular research activities related to those conditions. For example, if at the time of continuing review the IRB requires the investigator to change the research protocol to include a specific new procedure for screening prospective subjects, the IRB could approve the research with the following condition: research activities involving currently enrolled subjects may continue, but no new subjects may be enrolled until a designated IRB member reviews a revised protocol and verifies that the protocol includes the new screening procedure.
Likewise, if at the time of continuing review, or at the time of review of proposed changes to previously approved research, the IRB requires that the investigator within 30 days (a) change the informed consent document to include a description of a newly identified risk, and (b) submit a written plan for informing currently enrolled subjects about the new risk, the IRB could approve the research with the following condition: research activities involving currently enrolled subjects may continue, but no new subjects may be enrolled until a designated IRB member reviews a revised informed consent document and verifies that the description of the new risk has been added. Alternatively, the IRB could stipulate that no further research activities involving human subjects (including activities of already enrolled subjects) may occur after the date of the IRB’s continuing review or the review of the protocol changes until the investigator has submitted, and the designated IRB member has reviewed and accepted as satisfactory, the revised informed consent document and the written plan for informing currently enrolled subjects about the new risk.
Note that OHRP would not consider such suspensions of subject enrollment or of activities involving already enrolled subjects at the time of continuing review to be suspensions of IRB approval that needs to be reported to appropriate institutional officials, the head (or designee) of the agency conducting or supporting the research, and OHRP under HHS regulations at 45 CFR 46.103(a) and 46.103(b)(5).
I. What must the IRB records include regarding the documentation of conditions of IRB approval of research?
When the IRB approves research with conditions, the IRB must document, both to the investigator and in the IRB minutes for research reviewed at a convened meeting or elsewhere in the IRB records for research reviewed under an expedited review procedure, the following:
- All conditions that must be satisfied by the investigator (45 CFR 46.102(h), 45 CFR 46.109(d), and 45 CFR 46.115);
- The date when the IRB chairperson (and/or other individual(s) designated by the IRB) determines that all conditions of IRB approval have been satisfied, the date when initial approval becomes effective, and the date by which continuing review must occur;
- In the case of initial review, any conditions under which some research activities may be initiated (for example, the investigator may initiate research in non-pregnant adults, but not in pregnant women or children); and
- In the case of continuing review and the review of proposed changes to previously approved research, any conditions that need to be satisfied before an investigator can continue particular research activities related to those conditions (45 CFR 46.115(a)).
All correspondence between the IRB and the investigator regarding the conditions of approval set forth by the IRB must be maintained in the IRB records (45 CFR 46.115(a)(4)).
Copies of all research proposals reviewed by the IRB and approved sample consent documents, including any revised protocol or informed consent documents submitted by the investigator in order to satisfy the conditions of approval stipulated by the IRB, also must be maintained in the IRB records (45 CFR 46.115(a)(1)).
If you have specific questions about how to apply this guidance, please contact OHRP by phone at (866) 447-4777 (toll-free within the U.S.) or (240) 453-6900, or by e-mail at [email protected] .
Last revised: September 1, 2010
Disclaimer Policy: Links with this icon ( ) mean that you are leaving the HHS website.
- The Department of Health and Human Services (HHS) cannot guarantee the accuracy of a non-federal website.
- Linking to a non-federal website does not mean that HHS or its employees endorse the sponsors, information, or products presented on the website. HHS links outside of itself to provide you with further information.
- You will be bound by the destination website's privacy policy and/or terms of service when you follow the link.
- HHS is not responsible for Section 508 compliance (accessibility) on private websites.
For more information on HHS's web notification policies, see Website Disclaimers .

Affiliate 💸
Get started free
Research Project Guide
6 Tips On How To Start A Research Project Successfully
Discover practical tips on how to start research project successfully and set yourself up for success from the very beginning!
Oct 21, 2024

Starting a research project can feel like standing at the base of a mountain, unsure where to begin the climb. Whether dealing with a tight deadline or aiming for efficiency, knowing how to start a research project is crucial. This guide will provide practical insights to help you conduct fast research and write efficiently to tackle your project confidently and quickly. One innovative tool to help you achieve these goals is Otio's AI research and writing partner, designed to streamline the research process and enhance your writing efficiency.
Table Of Contents
What is a research project, 9 best tools for research projects, tips for starting an independent research project, supercharge your researching ability with otio — try otio for free today.

A research project is a structured exploration to answer specific questions or achieve particular objectives. Picture it as a well-organized journey, with clear steps that guide you from start to finish. You’ll begin with a literature review to understand what’s already known. Then, you’ll design your study, gather and analyze data, and report your findings. These projects are crucial in addressing problems, forming hypotheses, and creating strategies based on available resources like staff and funding.
The research must include a defined problem, a solid theoretical foundation, and a data collection and analysis process, regardless of the scientific field. According to UNESCO , there are roughly 7.8 million researchers worldwide, and scientific publications have surged by 23% since 2008.
Related Reading
• How to Find Academic Sources • How to Analyze Quantitative Data • How to Start a Research Project • How to Organize a Research Paper • Argumentative Essay Topics • Research Methodology Types • How Long Does It Take to Write a Research Paper • How to Create a Research Question • Can Ai Write a Paper for Me • Methods Section of Research Paper

1. Streamline Your Research with Otio
Research today is a minefield of distractions. Content overload leaves you juggling bookmarks, notes, and read-it-later apps. Otio creates a streamlined workspace where you can gather everything from tweets to YouTube videos in one place. Its AI-generated notes transform your resources into usable information, and its chat-like interface lets you interact with your sources intuitively. Give Otio a shot and see how it speeds up your research process .
2. Finding the Right Supervisor
A good supervisor is your guide through the research process. They'll help you design your study, choose the proper methods, and interpret your data. Talk to faculty members, trusted professors, or colleagues to find your match. Once you have a list of potential advisors, email an introductory email and request a meeting.
3. Refining Your Research Topic
Once you've found a supervisor, they can help narrow down the topic. The more specific your focus, the better you can direct your research and dig deep into your subject matter. A refined topic will also save you time by keeping your scope manageable and ensuring that you're concentrating on the most relevant aspects.
4. Crafting a Strong Thesis
Your thesis is the backbone of your project, providing direction and focus. It allows you to collect and organize information more efficiently and guides your readers through your argument. A clear and concise thesis statement is essential for helping your audience understand your project's purpose.
5. Developing a Research Timeline
Creating a timeline helps you structure your project and stay on track. It ensures you're organized and accounted for every step, from research to writing to editing. A timeline can also keep you motivated and prevent you from getting sidetracked.
6. Organizing Your Research with an Outline
Outlines provide structure and clarity, allowing you to organize your thoughts logically. They serve as a roadmap for your research, keeping you focused on the critical points and helping you identify gaps in your research before you start writing.
1. Otio: Streamline Your Research Workflow
Otio provides a unified AI -powered workspace for researchers, allowing them to manage the overwhelming influx of data from bookmarks, tweets, and videos. It enables users to extract key insights and produce draft outputs swiftly .
2. Zotero: Your Research Assistant
Zotero is a tool designed to simplify research by allowing users to efficiently collect, organize, annotate, and share data. It integrates smoothly with browsers, allowing users to save articles and publications conveniently.
Pros
User-friendly interface
The free version includes all features and updates
Plugin support for popular word processors
Cons
Limited free cloud storage
No official Android app is available
3. EndNote: Automate Your Citation Process
EndNote streamlines the referencing process by automatically formatting citations and creating a bibliography as you write. It’s a cloud-based tool that enables collaboration and smooth work across devices.
Compatible with library databases
Supports various citation styles
Efficiently imports multiple references
Primarily desktop-focused with limited online features
4. elink.io: Simplify Content Curation
elink.io is a bookmarking and curation tool that helps researchers save and organize web content effortlessly. It allows users to compile and share their collections with others, enhancing collaboration.
Easy to use with quick deployment
Eye-catching graphics and custom uploads
Smooth integration with SurveyMonkey
Customer service issues reported
Inconsistent pricing
5. Grammarly: Enhance Your Writing Quality
Grammarly is an AI-powered typing assistant that identifies spelling, grammar, and punctuation errors and suggests replacements. It’s a free basic version with a premium upgrade for more advanced features.
Advanced features for fluency and consistency
User-friendly interface
Non-intrusive writing experience
Inconsistent accuracy in context-dependent cases
May unnecessarily lengthen sentences
6. Asana: Manage Your Research Projects
Asana is a task management platform that connects development, copywriting, design, research teams, and product managers. It offers many features, including time tracking and Jira integration.
Time-tracking capabilities
Securely stores information about projects
User-friendly dashboard for task management
Overwhelming for small teams or simple projects
No built-in time-tracking
7. SurveyMonkey: Create Surveys with Ease
SurveyMonkey is a leading online survey tool with millions of users. It offers hundreds of templates for researchers to set up and deploy surveys for various purposes quickly.
Easy survey creation and multimedia integration
Valuable reporting toolkit
Excellent customer service
Too many features for some users
Limited user access options
8. ProofHub: Stay Organized and Focused
ProofHub is an all-in-one project and team management application that helps research teams efficiently plan their projects. It offers features like Kanban boards, Gantt charts, and time-tracking.
Affordable for large teams
Customizable project reports
Mobile apps for iOS and Android
Limited support
Costly for individuals and new teams
9. RefnWrite: Improve Your Academic Writing

RefnWrite is a tool that helps researchers improve their academic writing with a library of phrases and templates. It uses AI to suggest phrases, cross-reference previous work, and more.
Comprehensive phrase bank and templates
Powerful paraphrasing tool
Smooth integration with Microsoft Word
The learning curve for advanced features
Limited free trial

1. Spotting the Knowledge Gap: Your Starting Line
Kick off your independent research by spotting a gap in existing knowledge. This is an underexplored area ripe for discoveries. While some might immediately see this gap, others could need to dig deeper. A thorough literature review on your topic of interest can help. Many academic papers have insights into unanswered questions in their discussion or conclusion sections, making them an excellent resource for formulating your research question. Plus, this process can help you think about methodologies to use.
2. Securing a Mentor: Your Research Sidekick
Sure, you’re going for independence in your research, but mentors are assets, not obstacles. Even if you know a lot about your topic, you’re still in learning mode. Having an expert guide you through feedback and advice can be a game-changer and might even be necessary for progress. Once you have a research question, collaborate with faculty or professionals open to supporting you. For example, in a project I did on fentanyl overdose deaths, I needed access to autopsy reports. I got the needed resources by proposing my idea to a local forensic pathologist and gaining her mentorship.
3. Shaping Your Project: Setting Goals and Plans
With your research question ready, setting your project’s goals is time. Are you aiming for publication? Developing a product? Winning a competition? With these goals in mind, outline your objectives, methods, and timeline. Check out programs that support research projects, like the Holster Scholar Program and the UConn IDEA Grant. While exploring options, be realistic about the time and resources your project will need.
4. Making the Move: Get Going!
Independent research projects allow you to explore your passions and build essential skills. While the journey can be arduous, the knowledge and skills you gain are priceless. Enjoy the ride.
Let Otio be your AI research and writing partner. Try Otio for free today !
• Data Collection Tools • Title Page for Research Paper • How to Cite a Research Paper • Research Paper Abstract Example • How to Write Results in a Research Paper • How to Write a Psychology Research Paper • Best AI for Data Analysis • How to Write a Discussion in a Research Paper • Research Questions Examples • Best AI for Writing Research Papers • Ai Visualization Tools
Today, researchers and students face a storm of information. Too many settle for complex bookmarking and note-taking apps to manage their workflow, but this is not sustainable. As content creation becomes more accessible, the overload will only increase. What’s the solution? Otio offers a single AI -native workspace covering all research stages—from collecting diverse data sources to producing first drafts. Think about it: why scatter your efforts when one app can guide you through your entire research process?
A Researcher’s Best Friend: Collecting and Organizing Data
Otio is your research partner , helping you gather everything from tweets and books to videos. You don’t have to skip between different platforms, losing precious time. Otio lets you bring it all together in one place. Consider having a treasure trove of information, all neatly organized and ready to explore. This means less time hunting for data and more time connecting ideas.
Decoding the Noise: Extracting Key Takeaways
Once you have your data, you need to make sense of it. Otio uses AI to generate detailed notes on your bookmarks. Whether a YouTube video or an academic article, the platform highlights the most critical points. You can chat with your sources or entire knowledge bases, just like ChatGPT. This means you can ask questions and get answers fast. You won’t have to sift through endless content pages to find what you need.
From Reading List to Research Draft: Creating with Confidence
One of Otio’s most loved features is its ability to help you draft your work. Once you’ve collected and understood your data, the platform assists you in writing your research paper or essay. You can go from a reading list to a rough draft quickly, allowing you to focus on refining your ideas . This means you can spend less time struggling with writer’s block and more time producing meaningful work.
• Note-taking AI for Students • Zotero vs Mendeley • Logseq vs Obsidian • Milanote vs Notion • Obsidian vs Evernote • Claude AI Alternative • Milanote vs Miro • Best Chat Gpt Alternatives • Writesonic vs Jasper

Oct 23, 2024
How To Analyze Quantitative Data In 9 Simple Steps

Oct 22, 2024
How To Find Academic Sources In 4 Simple Ways
Join over 80,000 researchers changing the way they read & write

Chrome Extension
© 2024 Frontdoor Labs Ltd.
Terms of Service
Privacy Policy
Refund Policy
Join over 50,000 researchers changing the way they read & write
Join thousands of other scholars and researchers
Try Otio Free
© 2023 Frontdoor Labs Ltd.
- Request Info
- Browse Degrees
- Life at SLU
- Give to SLU
- Search & Directory
Conducting Research After IRB Approval
An email will be sent from Saint Louis University's eIRB system letting you know when the study has been approved. IRB approval is documented by a full approval letter, which is accessed in the eIRB system.
Do not start your research project until the approval letter has been issued and all other applicable institutional or hospital approvals are in place.
Importantly, researchers are expected to carry out their research in accordance with the procedures and using the materials, approved by the IRB. Any deviations will require approval by the IRB prior to implementation of the change.
This page contains information on IRB expectations for conducting IRB-approved research and the forms and guidance you may need along the way.
Saint Louis University investigators are responsible for conducting research in an ethical and professional manner, and in accordance with federal regulations (both the general regulations pertaining to human subjects protection in 45 CFR 46 and 21 CFR 50 and 56, and any specific regulations that may be imposed by the federal agency funding the research), local, state, and federal laws, Saint Louis University policies, and the IRB-approved protocol.
Any non-compliance must be promptly reported to the IRB.
The principal investigator (PI) is also responsible for the timely and proper administration of the research project. Beyond the scientific and clinical conduct of the study, responsibilities include:
- Fiscal management of the project
- Training and supervision of research team members
- Compliance with the sponsor’s terms and conditions (e.g., non-disclosure of sponsor confidential information)
- Submission of study amendments and continuing review applications in a timely manner
- Obtaining approval for protocol changes prior to implementation
- Timely submission of post-approval reporting requirements
- Disclosure of any potential conflicts of interest (for self and research team members)*
* Disclosures of potential conflicts of interest are reviewed and managed by institutional COI committees or designees; however, the IRB has the final authority to decide whether the potential conflict of interest and its management, if any, allows the research to be approved.
Please be aware of post-IRB approval submission requirements for more information on the types of events, when to submit, and the appropriate form to use. The following activities require further submission to the IRB. Submissions should be done in eIRB; for paper SLU IRB studies, links to paper forms are below.
Modifications to the approved research may not be implemented without prior IRB review and approval, except when necessary to eliminate immediate hazards to research participants. Modifications include revisions to any protocol, research procedure, recruitment plan, study instrument, research personnel, study site, or related study document. For studies not in eIRB: Change-in-Protocol/For Information Form .
Continuing Review
IRB review is required at least annually for all non-exempt research unless a shorter approval period was determined by the IRB. The purpose of continuing review is to monitor the progress of the study and ensure that it continues to meet the requirements for approval. It is the principal investigator's responsibility to complete the continuing review form in a timely manner or study approval may expire. For studies not in eIRB: Continuing Review Form/Notice of Study Closure .
Reportable Events
Serious adverse events, unanticipated problems, protocol violations, complaints and other events must be reported as detailed in the IRB Reportable Events Guidelines . For studies not in eIRB: Change-in-Protocol/For Information Form or Serious Adverse Event (SAE) Report Form .
- Decision Tree for Reporting Protocol Violations (PVs)
- Decision Tree for Reporting Serious Adverse Events (SAEs)
- Decision Tree for Reporting Unanticipated Problems (UPs)
Study Closure
If all research-related interventions or interactions with human subjects have been completed and all data collection and analysis of identifiable private information described in the IRB-approved protocol has been finished, the human subjects research study has been completed and the investigator should close the study. Studies should be closed in accordance with the SLU IRB Guidelines for Closure of Human Subjects Research Studies . Please note record retention requirements specified in that guidance. For studies not in eIRB: Continuing Review Form/Notice of Study Closure.
When faculty leave/are no longer SLU employees, they/their department are expected to close their study(ies), transfer the protocol(s) to another SLU investigator, or transfer them to their new institution. There have been exceptions made for Emeritus faculty, who can keep their protocols active with Dept Chair approval. Read our Guidelines for Closure of Human Subjects Research Studies for more information.
If terminating employment/association with SLU, PI should do one of the following:
- Close the study at SLU and submit a Final Report Form in eIRB; OR
- Transfer the protocol to another SLU investigator via an Amendment; OR
- Request transfer of research outside of SLU. Note: Research records must remain at SLU unless otherwise authorized in an agreement. See the SLU Policy on Research Records and Biological Specimens: Ownership, Retention, Transfer, and Destruction .
Industry-sponsored studies often are run with organizational binders provided by the study’s sponsor/CRO. For investigator-initiated studies, researchers can refer to the Regulatory Binder Guidance to put an organizational system in place. In addition, SLU provides some sample logs, though units are welcome to develop their own.
Sample Forms/Logs
- Delegation of Authority Log
- Eligibility Criteria Checklist
- Screening Enrollment Log
- Site Personnel Signature Log
- Study Specific Training
- Study Startup Checklist
- Subject Visit Tracking Log

IMAGES
VIDEO
COMMENTS
The IRB stamp of approval. For many students, obtaining Institutional Review Board approval is the first step they'll take toward making their research idea a reality. Here's how to make that process go smoothly. When University of Illinois at Chicago graduate student Jacklynn Fitzgerald first began working with her school's Institutional ...
As a high school student, whether or not you need to get IRB approval depends on your research goals. You may want to get your research published in a scientific journal or submit your project to a competition (e.g., research competitions for psychology and neuroscience).In these cases, you need IRB approval.
The NC State IRB Office asks three sequential questions to determine if IRB approval is necessary for a project and, if so, from where the IRB approval should be sought. If the answer is "yes" to the first question, we then proceed to the next question, and so on. If the answer to one of the first two questions is "no," then the study ...
Here are some tips for completing the Research Protocol to ensure that the IRB has the information it needs to review the study. Keep in mind that the IRB is reviewing the study to determine that it meets the criteria for approval. The more information the IRB has, the easier it can be to make the required determinations. 1.
Institutional Review Board Proposal Sample. Here is a sample proposal for an Institutional Review Board (IRB) submission: Title: Effects of Meditation on Stress Reduction in College Students. Introduction: Stress is a common issue among college students, and it can have negative effects on academic performance and overall well-being.
Your research advisor will help you determine which IRB should review your research protocol. JAASEP FALL, 2011 85. Information specific to your research institution may also be available via the Internet. Additionally, you may find that your advisor has received prior IRB approval via a grant-writing process.
The research may be approved by the IRB provided that the benefits outweigh the risks to participants. Factors that impact risk include: the procedure (possible harms); the person performing the procedure (training, experience, skill); the setting (privacy protections, availability of resuscitation equipment, etc.); and;
Before you request IRB review of a research study, you must complete CITI training with scores of 80% or greater in each module. If multiple people will collaborate on a research project, every one of them must complete CITI training. The ORSP Compliance website provides a link to the training and instructions for registering on the CITI site.
If your project is considered research under IRB rules, you must submit an application to the IRB office and receive approval before research can begin. Research under IRB regulations (as specified under the 'Common Rule' issued by the Office of Human Research Protections, U.S. Dept. of Health and Human Services) and Cornell policy is ...
If you submit a complete application to the IRB, you can generally expect the following turnaround times: If your project is exempt: 2-3 weeks (rolling review) If your project receives expedited review: 3-4 weeks for initial approval; 1-2 weeks for an amendment (rolling review) If your project needs full board review: a minimum of 4-6 weeks ...
Approval of Research. The ETSU and ETSU/VA IRB are required to make certain determinations before the IRB can approve any research. This includes all approval decisions, including initial full studies, initial expedited studies, changes to approved research, and continuing reviews, etc. Those required determinations are found in the rules that ...
Note: All members of the research team must complete training, including co-investigators, research assistants, and/or faculty advisors. STEP 3: Prepare for submission to the GSRD Office your protocol package, which should include the following as applicable to your research: 1. Request for Review of Research Involving Human Subjects 2.
If you have a question that is not answered below, or if you need to determine whether or not your project needs to be reviewed by the IRB, visit the IRB website at https://irb.truman.edu and/or contact the Grants Office, IRB Administrator, McClain Hall 203, 660-785-7245, or talk to the IRB member in your academic department.
If your research duration is long than one year, you will need to submit an Annual Review Form prior to the anniversary of your research project application's approval. Step 6: Submit a Close-Out Form. Once you have finished gathering data, you will need to submit a Research Project Closure Form to the IRB. This notifies the IRB that its ...
The need to obtain research ethical approval is common to all research involving human participants. This approval must be obtained before research participants can be approached and before data collection can begin. The process of ethical review is one way that research participants can be confident that possible risks have been considered ...
Procedures and Criteria for Approval. Navigate Research and Innovation. Initial Approval. The principal investigator may be asked to meet with the IRB should it be apparent that clarification or modification of statements in the application are required. No individual involved in the conduct and/or supervision of the research project shall ...
Likewise, certain course projects whose results are intended for dissemination might be eligible for blanket approval. If you are unsure whether the student research you supervise is subject to IRB review or if blanket approval is available, check with your IRB chair or compliance officer beforehand. Theory
Funding. As the largest public funder of biomedical research in the world, NIH supports a variety of programs from grants and contracts to loan repayment. Learn about assistance programs, how to identify a potential funding organization, and past NIH funding. Find a Fit for Your Research: NIH Institutes, Centers, and Offices. Grants Process.
When an IRB reviewing a research project under an expedited review procedure is unable to approve the project because the chairperson (or designated reviewer(s)) cannot make the determinations required for approval, the IRB chairperson (or designated reviewer(s)) can either refer the project to the IRB for further review and action at a ...
Check out programs that support research projects, like the Holster Scholar Program and the UConn IDEA Grant. While exploring options, be realistic about the time and resources your project will need. 4. Making the Move: Get Going! Independent research projects allow you to explore your passions and build essential skills.
Conducting Research After IRB Approval. An email will be sent from Saint Louis University's eIRB system letting you know when the study has been approved. IRB approval is documented by a full approval letter, which is accessed in the eIRB system. Do not start your research project until the approval letter has been issued and all other ...