

Crushing Can Experiment : Effect of Atmospheric Pressure
- December 3, 2020
- 7-9 Year Olds , Physics , Rainy Day Science
You may be used to crushing can using foot or hand. Have you crushed it using an implosion?
Today were are going to explore effect of Atmospheric Pressure with ‘Crushing Can Experiment’.

Air Pressure Can Crusher Experiment
The pressure created in the air surrounding us plays an important role while doing this activity.
Objective: To crush the empty soda can and explore simple science concepts like air pressure, equilibrium, water vapor, condensation, and unbalanced forces.
Hypothesis: If water in a can heated to reach its boiling point and then dipped by inverting in a cold bowl of water, this would create vacuum and result in decreased vapor pressure resulting in crushing of the can (implosion).
Safety Measures: Of course, the activity looks simple and easy while offering a lot of fun! But this experiment is in need of a burner or any other heat source and implosion happens suddenly. So, this experiment is not to be done by kids. However, under adult supervision, the activity can be explored.
Materials Mandatory to Gather:

- A Glass Bowl
- Empty Cans (we chose 3 empty soda cans)
- Burner or Stove or any other heating source
Simple Guide to the procedure of the Crushing Can Experiment
Let us step into the simple instructions guide!

Step-1: Clean all the Necessary Containers
Cleaning is the foremost and important step to do before we start any experiment. Because the remnants attached to the glass bowls and other containers may change the final results of the experiment.
So, in the process of cleaning, pick the empty soda cans and glass bowls. And rinse them thoroughly with clean water. Make sure they are clean and perfect for using in the experiment.
Step-2: Pour Cold Water into the glass bowl

Take the cleaned glass bowl and fill it with fresh ice cubes to its half. Then pour some normal water into the bowl. Such that we are making sure the water in the bowl are cold for longer time.
Or else you can use cold or chilled water directly from the freezer and pour it in the glass bowl.
Step-3: Fill the Empty Cans

Now it is time to fill the cleaned empty soda cans with a little amount of water. 2-3 table spoons of water is more than enough to pour into the cans.
Normal and regular water is preferred to use. But make sure the water you are using is fresh and clean.
Step-4: Heat the Cans

The next step is the precautionary step as we are bringing burner into the picture. Yes, switch on the burner and take the water filled cans on to the burner. You can see the videos or pictures attached to get an idea.
We took three empty cans and filled with 2 table spoons of normal water. Then, we brought all these three water filled cans on to the burner and set the right temperature to make it boil.
Wait for some time until the water inside the cans are boiled enough. It just takes a couple of minutes to do the job.
Note: If your child is performing this experiment on his/her own, then this is the step where adult supervision is compulsory to avoid unnecessary accidents that happen with the heat. So, adults please supervise your children to heat the cans on the burner.
Step-5: Take out the Heated Can

Once you feel the sounds of water bubbles formed due to enough heat supplied to the water inside the cans, wait for one more minute.
And then observe the water vapor fumes coming out of the cans. That is the time, you need to switch off the burner and take out the cans from the burner.
Do not forget to use the tongs in order to handle the heated cans.
Step-6: Place over the heated can upside down into the Glass Bowl

This is another important step to do with extra care!
Yes, to see the positive results, you need to perform this step with utmost care.
Soon after you remove the cans from the burner, using tongs place the cans over the glass bowl filled with cold water.
The trick here is you need to place the cans upside down into the glass bowl. That’s it! You can see and hear a loud noise of popping out sound from the can.
Can you guess why we hear that sound? That’s the sound of the crushing can!
As soon as the heated cans brought in to the glass bowl filled with cold water upside down, the cans get crushed and collapses on their own.
Crushing Can Experiment Calculations

In this modern world, we generally come in touch with 12oz drink cans like soda or beverages can.
Mostly, we find them in aluminum material. The approximate measures of these cans are like: 4.75 inches or 12cm in height and 2.5 inches or 6.5cm in diameter which in total makes the whole area of this cylindrical can to 49.5 square inches or 315 square cm.
The dimensions of the cans are also designed specially in order to withstand the outside pressures. A square inch of a soda can bears 80-90 pounds of force from outside.
One atmosphere pressure force is equals to 720lbs or 3200newtons. So, in order to crush a soda can, you need to give nearly 50 pounds of force.
Do you want to know what the science behind Crushed Can?
An empty aluminum soda can is full of air molecules. When you apply enough force or pressure on the can from outside, there happens an imbalance between the pressures outside and inside the can.
In fact, the pressure outside the can is stronger and more than the pressure inside the can.
At the time, the can from outside experiences enough pressure, it collapses and gets crushed immediately. This is how air pressure plays important role in crushing an aluminum can in our hands.
Let us know what is air pressure? Air pressure is the pressure or force created by the surrounding air on the surfaces within the atmosphere as gravity pulls. Hence, it is otherwise known as atmospheric pressure.

Science behind Crushing Can Experiment
As I already told you, the empty can is not really empty instead filled with air molecules.
When the can filled with little water and arranged for heating on the burner, the water inside the can starts boiling.
Once the water starts boiling, there happens the transformation of liquid state to gaseous state.
This means we can observe water vapors coming out of the can through the fumes. Evaporation is the process of converting liquids into gases, which is the result of heating cans on the burner.
Eventually, the can is filling with vapors replacing the air molecules. Soon after you remove the cans from the burner and bring it over the chilled water in a bowl, the water vapor condenses.
You can see the condensation process clearly when there is formation of a few drops of liquid back on the can inside.
Unfortunately, these little liquid droplets are not strong enough to produce enough pressure inside the can. That is the reason the pressure outside the can is much more and stronger than the pressure inside the can.
Hence, the strong pressure developed outside the can is good enough to crush the can from outside.
Here, we need to talk about “Implosion”. Implosion is nothing but a sudden process of something collapsing themselves towards inside violently.
Implosions happen when there is heavy pressure from outside an object rather inside and finally destroys the object inwards. Explosion is quite opposite to implosion.
Matter and energy plays important role in causing explosions and implosions.
In this experiment, due to imbalance of pressures between outside and inside surroundings of can, implosions happens.
Because to maintain and bring the balance between outside and inside pressures of can.

What happens after Implosion?
After implosion, just observe the inside of the can, you can observe water filled inside the can. This is the water dragged from the glass bowl due to stronger pressure build outside the can.
This high pressure created pulls the water from the glass bowl into the can where there is less pressure.
What is Gas Law and How it works?
Gas laws are the laws which establishes the association among pressure, volume, temperature, and the quantity of gas. In simple words, gas laws were designed around 16th-17 th century to learn the amazing properties of matters of gas regarding amount, volume, pressure, and temperature.
Generally, there are different types of gases available and all these gases behave differently while showing their chemical properties but strictly follow gas laws in the same way.
P= Pressure
n= Amount of Substance
R= Ideal Gas Constant
T= Temperature
The three principal and basic laws of gas includes: 1) Boyle’s Law
2) Charles Law
3) Avogadro’s Law
These three gas laws states different equations and properties but at the end they all come under Ideal Gas Law and General Gas Equation. Well, let us the three main gas laws in detail:
Boyle’s Law
Robert Boyle put Boyle’s Law into words in 1662 stating that the pressure of a gas is inversely proportional to volume of a gas. To keep it simple, if there is less volume of gas then there is more pressure on the gas at constant temperature. This law is otherwise known as Boyle–Mariotte law or Pressure-Volume Law.
V is inversely proportional to 1/P
Charles Law
Jacques Charlesformulated Charles Law that states that when pressure remains constant, the volume of stable amount of gas is directly proportional to temperature. In simple words, the rise in the volume of gas tends to increase the temperature as well. This law is otherwise known as Temperature-Volume Law.
Avogadro’s Law
Amedeo Avogadro established the Avogadro’s Law which states that volume of a gas is directly proportional to amount of gas at constant temperature and pressure. Modern Avogadro’s Law states that equal amount of volume of gas consists of equal amount of molecules at constant temperature and pressure. This law is otherwise known as Volume-Amount Law.
All these experimental gas laws are a part of Ideal Gas or General Gas Equation Law. Let us see what ideal or general gas equation law is.
Ideal Gas Law
Ideal Gas Law is also known as General Gas Equation Law. It states that it is a combination of all three gas laws and finally proves that pressure, volume, temperature, and amount of a gas relate each other. The gases that are fit to establish perfect relation between volume, temperature, pressure, and amount of gases are referred as ideal gases. The equation says:
When the aluminium can is hot, the pressure outside and inside the hot can are same. And when it is flipped upside down over the glass bowl containing cold water, immediately you can see sudden drop in temperature. Hence, the water molecules get cool rapidly causing imbalance in the outside and inside pressures around the can. The pressure outside the can is stronger and more compared to the pressure inside and hence the can pops out and collapses itself towards inside.
Crushing Can Experiment proves the Boyle’s Law, which is one of the major fundamental and experimental gas law of ideal gas equation law. Boyle’s law states that the volume of certain amount of gas is inversely proportional to pressure of a gas.
According to the sources and research studies, 10-20 pounds of force is necessary to crush an aluminium can. If you just want to open the mouth of the aluminium can, you need 1-2 pounds of force. Whereas nearly 50 pounds of force is necessary for crushing a steel beverage can. Remember that these numbers are just an estimated ones.
Take an empty aluminium soda can and pour two table spoons of normal fresh water. Now bring the can on to the burner and heat it until you hear the boiling water sound. When you observe the steam coming out of the can, switch off the burner. And pick the hot can using tongs and carefully flip it upside down over the glass bowl containing chilled water. That’s it, you can see the hot can crushing with a pop sound.
Jacques Charles formulated Charles Law that states that when pressure remains constant, the volume of stable amount of gas is directly proportional to temperature. In simple words, the rise in the volume of gas tends to increase the temperature as well. This law is otherwise known as Temperature-Volume Law. V T
Soda cans are generally encompasses of aluminium to keep its structure solid and strong enough to withhold the outside pressures. A regular soda can bears 80 pounds-90 pounds of pressure per every square inch. According to the research studies, 1pound-2pound force is necessary to open the mouth of the soda can whereas to crush it completely, nearly 50 pounds of force is necessary.
We have so many real life examples to discover Charles law around us but we just ignore to observe. Here is a classic example: the tyres of vehicles get deflated during chilled winter season whereas the same gets inflated during summer months: this phenomenon is due to Charles Law. During winter days, because of chilled temperature, the gas inside tyres get more cool and starts shrinking as well. While during summer months, due to hot temperature the sir inside tyres gets hot and starts expanding. This is the reason why tyres of some vehicles expand during summer and deflate during winter months.
Charles law does show its impact on human body but it is not much. You all might have observed shortened breath during winters and normal breathing during summer months. This is because of Charles law. Yes, during winter months, the chilled temperature outside causes the change in inside temperature of lungs. The inhaled cold air when passes through sinuses, gets warmed and expands in its volume. When the volume increases, you need to take shorter breaths in order to balance the increased volume. In this way, Charles law affects human body.
A: In real life, we can observe a lot of things happening around us which are actually related to ideal gas laws. Let us see a few of commonly happening things: 1) An inflated football shrinks when left aside during winter months—Proves Charles Law 2) Leave the slightly inflated rubber life raft under sun light for some hours and observe the swelling of the raft– Proves Charles Law 3) Increase in number of bubbles blowing out by a scuba diver especially when approaching the surface or river banks—Proves Boyle’s Law 4) Death of deep sea fish when brought to the surface or land– Proves Boyle’s Law 5) Expansion of lungs when filled with air and shrinks when air released—Proves Avogadro’s Law 6) Humid air is more thick than damp air—Proves Avogadro’s Law
Gases have low densities than solids and liquids because in solids and liquids the molecules are tightly and narrowly filled. Whereas gaseous molecules do not pack up closely and occupy more volumes because these molecules fall apart. This is the reason why gases have low densities.
Before heating, the air pressure outside and inside the open soda can measures equal. Because the open can is not really empty instead occupied with air molecules.
When you pour two table spoons of water inside can and kept on the burner, the water reaches its boiling point and leaves steam. This steam occupies the rest portion of the can inside and substitutes all the air molecules.
The process of conversion of a gas into a liquid is known as condensation. The reverse process of conversion of a liquid turning in to gas is known as evaporation. Evaporation and condensation are the nature’s fundamental phenomenon and goes hand in hand.
Crushing Can Experiment Worksheets
Simple worksheet for Class room : https://www.wlwv.k12.or.us/cms/lib/OR01001812/Centricity/Domain/2114/Can%20Crush%20Key.pdf
https://www.csub.edu/chemistry/_files/The%20Can%20CrusherAO.pdf
Explanation with method to do the experiment in large setup : https://www.flinnsci.com/api/library/Download/12267d76eaf04431b63a82777bb16195
Interesting Air Pressure Experiments
Balloon in a Bottle
Tornado In a Bottle
Drip Drop Water Bottle
Candle Rising Water Experiment
DIY Balloon Rocket
One comment
How is the can crushed if it is not sealed? Surely it would simply suck up the cold water you mention that is used for condensing the steam.
Leave a Reply Cancel Reply
Your email address will not be published. Required fields are marked *
Name *
Email *
Add Comment *
Save my name, email, and website in this browser for the next time I comment.
Post Comment
- PRO Courses Guides New Tech Help Pro Expert Videos About wikiHow Pro Upgrade Sign In
- EDIT Edit this Article
- EXPLORE Tech Help Pro About Us Random Article Quizzes Request a New Article Community Dashboard This Or That Game Happiness Hub Popular Categories Arts and Entertainment Artwork Books Movies Computers and Electronics Computers Phone Skills Technology Hacks Health Men's Health Mental Health Women's Health Relationships Dating Love Relationship Issues Hobbies and Crafts Crafts Drawing Games Education & Communication Communication Skills Personal Development Studying Personal Care and Style Fashion Hair Care Personal Hygiene Youth Personal Care School Stuff Dating All Categories Arts and Entertainment Finance and Business Home and Garden Relationship Quizzes Cars & Other Vehicles Food and Entertaining Personal Care and Style Sports and Fitness Computers and Electronics Health Pets and Animals Travel Education & Communication Hobbies and Crafts Philosophy and Religion Work World Family Life Holidays and Traditions Relationships Youth
- Browse Articles
- Learn Something New
- Quizzes Hot
- Happiness Hub
- This Or That Game
- Train Your Brain
- Explore More
- Support wikiHow
- About wikiHow
- Log in / Sign up
- Education and Communications
- Science Experiments
- Junior Science Experiments
How to Crush a Can with Air Pressure
Last Updated: May 1, 2024 Fact Checked
This article was co-authored by Meredith Juncker, PhD . Meredith Juncker is a PhD candidate in Biochemistry and Molecular Biology at Louisiana State University Health Sciences Center. Her studies are focused on proteins and neurodegenerative diseases. This article has been fact-checked, ensuring the accuracy of any cited facts and confirming the authority of its sources. This article has been viewed 215,297 times.
You can crush a soda can using nothing more than a heat source and a bowl of water. This is a great visual demonstration of some simple scientific principles, including air pressure and the concept of a vacuum. The experiment can be performed by teachers as a demonstration, or by mature students under supervision.
Crushing a Soda Can

- Continue only with adult supervision.

- If you smell something strange or metallic, move on to the next section right away. The water might have boiled away, or the heat might have been too high, causing the ink or aluminum on the can to melt. [3] X Research source
- If your stove burner cannot support the soda can, use a hot plate, or use tongs with heat-resistant handles to hold the soda can over the stove.

- Be prepared for a loud noise as the can is rapidly crushed! Because of the sound, avoid performing the experiment around children younger than kindergarten-age.
How it Works

- Despite the can losing some of the air inside it, it doesn't get crushed yet, because the water vapor that took the place of the air is pushing from the inside instead.
- In general, the more you heat a liquid or a gas, the more it expands. If it is an enclosed container so it can't keep expanding, it exerts more pressure. This is known as Gay-Lussac’s Law.

- Space that has nothing in it is called a vacuum .

Helping Students Learn from the Experiment

- If a student thinks the water (not the water vapor) inside the can was responsible for it getting crushed, have the students fill an entire can with water, and see if it is crushed.
- Try the same experiment with a sturdier container. The heavier material should take longer to be crushed, which will give the ice water more time to fill it.
- Try letting the can cool for a short time before putting it in the ice bath. This will result in more air being present in the can, and thus less severe crushing.

Expert Q&A

- Lower the can into the water with the tongs, rather than dropping it. Thanks Helpful 8 Not Helpful 8

- Older children (ages 12+) may be able to do the activity themselves, but only under adult supervision! Never allow more than one person to do the demonstration at a time, unless there is more than one supervisor present. Thanks Helpful 7 Not Helpful 8
- The can and the water inside will be hot. Have participants stand back as the can is flipped into the water, to avoid getting injured from hot water spray. Thanks Helpful 5 Not Helpful 5
Things You'll Need
- Empty aluminum soda cans
- Tongs large enough to comfortably handle the hot cans
- Stove, hot plate, or Bunsen burner
- Bowl of cold water
You Might Also Like

- ↑ https://www.physics.colostate.edu/physics-demos/can-crush-by-air-pressure/
- ↑ https://www.youtube.com/watch?v=mHzb8QMeZmI
- ↑ https://circus.physics.ucsb.edu/2021/12/07/crushing-soda-cans-with-air-pressure/
- ↑ http://www.stevespanglerscience.com/lab/experiments/incredible-can-crusher
About This Article

- Send fan mail to authors
Reader Success Stories
Saimon Mengisteab
Mar 27, 2018
Did this article help you?

Dec 7, 2016

Featured Articles

Trending Articles

Watch Articles

- Terms of Use
- Privacy Policy
- Do Not Sell or Share My Info
- Not Selling Info
Get all the best how-tos!
Sign up for wikiHow's weekly email newsletter
STEM Little Explorers
Knowing through exploring.
Home » Articles » STEM » STEM Science » How to Demonstrate Air Pressure with Can Crush Experiment

How to Demonstrate Air Pressure with Can Crush Experiment
You are probably familiar with blood pressure and its health-related symptoms. but do you know what pressure really is and what effect can it have on our environment join us today while we explore this topic with a simple can crush experiment, article contents.
What is the air pressure?
Atmospheric or air pressure is a force applied on a surface by the air above because of the influence of gravity . Gravity is pulling down the air molecules and they are pressing their weight on top of the Earth’s surface.
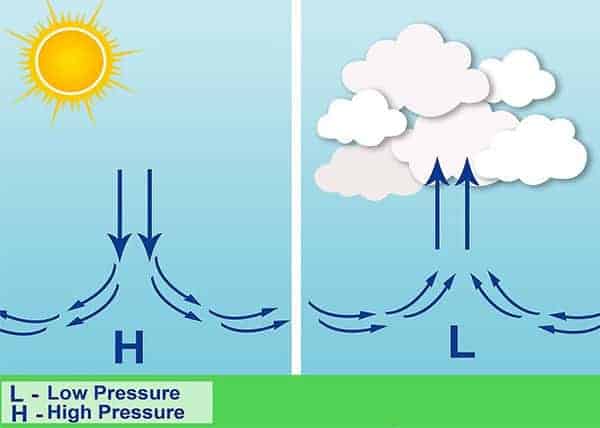
The pressure is highest at sea level , where there is the highest density of those air molecules. As the altitude increases, the atmospheric pressure drops.
The amount of oxygen in the air also decreases with the pressure decreasing. The higher we go, the lower the pressure and amount of oxygen. That is why at high altitudes, we can feel dizzy and sick due to the lack of oxygen.
Besides altitude, the amount of humidity is an important influence on air pressure. Water molecules in the air, contained in the water vapor, weigh less than the oxygen (and other gas molecules) so as the air becomes more humid, it’s lighter and therefore the pressure is lower.
At sea level, air pressure affecting our body is approximately 15 tons! But that pressure won’t crush us since there is also an inner pressure of equal size so they nullify each other.
The atmospheric pressure is measured using a barometer . As the weight of the atmosphere changes, mercury in a glass tube rises or falls. The mercury rises when the atmospheric pressure is high.
Materials Needed for the Air Pressure Experiment

- Empty can. A standard soda can or beer can will do great for our experiment. These aluminum cans can be easily obtained in any grocery store since many beverages come packed in cans.
- Bowl of Water . Put some cold water into a bowl or a basin. It doesn’t have to be ice cold, just regular cold water from the pipe will be enough.
- Kitchen Tongs . We will need something to hold a hot can, and kitchen tongs are the best tool we can use for that.
- Heater or stove. For getting the water inside of the can boiling, we need a heater. We can use our kitchen stove to achieve our goal.
- Optional: Spoon and Funnel . The spoon and funnel will help us in adding a little bit of water inside of the can. We can just add water directly from the pipe so this is optional. But for demonstration purposes, it is good to show adding water in the can.
Instructions on doing the Can Crush Experiment
If you’re interested in a video guide, you can watch the video at the beginning of the article. And for step-by-step instructions and explanations, continue reading further.
- Put one or two tablespoons of water (around 15 milliliters) into the empty can . Here you can use a funnel and spoon to demonstrate the procedure. Or you can add the same amount of water directly from the pipe if you are not demonstrating the experiment to other people.
- Prepare cold water in some container that is big enough to sink the can in it. We will do that in just a minute.
- Heat the can until the water inside starts to boil . For this, you can use the stove or a heater. Since the amount of water inside the can is small to hear water bubbling, you can tell that it is boiling when you notice steam coming out of the can.
- Use the kitchen tongs to grab the can. Be careful, the can is very hot so use tongs to not get burned. Turn the can upside down and sink it in the cold water container .
- You will instantly hear a loud pop and the can will crush because of the pressure difference.
Science Behind The Air Pressure Experiment
So what happened to the can? Why did it get crushed? By heating up our can, we boiled the water inside it. The process of boiling turned the water into vapor. And since the water vapor molecules are much more spread out than the water molecules, they take more space and are forcing the molecules of air out from the can.
And when we put the can in the cold water, we suddenly cooled it. That cooling caused the water vapor in the can to condense, creating a partial vacuum . Because of that, the pressure outside of the can became much greater than the pressure inside, and that pressure difference crushed the can .
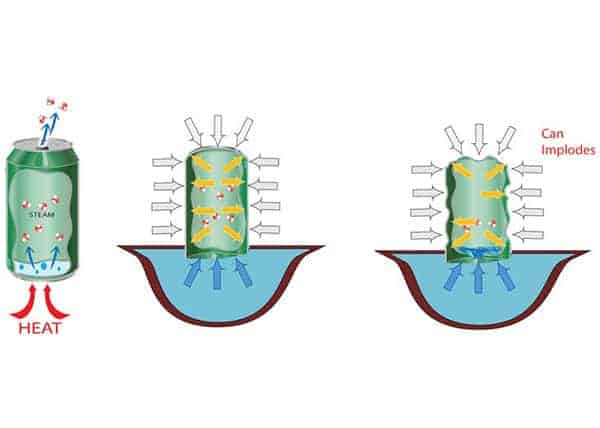
So in summary, a can will get crushed when the pressure outside is greater than the pressure inside of the can. Also, the pressure difference must be greater than the one the can is able to withstand . For example, we can easily crush an aluminum can with our hands. When we squeeze the can, the pressure outside becomes greater than the pressure inside. If we squeeze hard enough the can collapses.
This phenomenon also has a name – implosion! As you may guess, an implosion is the opposite of an explosion. Implosion is a process in which objects are destroyed by collapsing on themselves. Implosion reduces the volume of an object and concentrates matter and energy into a smaller space.
We may also ask ourselves: “Won’t the water from the bowl fill the can through the hole when we sink the can?” Some water may actually come inside of the can, but the water cannot flow into the can fast enough to fill it before the air outside crushes it.
What will you develop and learn by doing the Can Crush experiment?
- Knowledge from chemistry and physics
- What is the atmospheric pressure and how does it work
- How is pressure affecting the human body
- What is Implosion
- How to conduct an experiment
We hope you enjoyed this science activity as much as we did. And if you are searching for more similar science experiments, we have the right ones for you:
- For another method to demonstrate air pressure, check out How to Demonstrate Air Pressure with Balloon experiment.
- To learn more about oxygen effects, check out the Apple Oxidation Experiment activity .
- Check the Diffusion experiment to learn the different properties in hot and cold water.
- For Heat effects, you can check activity What is heat conduction and how to demonstrate it .
- And to learn more about heat effects on different materials, check the Shrinking bag experiment .
And until next time, happy exploring!
If you’re searching for some great STEM Activities for Kids and Child development tips, you’re in the right place! Check the Categories below to find the right activity for you.

STEM Science
Videos, guides and explanations about STEM Science in a step-by-step way with materials you probably already have at your home. Find new Science ideas.


STEM Technology
Videos, guides and explanations about STEM Technology in a step-by-step way with materials you probably already have at your home. Find new Technology ideas.

STEM Engineering
Videos, guides and explanations about STEM Engineering in a step-by-step way with materials you probably already have at your home. New Engineering ideas!

Videos, guides and explanations about STEM Math in a step-by-step way with materials you probably already have at your home. Find new Mathematics ideas.

Find out all about development psychology topics that you always wanted to know. Here are articles from child psychology and development psychology overall.

First year of Child’s Life
Following a Child’s development every month from its birth. Personal experiences and tips on how to cope with challenges that you will face in parenting.
5 thoughts on “ How to Demonstrate Air Pressure with Can Crush Experiment ”
- Pingback: How to Demonstrate Air Pressure with Balloon - STEM Little Explorers
- Pingback: How to Shrink a Bag with Microwaves? - STEM Little Explorers
- Pingback: How to make a Match Head Rocket | STEM Little Explorers
- Pingback: How to Make a Potato Battery | STEM Little Explorers
- Pingback: What is Heat Conduction and how to demonstrate it | STEM Little Explorers
Leave a Reply Cancel reply
You must be logged in to post a comment.
Get Fresh news from STEM fields
I'm not interested in STEM
Get Your ALL ACCESS Shop Pass here →

Crushed Can Experiment
Love exploding experiments? YES!! Well here’s another one the kids are sure to love except this one is an imploding or collapsing experiment! All you need are a coke can and water. Learn about atmospheric pressure with this incredible can crusher experiment. We love easy science experiments for kids !

How to Crush a Can with Air Pressure
This simple science experiment has been on our to-do list for a while now because we wanted to know if air pressure can really crush a can! This soda can experiment is a great way to get your kiddos excited about science! Who doesn’t love something that implodes?
Check out our chemistry experiments and physics experiments !
Grab an empty soda can (Suggestion – use the soda for our pop rocks and soda experiment ) and find out what happens when you put a hot can in cold water! Make sure to have an adult involved with heating the can!
F ree printable STEM activities pack!
Can Crusher Experiment
Also check out how changes in pressure can suck an egg into a bottle.
- Empty aluminum can
- Heat source Eg stove burner
- Bowl of ice water
INSTRUCTIONS:
STEP 1. Prepare a bowl with ice and water,

STEP 2: Put about two tablespoons of water in an empty aluminum can.
STEP 3: Set the can on a stove burner or over a flame until the the water in the can turns to steam.
THIS STEP SHOULD ONLY BE DONE BY AN ADULT!

STEP 4: Use an oven mitt or tongs to carefully remove the steaming can from the heat source and immediately turn the can upside down into a bowl of cold water.

Prepare for a loud POP as the can implodes!

Why Does a Hot Can Crush in Cold Water
Here’s how the collapsing can experiment works. As the water in the can gets hot, it changes to steam. The steam or water vapor is a gas and so it spreads out and fills the inside of the can. This a great example of states of matter phase change, and a physical change !
When you flip the can and put it in cold water, the steam condenses quickly or cools and changes to a liquid state. This reduces the number of gaseous molecules in the can, and so the air pressure inside becomes lower.
Air pressure is the the force exerted onto a surface by the weight of the air. The difference between the low air pressure inside and the pressure of the air outside creates an inward force on the walls of the can, causing it to implode!
What does implode mean? Implode refers to exploding violently inwards rather than outwards.
MORE FUN EXPLODING EXPERIMENTS
Why not try one of these science experiments below!

Printable Science Projects For Kids
If you’re looking to grab all of our printable science projects in one convenient place plus exclusive worksheets and bonuses like a STEAM Project pack, our Science Project Pack is what you need! Over 300+ Pages!
- 90+ classic science activities with journal pages, supply lists, set up and process, and science information. NEW! Activity-specific observation pages!
- Best science practices posters and our original science method process folders for extra alternatives!
- Be a Collector activities pack introduces kids to the world of making collections through the eyes of a scientist. What will they collect first?
- Know the Words Science vocabulary pack includes flashcards, crosswords, and word searches that illuminate keywords in the experiments!
- My science journal writing prompts explore what it means to be a scientist!!
- Bonus STEAM Project Pack: Art meets science with doable projects!
- Bonus Quick Grab Packs for Biology, Earth Science, Chemistry, and Physics

Subscribe to receive a free 5-Day STEM Challenge Guide
~ projects to try now ~.

Colorado State University
College of natural sciences, department of physics, can crush by air pressure.

| Demo Number | 361 |
|---|---|
| Location | Shelf 15 |
| Description | The heated air inside the can contracts when exposed to the cold water in the bowl. The atmospheric pressure crushes the can. |
| Related Demos | |
| Tips |
Want to check out this demo?
Visit the reservation page , main demo categories: 0 homepage (1) 1 mechanics (107) 2 fluid mechanics (35) 3 oscillations and waves (55) 4 thermodynamics (35) 5 electricity & magnetism (110) 6 optics (49) 7 modern physics (18) 9 equipment (59) in storage (31) new demos in the last year (1), this demo is part of the following categories: 2b30 atmospheric pressure.
- AR124 Engineering Building 1875 Campus Delivery Fort Collins, Colorado 80523-1875
- In-Office Hours: Mon - Fri 8:00am - 4:30pm
- Telephone: 970-491-6206
- Email: [email protected]
Contact Details
- www.natsci.colostate.edu
- Biochemistry & Molecular Biology
- Computer Science
- Mathematics
Support the College
- Apply to CSU
- Contact CSU
- Equal Opportunity
- Privacy Statement
Explaining the Can Crush on a Particle Level
Throughout the KMT unit, we have been presented with experiments that involve the following variables: temperature, volume, pressure, and number of particles. The Can Crusher is not different in this aspect. Temperature in our experiment is seen both in the water temperature, and in the amount of time the can is left on the fire. Volume is seen in both the can volume and in the measurement of percent crush. The number of particles is seen in the initial water amount added to the can to start the experiment, and finally, pressure is obviously a factor (though not measured directly) throughout the experiment.
What is different in the Can Crusher experiment is that none of the variables (temperature, volume, pressure, or number of particles) are actually held constant throughout the experiment. In this case, identifying a simple relationship between two variables is impossible. In other words, the can crushing cannot be explained by simply stating that as pressure increases, volume decreases. The temperature was not held constant and neither were the number of particles in the can (the can remains open to the air as it is being heated, so particles in the can are allowed to escape). Stating that as temperature decreases, volume decreases cannot explain the entire phenomenon either as pressure is also changing through the experiment.
Explaining the can crush will require that you focus on what particles are doing on a microscopic level throughout the entire process. The can crush simulation includes a check box labeled "see-inside"; the simulation is included again below so you can perform a trial (or trials) while this box is checked. Be sure to observe the particles in the can throughout the entire can crush, then answer the questions below.
Describe the movement of the particles from the moment you place the can in the fire until the can has been in the fire for at least 60 seconds.
What effect would this movement (from question 6.1 above) have on pressure inside the can? Explain why.
How does the particle movement in the can change as the can is taken from the fire and flipped upside down into the ice bath?
What effect would this movement (from question 8.3 above) have on pressure inside the can? Explain why.
Describe how the particles are moving in the can after it is crushed.
Based on the particle movement after the can is crushed, what has happened to the internal pressure in the can? Why?
Notice the graph of particles in the cross-section at the right of the simulation. If you leave the can (with any initial amount of water in the can) in the fire long enough, particles will start to leave the cross-section. Explain this particle behavior.
Session Expiring
Your session is expiring in second(s), privacy policy.
Privacy Policy Lorem ipsum
Terms of Use
Curriculum materials on this site are licensed under a Creative Commons Attribution-Noncommercial-Share Alike 3.0 United States License subject to the following additional terms. The CT-STEM website and curriculum materials are distributed free of charge for non-commercial educational use. For any other uses of this website and materials, in original or modified form, including but not limited to distribution in whole or in part, specific prior permission must be obtained from the CT-STEM Project Investigators . The website and curriculum materials shall not be used, rewritten, or adapted as the basis of a commercial software product or as part of course materials that are licensed or sold commercially without first obtaining appropriate licenses from Northwestern University. We make no representations about the suitability of this website for any purpose. It is provided "as is" without express or implied warranty.
Funding Agencies
This work is supported by the National Science Foundation under NSF Grants CNS-1138461 and CNS-1441041 and by the Spencer Foundation. However, any opinions, findings, conclusions, and/or recommendations are those of the investigators and do not necessarily reflect the views of the funders.
Notification
Choose an Account to Log In

Notifications
Can crusher experiment.

This can crusher experiment is the perfect way to demonstrate the wonders of pressure and condensation. All you need is the power of air and water to produce the amazing end result: a crushed soda can!
What You Need:
- Empty soda can with tab removed
- Mixing bowl
Caution! Experiment involves heat and boiling water. Adult supervision required.
What You Do:
- Rinse the soda can. Then, place a couple tablespoons of water inside it—just enough to cover the bottom of the can.
- Place the can directly in a pan (use an old pan if you are worried about any damage caused by heating the can) and place on a stovetop burner. Turn the burner to medium–high heat. Allow the water in the can to heat.
- Fill the mixing bowl with about two inches of ice water.
- Once the water in the can is boiling (steam will be coming out of the can and you should be able to hear the “popping” sound of boiling water), use the tongs to remove the can from the pan and quickly (without splashing boiling water!) bring the can to the bowl of water, turn it upside down, and immerse the can in the cold water. The can should quickly be “crushed” by the cold water!
- Still holding the can with the tongs, pull the can out of the water and observe how much water pours out of the can.
- Tip: If the can doesn’t crunch on the first attempt, repeat the experiment. Consider using a different can, placing less water in the can, making sure the water in the bowl is very cold, or heating the can in pan longer.
Challenge your child to explain why the can was crushed. If she mentions pressure and/or condensation she's off to a great start! Here's what happened:
Boiling the water in the can decreases the air pressure inside, creating a partial vacuum. The water vapor produced by boiling the water pushes air out of the can, creating a lower pressure system inside of the can. Immersing the can in the cold water causes the can to implode because the pressure exerted by the water and air outside the can is greater than the pressure inside the can.
Condensation also occurs when the water vapor inside the can (a gas) quickly cools when the can is immersed in the cold water. The water vapor rapidly condenses and turns the water vapor into liquid water again. The molecules of the liquid water droplets take up less space inside the can than the molecules of the gaseous water vapor, once again causing the can to be crushed by the greater external pressure exerted by the water in the bowl and air pressure around the can.
Hopefully your child observed that more water came out of the can than was originally placed inside. The extra water was forced into the can as a result of the greater air pressure outside of the can.
Related learning resources
Add to collection, create new collection, new collection, new collection>, sign up to start collecting.
Bookmark this to easily find it later. Then send your curated collection to your children, or put together your own custom lesson plan.
Incredible Can Crush
Activity length, 15-20 mins., states of matter, activity type, discrepant event (demonstration only).
In this demonstration, students see firsthand how matter changes states and the incredible impact of atmospheric pressure on objects.
Air is made up of matter and takes up space and has mass. If the amount of air inside an object changes, the atmospheric pressure outside of the object may have a greater impact on the object itself.
Lowering the pressure inside a pop can by heating it up creates a situation in which the pressure of the atmosphere can crush the pop can. This happens because the small amount of water that is placed inside the pop can becomes water vapour when it is heated up and displaces the air inside the can. Then, when the pop can is quickly cooled, the water vapour returns to a liquid state and since the air pressure outside of the can is greater than that on the outside, the pop can is quickly crushed.
Describe the properties of a solid, a liquid, and a gas.
Describe the transitions between different states of matter.
Per Class or Demo: empty pop can tongs container cold water hot plate
Key Questions
- What happens to water inside the pop can when it is heated up?
- What happens to the air inside the pop can when the water becomes steam?
- Why does the pop can get crushed when it is quickly cooled?
Preparation:
- Rinse out an empty pop can for the demonstration.
- Set aside a container filled with ice-cold water.
- Add approximately 10mL (1 tablespoon) of water to the empty pop can.
- Discuss what you are going to do with your students and ask them to record predictions for each stage of the experiment
Demonstration:
- Use the hot plate to heat up the water inside the pop can.
- As the water heats up and comes to a boil, it will become visible as steam. The steam will fill up the interior of the can and displace the air inside the pop can. Any remaining air will heat up as well.
- Use the tongs to remove the can from the heat and quickly turn it over, mouth first, into the container of cold water. The pop can should become crushed because the very little remaining air quickly cools and the steam becomes liquid. Since the atmospheric pressure is greater than the pressure of the leftover air inside the can, the pop can is crushed.
- Turn off the hot plate and discuss what happened as a group.
Teacher tip: Practice this demonstration before trying it in front of the class.
About the sticker
Artist: Jeff Kulak
Jeff is a senior graphic designer at Science World. His illustration work has been published in the Walrus, The National Post, Reader’s Digest and Chickadee Magazine. He loves to make music, ride bikes, and spend time in the forest.
Comet Crisp
T-Rex and Baby
Artist: Michelle Yong
Michelle is a designer with a focus on creating joyful digital experiences! She enjoys exploring the potential forms that an idea can express itself in and helping then take shape.
Buddy the T-Rex
Science Buddies
Artist: Ty Dale
From Canada, Ty was born in Vancouver, British Columbia in 1993. From his chaotic workspace he draws in several different illustrative styles with thick outlines, bold colours and quirky-child like drawings. Ty distils the world around him into its basic geometry, prompting us to look at the mundane in a different way.
Western Dinosaur
Time-Travel T-Rex
Related Resources
States of matter can be more than just your average solids, liquids and gases when water turns into snow, is…, in these activities students explore the impressive force of air and learn how air pressure affects their daily lives., related school offerings.

Exploring Matter

Changing Matter
We believe that now, more than ever, the world needs people who care about science. help us fund the future and next generation of problem solvers, wonder seekers, world changers and nerds..
- Air Pressure
- Newton's 2nd Law - Unbalanced Forces
- Condensation's Effect on Pressure
- Equilibrium
- As the water is heated and boiled, steam displaces the air in the container above the water. When the soda can is plunged into cold water, the vapor condenses quickly, leaving a vacuum in most of the can. The resulting large discrepancy between the outside and inside air pressure leads to a large net inward force on the can, ending with its rapid crushing.
- The one gallon can operates similarly, but its capping and the fact that it is not plunged into cold water results in a slower condensation of the moisture inside the can. The one gallon can is much sturdier but atmospheric pressure is such that not even this container can withstand its forces. The can collapses as a vacuum is formed.
- The can has air pressure exerted on it both from outside and inside and those forces are responsible for the can's subsequent demise.
- Newton's second law deals with the sum of the forces. As long as the forces inside and outside are balanced and equal, the can undergoes no change. When the forces are unbalanced, changes in the shape of the metal container can and will take place.
- Pressure in a container is the sum of the partial pressures of the constituent gases. If one gas is removed (water vapor condensing) the total pressure of the gases in the container declines.
- As the container collapses, the volume of the container decreases. The pressure of a gas is related to the volume of its container. At some point the diminished volume results in an adequate pressure inside the container to prevent continued collapse.
1b. Students know that when forces are balanced, no acceleration occurs; thus an object continues to move at a constant speed or stays at rest (Newton’s first law). 1c. Students know how to apply the law F=ma to solve one-dimensional motion problems that involve constant forces (Newton’s second law).
H.S. Chemistry
4c. Students know how to apply the gas laws to relations between the pressure, temperature, and volume of any amount of an ideal gas or any mixture of ideal gases.
4i.* Students know how to apply Dalton’s law of partial pressures to describe the composition of gases and Graham’s law to predict diffusion of gases.
- Students know that an aluminum can can be crushed by hand but stronger cans such as a one gallon can, cannot.
- Students know that as one dives deep into a pool, they feel pressure against their ears.
- Steam can return to a liquid when cooled.
- Does atmospheric pressure exert enough force to crush cans, even strong ones?
- Can students relate air pressure (14.7 lbs/in 2 ) to a force when applied over an area?
- Since students don't experience pressure's effects often they overlook it.
- The quantitative value of air pressure, 14.7 lbs/in, seems small and unable to have an effect.
- Steam is not a gas.
- Gases exert little pressure.

- Illustrations
- Problem Sets
- Calculators
You are here
Not gay-lussac's law.
Crushing a can by heating it, sealing it and cooling it rapidly is an excellent demonstration of the existance of atmospheric pressure. Many youtube videos describe the crushed can as an illustration of either Charles' Law or Gay-Lussac's Law. I don't agree.
There are many variations on the theme, but the basic experiment involves heating a small amount of water inside of a metal container (usualy a soda can). The can is then sealed and cooled rapidly. This results in a drop in pressure inside of the can. The drop in pressure inside of the can allows the higher external pressure from the atmosphere to crush the can.
Why does this setup not demonstrate Charles' or Gay Lussac's Laws?
Charles' Law and Gay-Lussac's Law each explore two and only two parameters. In both cases, all but the two parameters of interest are held constant.
- Gay-Lussac's Law explores the relationship between pressure and temperature assuming volume * and number of molecules of gas remain constant.
- Charles' Law explores the relationship between temperature and volume assuming pressure and number of molecules of gas remain constant.
In the soda can experiment, the boiling water fills the can with water vapor, driving out other gases. When the can is sealed, most of container is filled with water vapor. As it is then cooled, the water vapor condenses, drastically reducing the amount of gas particles in the can. It is the drop in the amount of gas rather than a change in temperature of a fixed volume of gas that allows the pressure difference between the inside and outside of the can to crush it.
- charles' Law
- temperature
Search form
Latest illustrations.
- Ocean Gyres and Geostrophic Flow
- Langmuir Circulation
- Test sensitivity - specificity calculator
- How earthquakes show us the inside of the Earth
- Surface currents, the Ekman spiral, and Ekman transport
© Andrew Staroscik 2011-2022
Get the best experience and stay connected to your community with our Spectrum News app. Learn More
Continue in Browser
Get hyperlocal forecasts, radar and weather alerts.
Please enter a valid zipcode.

Project Weather School: Crushing Can Experiment -- How Temps Affect Air Pressure
Pressure: How Temperature Differences Affect Pressure
ORLANDO, Fla. -- Pressure means everything when it comes to forecasting weather and learning about global patterns. High pressure gives us sunny weather and low pressure gives us stormy weather. This lesson will be broken into two parts over the next two weeks. This week will focus on the relationship between temperature and pressure. Next week we will learn about how pressure controls our weather patterns.
First, let’s start off by defining air pressure. It is the weight (force) of the Earth’s atmosphere pressing down on any object on the Earth’s surface. There are many units to describe air pressure. The two most common metric units meteorologist use are “Inches of Mercury (Hg)” or “millibars (mb).” You may hear meteorologist refer to these units when describing the strength of a hurricane. There is a cool tool used to measure air pressure and it is called a barometer.
BELOW: Take Nick's Weather Quiz!
Here’s a fun fact! The average atmospheric pressure is 1013.25 mb or 29.92”Hg. What does this mean? It translates down to 14.7 lbs per square inch! Think about that. Take out your pencil and let’s do some quick math. Let’s calculate the approximate weight of the atmosphere over a standard piece of paper with the dimensions of 8.5” x 11”?
Find the area of the piece of paper which equals 93.5 square inches. Multiply that value by 14.7 lbs per square inch and you get a weight of 1,374 pounds of air over that sheet of paper. Now that is a lot of weight!
Did you know more than 2,000 pounds of air is resting on our heads every day? So why aren’t we crushed by it? Thank goodness for a strong vertebrae! Our bodies exert pressure too and these forces are balanced. Equilibrium is a great thing!
Now that you understand the standard pressure of our atmosphere and what it means, let’s talk about how this influences weather. We know that pressure varies greatly around our planet and the pressure difference generates wind and storms.
Why do we have a change in pressure around the planet? This has to do with temperature and the heating of the planet from the sun. The tilt of our planet causes the globe to heat up at different rates. Some areas like the equator are warmer than others, especially the poles. Cold air is more dense, therefore it has a higher pressure. Warm air is less dense and has a lower pressure associated with it.
As the sun heats the ground, the air near the ground warms. Remember, heat is less dense than cold air so the warm air will rise. This rising motion creates a natural vacuum lowering the air pressure at the Earth’s surface.
Think of a hot air balloon. When you heat the air inside the balloon, it causes the balloon to rise because the heated air is less dense than the colder air around it.
Cold air on the other hand can create large areas of high pressure because cold air is more dense and hovers near the ground. Think back to last week’s lesson when we demonstrated how cold fronts work. The sinking air can create areas of high pressure at the Earth’s surface.
When high pressure is in control, the air sinks. Sinking air compresses the atmosphere and inhibits clouds to form. Sinking air also pushes down toward the ground so the weight above you is greater than on a standard day.
The opposite is true with a low pressure system. With low pressure, air rises, cools, and condenses into storm clouds, which may lead to a rainy day. Since the rising column of air weighs less, the air pressure is lower. Think back to our water cycle lesson. Water vapor rises, cools, condenses into a cloud, and later produces rain.
Let’s demonstrate how temperature pressure relate to one another in this crushing experiment.
Experiment: A collapsing can
Purpose: To demonstrate how pressure changes with temperature
What you need:
- ADULT SUPERVISION
- Empty soda can
- Stove top of burner
- Large metal or glass bowl filled with ice water
- Kitchen Tongs
- Goggles, gloves, and apron (protective gear)
Procedure:
1. Fill the empty metal or glass bowl with ice water
2. Turn the stove top to a medium heat
3. Fill the empty soda can with 2 inches of water
4. Place the soda can on the stove top
5. Let the water boil. This is when you will see steam escaping the top of the can
6. With an adult, turn the stove off and use the tongs to take the hot soda can and swiftly flip it over into the bowl filled with ice cold water.
7. Observe your findings
Results: The can crushed immediately after placing it in the bowl of ice cold water.
Conclusion: The heating of the can turned some of the water into water vapor. The warm water vapor was less dense than the surrounding environment causing it to rise out of the can. It was visible as steam. Heating the can causes the water particles to expand and therefore the total volume of water inside the can decreased as much of it was lost due to the water vapor escaping through the top.
When the can was flipped into a pool of ice cold water, the can collapsed on itself. The water vapor that was left inside the can quickly cooled and condensed into water droplets, creating a vacuum. Suddenly the pressure outside the can is greater than inside the can, causing it to collapse on itself!
Next week, we will learn how pressure differences causes the wind to blow.
LATEST NEWS

IMAGES
COMMENTS
Air Pressure Can Crusher Experiment - To understand effect of Atmospheric pressure and how it crushes a heated can when dipped in cold water (Based on Gas Law)
Collapsing can or can crusher experiment is a demonstration of an aluminum can being crushed by atmospheric pressure. Due to the low pressure inside a can as compared to the pressure outside, the pressure outside exerts a force on the can causing the can to collapse.
You can crush a soda can using nothing more than a heat source and a bowl of water. This is a great visual demonstration of some simple scientific principles, including air pressure and the concept of a vacuum. The experiment can be...
Did you know you can crush a can with only air? Learn about implosion, air pressure and demonstrate it with your own can crush experiment.
Teach your children about air pressure with this soda can crush experiment. This can crushing science experiment will shock and amaze your kids!
Have fun with this incredible can crushing experiment. Learn about air pressure with a soda can for an easy science experiment.
The heated air inside the can contracts when exposed to the cold water in the bowl. The atmospheric pressure crushes the can. Fill the bowl with cold water. Put about 1oz or 30mL in the can. Place the can on the hot plate and allow the water to heat. Once Steam is visible from the top of the can, quickly with gloves or tongs, flip the can ...
Discover the wonders of atmospheric pressure on an aluminum can.This article will teach you how to conduct this experiment and explain how and why it works.
An explanation of the can crush experiment. The role of atmospheric pressure as well as the liquid to gas phase change of water are explored. The can crush experiment can be confusing at first ...
How to Demonstrate Air Pressure with Can Crush Experiment STEM Little Explorers 5.44K subscribers Subscribed 101 90K views 6 years ago #implosion #airpressure #cancrush
The Can Crusher is not different in this aspect. Temperature in our experiment is seen both in the water temperature, and in the amount of time the can is left on the fire. Volume is seen in both the can volume and in the measurement of percent crush. The number of particles is seen in the initial water amount added to the can to start the ...
We will show you how you can do the can crush experiment at home with a bunch of simple household items and at the end you will have violent can crush effect.
This crushing cans science experiment is an exciting way to demonstrate the power of air pressure. Make soda cans instantly collapse.
This can crusher experiment is the perfect way to demonstrate the wonders of pressure and condensation. All you need is the power of air and water to produce the amazing end result: a crushed soda can!
This experiment will show students one example of differences in pressure in the environment. By heating aluminum cans and subjecting them to a colder environment, a vacuum effect will be created inside the can. This will instantly crush the can without having to touch it!
In this demonstration, students see firsthand how matter changes states and the incredible impact of atmospheric pressure on objects. Air is made up of matter and takes up space and has mass. If the amount of air inside an object changes, the atmospheric pressure outside of the object may have a greater impact on the object […]
When the soda can is plunged into cold water, the vapor condenses quickly, leaving a vacuum in most of the can. The resulting large discrepancy between the outside and inside air pressure leads to a large net inward force on the can, ending with its rapid crushing. The one gallon can operates similarly, but its capping and the fact that it is ...
In this air pressure experiment, we will show you how you can crush an empty soda can using nothing else but a heat source and water. Your kids will love the...
NEVER eat or drink anything while doing any experiment. REMEMBER experiments may require marbles, small balls, balloons, and other small parts. Those objects could become a CHOKING HAZARD. Adults are to perform those experiments using these objects. Any child can choke or suffocate on uninflated or broken balloons.
Crushing a can by heating it, sealing it and cooling it rapidly is an excellent demonstration of the existance of atmospheric pressure. Many youtube videos describe the crushed can as an illustration of either Charles' Law or Gay-Lussac's Law. I don't agree. There are many variations on the theme, but the basic experiment involves heating a small amount of water inside of a
Soda Can Crusher Lab. Amanda has taught high school science for over 10 years. She has a Master's Degree in Cellular and Molecular Physiology from Tufts Medical School and a Master's of Teaching ...
How to explain to your kids that we are surrounded by the air. I think our Air Pressure Can Crusher is a good idea. It is super simple to demonstrate it and the required material can be found in ...
Project Weather School: Crushing Can Experiment -- How Temps Affect Air Pressure By Meteorologist Nick Merianos Orlando UPDATED 1:09 PM ET Aug. 27, 2020