LOGIN

Annual Report
- Board of Directors
- Nomination Process
- Organizational Structure
- ATS Policies
- ATS Website
- MyATS Tutorial
- ATS Experts
- Press Releases
Member Newsletters
- ATS in the News
- ATS Conference News
- Embargo Policy
ATS Social Media
Breathe easy podcasts, ethics & coi, health equity, industry resources.
- Value of Collaboration
- Corporate Members
- Advertising Opportunities
- Clinical Trials
- Financial Disclosure
In Memoriam
Global health.
- International Trainee Scholarships (ITS)
- MECOR Program
- Forum of International Respiratory Societies (FIRS)
- 2019 Latin American Critical Care Conference
Peer Organizations
Careers at ats, affordable care act, ats comments and testimony, forum of international respiratory societies, tobacco control, tuberculosis, washington letter.
- Clinical Resources
- ATS Quick Hits
- Asthma Center
Best of ATS Video Lecture Series
- Coronavirus
- Critical Care
- Disaster Related Resources
- Disease Related Resources
- Resources for Patients
- Resources for Practices
- Vaccine Resource Center
- Career Development
- Resident & Medical Students
- Junior Faculty
- Training Program Directors
- ATS Reading List
- ATS Scholarships
- ATS Virtual Network
ATS Podcasts
- ATS Webinars
- Professional Accreditation
Pulmonary Function Testing (PFT)
- Calendar of Events
Patient Resources
- Asthma Today
- Breathing in America
- Fact Sheets: A-Z
- Fact Sheets: Topic Specific
- Patient Videos
- Other Patient Resources
Lung Disease Week
Public advisory roundtable.
- PAR Publications
- PAR at the ATS Conference
Assemblies & Sections
- Abstract Scholarships
- ATS Mentoring Programs
- ATS Official Documents
- ATS Interest Groups
- Genetics and Genomics
- Medical Education
- Terrorism and Inhalation Disasters
- Allergy, Immunology & Inflammation
- Behavioral Science and Health Services Research
- Clinical Problems
- Environmental, Occupational & Population Health
- Pulmonary Circulation
- Pulmonary Infections and Tuberculosis
- Pulmonary Rehabilitation
- Respiratory Cell & Molecular Biology
- Respiratory Structure & Function
- Sleep & Respiratory Neurobiology
- Thoracic Oncology
- Joint ATS/CHEST Clinical Practice Committee
- Clinicians Advisory
- Council of Chapter Representatives
- Documents Development and Implementation
- Drug/Device Discovery and Development
- Environmental Health Policy
- Ethics and Conflict of Interest
- Health Equity and Diversity Committee
- Health Policy
- International Conference Committee
- International Health
- Members In Transition and Training
- View more...
- Membership Benefits
- Categories & Fees
- Special Membership Programs
- Renew Your Membership
- Update Your Profile
- ATS DocMatter Community
- Respiratory Medicine Book Series
- Elizabeth A. Rich, MD Award
- Member Directory
- ATS Career Center
- Welcome Trainees
- ATS Wellness
- Thoracic Society Chapters
- Chapter Publications
- CME Sponsorship
Corporate Membership
Clinical education, professionals.
- Respiratory Health Awards
- Clinicians Chat
- Ethics and COI
- Pulmonary Function Testing
- ATS Resources
- Live from the CCD
- Pediatric Division Directors

Interpretation of Arterial Blood Gases (ABGs) David A. Kaufman, MD Chief, Section of Pulmonary, Critical Care & Sleep Medicine Bridgeport Hospital-Yale New Haven Health Assistant Clinical Professor, Yale University School of Medicine (Section of Pulmonary & Critical Care Medicine)
Introduction:
Interpreting an arterial blood gas (ABG) is a crucial skill for physicians, nurses, respiratory therapists, and other health care personnel. ABG interpretation is especially important in critically ill patients.
The following six-step process helps ensure a complete interpretation of every ABG. In addition, you will find tables that list commonly encountered acid-base disorders.
Many methods exist to guide the interpretation of the ABG. This discussion does not include some methods, such as analysis of base excess or Stewart’s strong ion difference. A summary of these techniques can be found in some of the suggested articles. It is unclear whether these alternate methods offer clinically important advantages over the presented approach, which is based on the “anion gap.”
6-step approach:
Step 1: Assess the internal consistency of the values using the Henderseon-Hasselbach equation:
[H+] = 24(PaCO 2 ) [HCO 3 -]
If the pH and the [H+] are inconsistent, the ABG is probably not valid.
|
| |
| 7.00 | 100 |
| 7.05 | 89 |
| 7.10 | 79 |
| 7.15 | 71 |
| 7.20 | 63 |
| 7.25 | 56 |
| 7.30 | 50 |
| 7.35 | 45 |
| 7.40 | 40 |
| 7.45 | 35 |
| 7.50 | 32 |
| 7.55 | 28 |
| 7.60 | 25 |
| 7.65 | 22 |
Step 2: Is there alkalemia or acidemia present?
pH < 7.35 acidemia pH > 7.45 alkalemia
- This is usually the primary disorder
- Remember: an acidosis or alkalosis may be present even if the pH is in the normal range (7.35 – 7.45)
- You will need to check the PaCO 2 , HCO 3 - and anion gap
Step 3: Is the disturbance respiratory or metabolic? What is the relationship between the direction of change in the pH and the direction of change in the PaCO 2 ? In primary respiratory disorders, the pH and PaCO2 change in opposite directions; in metabolic disorders the pH and PaCO 2 change in the same direction.
| Acidosis | Respiratory | pH ↓ | PaCO ↑ |
| Acidosis | Metabolic& | pH ↓ | PaCO ↓ |
| Alkalosis | Respiratory | pH ↑ | PaCO ↓ |
| Alkalosis | Metabolic | pH ↑ | PaCO ↑ |
Step 4: Is there appropriate compensation for the primary disturbance? Usually, compensation does not return the pH to normal (7.35 – 7.45).
|
|
|
|
| Metabolic acidosis | PaCO = (1.5 x [HCO -]) +8 | ± 2 |
| Acute respiratory acidosis | Increase in [HCO -]= ∆ PaCO /10 | ± 3 |
| Chronic respiratory acidosis (3-5 days) | Increase in [HCO -]= 3.5(∆ PaCO /10) |
|
| Metabolic alkalosis | Increase in PaCO = 40 + 0.6(∆HCO -) |
|
| Acute respiratory alkalosis | Decrease in [HCO -]= 2(∆ PaCO /10) |
|
| Chronic respiratory alkalosis | Decrease in [HCO -] = 5(∆ PaCO /10) to 7(∆ PaCO /10) |
|
If the observed compensation is not the expected compensation, it is likely that more than one acid-base disorder is present.
Step 5: Calculate the anion gap (if a metabolic acidosis exists): AG= [Na+]-( [Cl-] + [HCO 3 -] )-12 ± 2
- A normal anion gap is approximately 12 meq/L.
- In patients with hypoalbuminemia, the normal anion gap is lower than 12 meq/L; the “normal” anion gap in patients with hypoalbuminemia is about 2.5 meq/L lower for each 1 gm/dL decrease in the plasma albumin concentration (for example, a patient with a plasma albumin of 2.0 gm/dL would be approximately 7 meq/L.)
- Elevation in AG is not explained by an obvious case (DKA, lactic acidosis, renal failure
- Toxic ingestion is suspected
- The OSM gap should be < 10
Step 6: If an increased anion gap is present, assess the relationship between the increase in the anion gap and the decrease in [HCO 3 -].
Assess the ratio of the change in the anion gap (∆AG ) to the change in [HCO3-] (∆[HCO 3 -]): ∆AG/∆[HCO 3 -]
This ratio should be between 1.0 and 2.0 if an uncomplicated anion gap metabolic acidosis is present.
If this ratio falls outside of this range, then another metabolic disorder is present:
- If ∆AG/∆[HCO 3 -] < 1.0, then a concurrent non-anion gap metabolic acidosis is likely to be present.
- If ∆AG/∆[HCO 3 -] > 2.0, then a concurrent metabolic alkalosis is likely to be present.
It is important to remember what the expected “normal” anion gap for your patient should be, by adjusting for hypoalbuminemia (see Step 5 , above.)
Table 1 : Characteristics of acid-base disturbances
|
|
|
|
|
| Metabolic acidosis | ↓ | ↓ in HCO - | ↓ in PaCO |
| Metabolic alkalosis | ↑ | ↑ in HCO - | ↑ in PaCO |
| Respiratory acidosis | ↓ | ↑ in PaCO | ↑ in [HCO -] |
| Respiratory alkalosis | ↑ | ↓ in PaCO | ↓ in [HCO -] |
Table 2 : Selected etiologies of respiratory acidosis
- other obstructive lung disease
- CNS depression
- Sleep disordered breathing (OSA or OHS)
- Neuromuscular impairment
- Ventilatory restriction
- Increased CO2 production: shivering, rigors, seizures, malignant hyperthermia, hypermetabolism, increased intake of carbohydrates
- Incorrect mechanical ventilation settings
Table 3 : Selected etiologies of respiratory alkalosis
- CNS stimulation: fever, pain, fear, anxiety, CVA, cerebral edema, brain trauma, brain tumor, CNS infection
- Hypoxemia or hypoxia: lung disease, profound anemia, low FiO2
- Stimulation of chest receptors: pulmonary edema, pleural effusion, pneumonia, pneumothorax, pulmonary embolus
- Drugs, hormones: salicylates, catecholamines, medroxyprogesterone, progestins
- Pregnancy, liver disease, sepsis, hyperthyroidism
Table 4 : Selected causes of metabolic alkalosis
- Vomiting, gastric suction, villous adenoma, diarrhea with chloride-rich fluid
- Loop and thiazide diuretics, post-hypercapnia (especially after institution of mechanical ventilation)
- Renal loss of H+: edematous states (heart failure, cirrhosis, nephrotic syndrome), hyperaldosteronism, hypercortisolism, excess ACTH, exogenous steroids, hyperreninemia, severe hypokalemia, renal artery stenosis, bicarbonate administration
Table 5 : Selected etiologies of metabolic acidosis
- Methanol intoxication
- Diabetic ketoacidosis a , alcoholic ketoacidosis, starvation ketoacidosis
- Paraldehyde toxicity
- Type A: tissue ischemia
- Type B: Altered cellular metabolism
- Ethanol b or ethylene glycol b intoxication
- Salicylate intoxication
a Most common causes of metabolic acidosis with an elevated anion gap b Frequently associated with an osmolal gap
- Diarrhea, ileostomy, proximal colostomy, ureteral diversion
- proximal RTA
- carbonic anhydrase inhibitor (acetazolamide)
- Chronic renal disease
- Aldosterone inhibitors or absence
- NaCl infusion, TPN, NH 4 + administration
Table 6 : Selected mixed and complex acid-base disturbances
|
|
|
|
| Respiratory acidosis with metabolic acidosis | ↓in pH
| |
| Respiratory alkalosis with metabolic alkalosis | ↑in pH
| |
| Respiratory acidosis with metabolic alkalosis | pH in normal range
| |
| Respiratory alkalosis with metabolic acidosis | pH in normal range
| |
| Metabolic acidosis with metabolic alkalosis | pH in normal range
|
Suggested additional reading:
- Rose, B.D. and T.W. Post. Clinical physiology of acid-base and electrolyte disorders , 5th ed. New York: McGraw Hill Medical Publishing Division, c2001.
- Fidkowski, C And J. Helstrom. Diagnosing metabolic acidosis in the critically ill: bridging the anion gap, Stewart and base excess methods. Can J Anesth 2009;56:247-256.
- Adrogué, H.J. and N.E. Madias. Management of life-threatening acid-base disorders—first of two parts. N Engl J Med 1998;338:26-34.
- Adrogué, H.J. and N.E. Madias. Management of life-threatening acid-base disorders—second of two parts. N Engl J Med 1998;338:107-111.

The American Thoracic Society improves global health by advancing research, patient care, and public health in pulmonary disease, critical illness, and sleep disorders. Founded in 1905 to combat TB, the ATS has grown to tackle asthma, COPD, lung cancer, sepsis, acute respiratory distress, and sleep apnea, among other diseases.
AMERICAN THORACIC SOCIETY 25 Broadway New York, NY 10004 United States of America Phone: +1 (212) 315-8600 Fax: +1 (212) 315-6498 Email: [email protected]
Privacy Statement | Term of Use | COI Conference Code of Conduct

- Remote Access
- Save figures into PowerPoint
- Download tables as PDFs

Chapter 44: Interpretation of Arterial Blood Gases
Kristen Carey Rock; Maurizio Cereda
- Download Chapter PDF
Disclaimer: These citations have been automatically generated based on the information we have and it may not be 100% accurate. Please consult the latest official manual style if you have any questions regarding the format accuracy.
Download citation file:
- Search Book
Jump to a Section
Introduction, acid–base abnormalities.
- INTERPRETATION OF PAO 2
- BLOOD GAS INTEPRETATION DURING CARDIOPULMONARY BYPASS
- BLOOD GAS INTEPRETATATION IN SPECIAL POPULATIONS
- VENOUS BLOOD GASES
- PITFALLS IN ABG INTERPRETATION
- Full Chapter
- Supplementary Content
The arterial blood gas (ABG) is one of the most powerful and frequently used tests in critical care and in the operating room. An ABG may be ordered to obtain information about the patient’s acid/base status, arterial carbon dioxide tensions (PaCO 2 ) and arterial oxygen (PaO 2 ) tensions. Frequently, other information such as the calculated sodium bicarbonate, base deficit, hemoglobin, basic metabolic profile, dyshemoglobins (methemoglobin and carboxyhemoglobin), and lactic acid levels may also be measured in conjunction with traditional ABG values. However, this chapter will focus only on the information obtained from a traditional ABG (pH, PaCO 2 , PaO 2 ).
The clinician may choose to obtain an ABG in a variety of clinical scenarios. In the intensive care unit, the ABG can diagnose a variety of metabolic acid/base disorders, perturbations of ventilation and hypoxemia. After a therapy has been initiated, a repeat ABG can determine the efficacy of the intervention (e.g., when mechanical ventilation is initiated for respiratory failure.) The ABG can also suggest the degree of degree of respiratory and renal compensation for a given acid/base disorder. In the operating room, ABGs are particularly helpful when acid/base status may change dynamically due to the procedure being performed, such as during operations requiring cardiopulmonary or veno-veno bypass, one-lung ventilation, transplant surgery, certain urologic procedures, and trauma. ABGs are also helpful when tight control of the partial pressure of CO 2 is important for improved patient outcomes, such as in neurosurgical cases where carbon dioxide’s effect on intracranial pressure (ICP) can be critical.
The normal range for pH is 7.35–7.45. Lower values indicate an acidosis. Higher values signify an alkalosis. The next step in pH interpretation is to determine whether the acidosis or alkalosis is metabolic or respiratory in origin.
Metabolic Acidosis
Although the cause of a metabolic acidosis cannot be determined solely by the ABG, a metabolic acidosis can be identified with a pH value of less than 7.35 with a PaCO 2 below 40 mmHg. It can also be characterized as a decrease in the strong ion difference. A metabolic acidosis signifies an overproduction, ingestion or inadequate excretion of hydrogen (H + ) ions in a variety of forms. If the cause is an increase in anions or nonvolatile acids not usually present in the blood, the acidosis is termed an “anion gap” acidosis. The anion gap is the difference between primary measured cations (sodium [Na + ] and potassium [K + ]) and the primary measured anions (chloride [Cl – ] and bicarbonate [HCO 3 – ]) in serum. A normal anion gap is less than 11 mEq/L. The normal gap does not reflect a permanent imbalance between cations and anions, but rather acknowledges the contribution of albumin as a significant negative change contribution to electrical neutrality.
Get Free Access Through Your Institution
Pop-up div successfully displayed.
This div only appears when the trigger link is hovered over. Otherwise it is hidden from view.
Please Wait
Warning: The NCBI web site requires JavaScript to function. more...
An official website of the United States government
The .gov means it's official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you're on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- Browse Titles
NCBI Bookshelf. A service of the National Library of Medicine, National Institutes of Health.
StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.

StatPearls [Internet].
Arterial blood gas.
Danny Castro ; Sachin M. Patil ; Muhammad Zubair ; Michael Keenaghan .
Affiliations
Last Update: January 8, 2024 .
- Introduction
Blood gas analysis is a commonly used diagnostic tool to evaluate the partial pressures of gas in blood and acid-base content. Understanding and using blood gas analysis enables providers to interpret respiratory, circulatory, and metabolic disorders. [1]
A "blood gas analysis" can be performed on blood obtained from anywhere in the circulatory system (artery, vein, or capillary). An arterial blood gas (ABG) explicitly tests blood taken from an artery. ABG analysis assesses the patient's partial pressures of oxygen (PaO 2 ) and carbon dioxide (PaCO 2 ). [2] PaO 2 provides information on the oxygenation status, and PaCO 2 offers information on the ventilation status (chronic or acute respiratory failure). PaCO 2 is affected by hyperventilation (rapid or deep breathing), hypoventilation (slow or shallow breathing), and acid-base status. [3] Although oxygenation and ventilation can be assessed non-invasively via pulse oximetry and end-tidal carbon dioxide monitoring, respectively, ABG analysis is the standard. [4]
When assessing the acid-base balance, most ABG analyzers measure the pH and PaCO 2 directly. [2] A derivative of the Hasselbach equation calculates the serum bicarbonate (HCO 3 ) and base deficit or excess. This calculation frequently results in a discrepancy from the measured value due to the blood CO 2 unaccounted for by the equation. [5] The measured HCO 3 uses a strong alkali that liberates all CO 2 in serum, including dissolved CO 2 , carbamino compounds, and carbonic acid. [6] The calculation only accounts for dissolved CO 2 ; this measurement uses a standard chemistry analysis and will likely be called a "total CO 2 ". Therefore, the difference will amount to around 1.2 mmol/L. However, a more considerable difference may be seen in the ABG compared to the measured value, especially in critically ill patients. [7]
The calculation has been disputed as both accurate and inaccurate based on the study, machine, or calibration used and must be interpreted appropriately based on institutional standards. [6]
Emergency medicine, intensivist, anesthesiology, and pulmonology clinicians frequently order arterial blood gases, which may also be used in other clinical settings. Healthcare professionals evaluate many diseases using an ABG, including acute respiratory distress syndrome (ARDS), severe sepsis, septic shock, hypovolemic shock, diabetic ketoacidosis, renal tubular acidosis, acute respiratory failure, heart failure, cardiac arrest, asthma, and inborn errors of metabolism. [3]
- Pathophysiology
By obtaining an ABG and analyzing the pH, partial pressures, and comparing it to measured serum bicarbonate in a sick patient, multiple pathological conditions can be diagnosed. [1] The alveolar-arterial oxygen gradient is a useful measure of lung gas exchange, which can be abnormal in patients with a ventilation-perfusion mismatch. [8]
- Specimen Requirements and Procedure
Whole blood is the required specimen for an arterial blood gas sample. The specimen is obtained through an arterial puncture or acquired from an indwelling arterial catheter. A description of these procedures is beyond the scope of this article; please refer to the StatPearls article “Arterial Lines” and other references for more information. Once obtained, the arterial blood sample should be placed on ice and analyzed as soon as possible to reduce the possibility of erroneous results. [9]
Differences in measured blood gas values between arterial and venous blood are most pronounced for PO 2 , as PO 2 is the only clinical reason for obtaining arterial collections. [10] PO 2 is generally approximately 60 mm Hg lower in venous blood after O 2 is released in the capillaries, whereas PCO 2 is 2 to 8 mm Hg higher in venous blood. pH is generally only 0.02 to 0.05 pH units lower in a venous sample. [11] Proper specimen collection is paramount in obtaining accurate blood analysis results for gas and pH. Placement of indwelling catheters with heparin locks for short- and long-term intravenous therapies is typical. Failure to flush the lock properly has unpredictable effects on measured quantities and is often indicated by bizarre, non-physiologic results. [12]
Arterial or venous specimens must be collected anaerobically with lyophilized heparin anticoagulant in 1- to 3-mL sterile syringes. Evacuated lithium heparin sample tubes (vacuum tubes) used for plasma collection are not acceptable for analysis as these evacuated tubes still contain a significant amount of oxygen and will impact the accuracy of the measured whole blood PO 2 . [13] Syringes containing lyophilized heparin are preferable to those containing liquid heparin, as liquid heparin has atmospheric PO 2 and PCO 2 values that dilute the sample; when the syringe is not filled, the effect is most significant. [14] An increasing ratio of liquid heparin to blood can have an increasingly marked effect on measured PCO 2 and the parameters calculated from it. Variations in syringe manufacturing can create significant differences in pre-analytical effects on the sample. [15]
The anaerobic collection technique means no blood exposure to atmospheric air. [16] The PCO 2 of air is approximately 0.25 mm Hg, much less than that of blood (40 mm Hg). Thus, the CO 2 content and PCO 2 of blood exposed to air will decrease, and blood pH, a function of PCO 2 , will rise. The PO 2 of atmospheric air (155 mm Hg) is approximately 60 mm Hg higher than that of arterial blood and approximately 100 mm Hg higher than venous blood. Hence, blood exposed to atmospheric air in a patient’s breathing room absorbs oxygen, while blood with a PO 2 exceeding 150 mm Hg, a condition observed in patients undergoing oxygen therapy, releases oxygen. [17]
Blood exposure to air can occur simply from the air in the needle and the syringe hub dead space. The error will be minimal if the resulting bubble is ejected immediately after drawing by holding the syringe tip up and ejecting a small drop of blood. [18] The potential effect of small bubbles on blood gas results was demonstrated in one study in which a 100-mL bubble of room air was added to 10, 2-mL blood samples with PO 2 values between 25 and 40 mm Hg. PO 2 increased an average of 4 mm Hg in these samples in only 2 minutes, whereas PCO 2 decreased by 4 mm Hg. Before analysis, mixing the sample by vigorously rolling the syringe between the palms should be done to establish a homogeneous sample. [19] Arterialized capillary blood is sometimes an acceptable alternative to arterial blood when an arterial cannula is unavailable or repeated arterial punctures must be avoided. [20]
Automated blood gas analyzers are commonly used to analyze blood gas samples, and results are obtained within 10 to 15 minutes. Automated blood gas analyzers, directly and indirectly, measure specific components of the arterial blood gas sample (see above). [1]
ABG components include the following:
- pH = measured acid-base balance of the blood
- PaO 2 = measured the partial pressure of oxygen in arterial blood
- PaCO 2 = measured the partial pressure of carbon dioxide in arterial blood
- HCO 3 = calculated concentration of bicarbonate in arterial blood
- Base excess/deficit = calculated relative excess or deficit of base in arterial blood
- SaO 2 = calculated arterial oxygen saturation (unless a co-oximetry is obtained, in which case it is measured)
A modified Allen test is necessary before an ABG is drawn from either upper extremity to check for adequate collateral flow. Alternatively, use pulse oximetry and duplex ultrasound. The arterial site commonly used is the radial artery, which is superficial and easily palpable over the radial styloid process. The next most common site is the femoral artery. The test is performed on the unilateral upper extremity chosen for the procedure (see Figure 1. Modified Allen Test). Have the patient flex the selected upper extremity at the elbow and clench a raised fist for 30 seconds. Apply pressure over the ulnar and radial arteries to occlude the blood flow. After 5 seconds, the patient may unclench the raised fist. The palm will now appear pale, white, or blanched. Then, pressure over the ulnar artery is released while the radial artery compression is maintained. In 10 to 15 seconds, the palm returns to its original color, indicating adequate ulnar collateral blood flow. If the palm does not return to its actual color, it is an abnormal test and unsafe to puncture the radial artery. Similarly, the radial collateral blood flow is assessed by maintaining ulnar artery pressure and releasing the radial artery pressure. [21]
- Testing Procedures
Operating a traditional blood gas instrument begins with the operator presenting a blood specimen at the sample probe. The sample is taken through the probe by a peristaltic pump that loads the chamber with a specific amount of the sample. The sample then resides in the chamber long enough to allow thermal equilibration and completion of measurements. On completion of the measurement, the pump pushes the sample to waste. [22] Because electrodes are not stable for very long, frequent calibration of pH, PCO 2 , and PO 2 is required. [23] Most instruments contain a barometer so that barometric pressure P(Amb) is always known to the microprocessor during calibration. Other instruments perform point-of-care or bedside testing. Almost all manufacturers now produce small, portable, stand-alone, easy-to-operate instruments designed for “satellite lab” operations; several hand-held devices that use disposable electrodes are also available. [24]
The sophistication of contemporary equipment and availability of high-quality calibrator materials have made reliable and accurate determination of blood pH and gases primarily due to meticulous maintenance, adherence to the manufacturer’s recommended procedures, control of the equipment, and proper collection and handling of specimens. [22] Software programs of the instrument’s microprocessor often provide display warnings and diagnostic routines that alert the operator and assist in troubleshooting. The manufacturer’s suggested maintenance schedule should be considered a minimum guideline, relying on experience to indicate maintenance frequency. [25]
Cleanliness of the sample chamber and path is essential. Automatic flushing to cleanse the sample chamber and path after each blood sample measurement is a feature of most instruments without disposable electrodes. Despite proper flushing, however, complete or partial clogging of the chamber or path may occur. [1] Fibrin threads and small clots may be present in the specimen or may form while the sample resides in the warm chamber. If allowed to remain, they can affect subsequent measurements or calibrations by interfering with the contact of blood, buffers, or gases with electrode membranes. [18] Visibility of the path through the heat sink helps detect clogs, dirt, and bubbles. Bubbles that fail to rinse out can be problematic if they settle on an electrode. [26]
- Results, Reporting, and Critical Findings
An acceptable normal range of ABG values of ABG components is the following, [27] [28] noting that the range of normal values may vary among laboratories and in different age groups from neonates to geriatrics:
- pH (7.35-7.45)
- PaO 2 (75-100 mm Hg)
- PaCO 2 (35-45 mm Hg)
- HCO 3 (22-26 mEq/L)
- Base excess/deficit (-4 to +2)
- SaO 2 (95-100%)
It is best to approach arterial blood gas interpretation systematically. Interpretation leads to understanding the degree or severity of abnormalities, whether the abnormalities are acute or chronic, and whether the primary disorder is metabolic or respiratory in origin. [29] Several articles have described simplistic ways to interpret ABG results. However, the Romanski method of analysis is most simplistic for all levels of providers. This method helps determine the presence of an acid-base disorder, its primary cause, and whether compensation is present. [30]
The first step is to look at the pH and assess for the presence of acidemia (pH < 7.35) or alkalemia (pH > 7.45). If the pH is in the normal range (7.35-7.45), use a pH of 7.40 as a cutoff point. In other words, categorize a pH of 7.37 as acidosis and a pH of 7.42 as alkalemia. Next, evaluate the respiratory and metabolic components of the ABG results, the PaCO 2 and HCO 3 , respectively. The PaCO 2 indicates whether the acidosis or alkalemia is primarily from a respiratory or metabolic acidosis/alkalosis. PaCO 2 > 40 with a pH < 7.4 indicates a respiratory acidosis, while PaCO 2 < 40 and pH > 7.4 indicates a respiratory alkalosis (but is often from hyperventilation from anxiety or compensation for a metabolic acidosis). Next, assess for evidence of compensation for the primary acidosis or alkalosis by looking for the value (PaCO 2 or HCO 3 ) inconsistent with the pH. Lastly, assess the PaO2 for any abnormalities in oxygenation. [29]
Example 1 [28] : ABG: pH = 7.39, PaCO 2 = 51 mm Hg, PaO 2 = 59 mm Hg, HCO 3 = 30 mEq/L and SaO 2 = 90%, on room air.
- pH is in the normal range, so use 7.40 as a cutoff point, in which case it is < 7.40, and acidosis is present.
- The elevated PaCO 2 indicates respiratory acidosis, and the elevated HCO 3 indicates a metabolic alkalosis.
- The value consistent with the pH is PaCO 2 . Therefore, this is a primary respiratory acidosis. The acid-base that is inconsistent with the pH is the elevated HCO3, indicating a metabolic alkalosis, so there is compensation signifying a non-acute primary disorder because it takes days for metabolic compensation to be effective.
- Last, the decreased PaO 2 indicates an abnormality with oxygenation. However, a history and physical will help delineate the severity and urgency of required interventions, if any.
Example 2 [28] : ABG: pH = 7.45, PaCO 2 = 32 mm Hg, PaO 2 = 138 mm Hg, HCO 3 = 23 mEq/L, the base deficit = 1 mEq/L, and SaO 2 is 92%, on room air.
- pH is in the normal range. Using 7.40 as a cutoff point, it is >7.40, so alkalemia is present.
- The decreased PaCO 2 indicates a respiratory alkalosis, and the HCO 3 is normal but on the low end of normal.
- The value consistent with the pH is PaCO 2 . Therefore, this is a primary respiratory alkalosis. The HCO 3 is in the normal range and, thus, not inconsistent with the pH, so there is a lack of compensation.
- Last, the PaO 2 is within the normal range, so there is no abnormality in oxygenation.
When evaluating a patient's acid-base status, it is important to include an electrolyte imbalance or anion gap in synthesizing the information. [31] For example, a patient who presents with diabetic ketoacidosis will eliminate ketones and close the anion gap but with persistent metabolic acidosis due to hyperchloremia due to the strong ionic effect, which is beyond the scope of this article.
- Clinical Significance
Arterial blood gas monitoring is the standard for assessing a patient’s oxygenation, ventilation, and acid-base status. Although ABG monitoring has been replaced mainly by non-invasive monitoring, it is still helpful in confirming and calibrating non-invasive monitoring techniques. [1]
Frequently performed is the evaluation of oxygenation in the context of severe sepsis, acute respiratory failure, and ARDS In the intensive care unit (ICU) and emergency room settings. Calculating an alveolar-arterial (A-a) oxygen gradient can aid in narrowing down the hypoxemia cause. [25] For example, a gradient’s presence or absence can help determine whether the abnormality in oxygenation is potentially due to hypoventilation, a shunt, V/Q mismatch, or impaired diffusion. The equation for the expected A-a gradient assumes the patient is breathing room air; therefore, the A-a gradient is less accurate at higher percentages of inspired oxygen. Determining the intrapulmonary shunt fraction, the fraction of cardiac output flowing through pulmonary units that do not contribute to gas exchange, is the best estimate of oxygenation status. Calculating the shunt fraction is traditionally done at a delivered FiO 2 of 1.0, but if performed at a FiO 2 lower than 1.0, venous admixture would be the more appropriate term. [1]
For simplicity, assessing oxygenation is more commonly performed by computing the ratio of PaO 2 and the fraction of inspired oxygen (PaO 2 /FiO 2 or P/F ratio). However, there are limitations in using the P/F ratio in assessing oxygenation, as the discrepancy between venous admixture and the P/F ratio at a given shunt fraction depends on the delivered FiO 2 . Researchers use the P/F ratio to categorize disease severity in ARDS. [32]
Another parameter commonly used in ICUs to assess oxygenation is the oxygenation index (OI). This index is considered a better indicator of lung injury, particularly in the neonatal and pediatric population, compared to the P/F ratio. This index also includes the level of invasive ventilatory support required to maintain oxygenation. [33] The OI is the product of the mean airway pressure (Paw) in cm H 2 O, measured by the ventilator, and the FiO 2 is the percentage divided by the PaO 2 . The OI is commonly used to guide management, such as initiating inhaled nitric oxide, administering surfactant, and defining the potential need for extracorporeal membrane oxygenation. [34]
A normal PaO 2 value does not rule out respiratory failure, particularly in the presence of supplemental oxygen. The PaCO 2 reflects pulmonary ventilation and cellular CO 2 production. It is a more sensitive marker of ventilatory failure than PaO 2 , particularly in the presence of supplemental oxygen, as it is closely related to the depth and rate of breathing. [27] Calculating the pulmonary dead space is a good indicator of overall lung function. Pulmonary dead space is the difference between the PaCO 2 and mixed expired PCO 2 (physiological dead space) or the end-tidal PCO 2 divided by the PaCO 2 . Pulmonary dead space increases when the pulmonary units’ ventilation increases relative to their perfusion, and shunting increases. Hence, pulmonary dead space is an excellent bedside indicator of lung function and one of the best prognostic factors in ARDS patients. [1] The pulmonary dead space fraction may also help diagnose other conditions, such as pulmonary embolism. [35]
Acid-base balance can be affected by the aforementioned respiratory system abnormalities. For instance, acute respiratory acidosis and alkalemia result in acidemia and alkalemia, respectively. Additionally, hypoxemic hypoxia leads to anaerobic metabolism, which causes metabolic acidosis that results in acidemia. Metabolic system abnormalities also affect acid balance, as acute metabolic acidosis and alkalosis result in acidemia and alkalemia. [25] Patients with diabetic ketoacidosis, septic shock, renal failure, drug or toxin ingestion, and gastrointestinal or renal HCO 3 loss exhibit metabolic acidosis. [28] Conditions such as kidney disease, electrolyte imbalances, prolonged vomiting, hypovolemia, diuretic use, and hypokalemia cause metabolic alkalosis. [36]
- Quality Control and Lab Safety
Healthcare providers can analyze an arterial blood gas and electrolytes (often called a shock panel) as a point-of-care test. Appropriately calibrate or standardize these machines to ensure accurate and precise readings for clinical decisions. Please refer to the user manuals to ensure the appropriate device calibration during discussion with the clinical laboratory team. [37]
Elements of good quality assurance of blood gas and pH measurements include the following:
- Proper maintenance of the instrument
- Use of control materials
- Verification of electrode linearity
- Checking barometer accuracy
- Accurately measuring temperature. [38]
External quality assurance (proficiency testing) mandated by federal law in the United States (Clinical Laboratory Improvement Amendments [CLIA] 1988) has assumed new importance for quality control of blood gas analysis. [39] These rules became effective in January 1991 and set criteria for satisfactory interlaboratory performance, which are as follows: pH, target value ± 0.04; PO 2 , target value ± 3 SD; and PCO 2 , target value ± 8% or ± 5 mm Hg, whichever is greater. [40] The significance of proficiency testing and the penalties for failure place strong incentives on consistent performance of internal control measures and effective response to quality control failures. [41]
At the same time, the pressure to control costs has raised the question of how often one should monitor interlaboratory performance effectively and determine the necessary concentrations of control materials. Per CLIA 1988, the answer is one concentration of control every 8 hours, with the entire range of control concentrations covered every 24 hours. [22] In many laboratories, however, the practical answer is to run on every instrument in use, at least once per shift, three concentrations of control for pH, PO 2 , and PCO 2 , always on completion of maintenance and troubleshooting procedures. Newer analyzers, particularly the smaller satellite laboratory and point-of-care instruments, frequently have an auto quality control (QC) feature or use electronic QCs. [42]
Auto QC consists of onboard QC material automatically analyzed by the instrument at designated intervals to fulfill regulatory requirements. Electronic QC, which is most common in devices with disposable electrode cartridges, consists of cartridges that verify the electronic specification of the instruments. [43]
- Enhancing Healthcare Team Outcomes
ABG should be used to assess a patient's ventilatory, acid-base, and oxygenation status. Additionally, blood gas analysis is recommended to assess a patient's response to therapeutic interventions and to monitor the severity and progression of documented cardiopulmonary disease processes. [44] Despite its clinical value, erroneous or discrepant values represent a potential drawback of blood gas analysis, so eliminating potential sources of error is paramount. [27] Therefore, attention to detail in the sampling technique and processing is essential.
Rigorous quality control of the automated blood gas analyzers is also necessary for accurate results. However, machine performance and quality assurance advances have now made most errors in point-of-care analysis of ABGs attributable to clinical providers. Several pre-analytic steps must be followed to obtain a valid, interpretable ABG. [27] In most hospital settings, ABG analysis is a process that involves multiple healthcare providers (eg, physicians, respiratory therapists, and nurses). Hence, interprofessional coordination, cooperation, and communication are vitally important.
The American Association for Respiratory Care has published Clinical Care Guidelines for Blood Gas Analysis and Hemoximetry that provide current best practices for sampling, handling, and analyzing ABGs. [44] Notable sources of erroneous values during blood draws include abnormal or misstated FiO 2 , barometric pressures, or temperatures. Temperature is a significant variable, leading to PaO 2 and O 2 saturation discrepancies, as do acid-base disturbances. Several physiological and clinical conditions, such as hyperleukocytosis and dyshemoglobinemias, can also lead to PaO 2 and O 2 saturation discrepancies. Sample dilution can be an additional error source, with liquid heparin and saline as potential culprits. [45]
The mode of sample transportation is also of significance as discrepant values can result from air contamination after pneumatic tube system transport, compared with manual transport of the specimen, especially in the presence of inadvertent air bubbles. [45] Therefore, procuring samples using suitable syringes filled with adequate amounts of blood without air bubbles, maintained at the correct temperatures, and appropriately and promptly transporting them for rapid analysis can minimize erroneous values.
- Review Questions
- Access free multiple choice questions on this topic.
- Comment on this article.
Modified Allen Test. This test is used to check the overall blood supply to the hand. Illustration by Katherine Humphreys
Disclosure: Danny Castro declares no relevant financial relationships with ineligible companies.
Disclosure: Sachin Patil declares no relevant financial relationships with ineligible companies.
Disclosure: Muhammad Zubair declares no relevant financial relationships with ineligible companies.
Disclosure: Michael Keenaghan declares no relevant financial relationships with ineligible companies.
This book is distributed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0) ( http://creativecommons.org/licenses/by-nc-nd/4.0/ ), which permits others to distribute the work, provided that the article is not altered or used commercially. You are not required to obtain permission to distribute this article, provided that you credit the author and journal.
- Cite this Page Castro D, Patil SM, Zubair M, et al. Arterial Blood Gas. [Updated 2024 Jan 8]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-.
In this Page
Bulk download.
- Bulk download StatPearls data from FTP
Related information
- PMC PubMed Central citations
- PubMed Links to PubMed
Similar articles in PubMed
- The importance of arterial blood gas analysis as a systemic diagnosis approach in assessing and preventing chronic diseases, from emergency medicine to the daily practice. [Eur Rev Med Pharmacol Sci. 2023] The importance of arterial blood gas analysis as a systemic diagnosis approach in assessing and preventing chronic diseases, from emergency medicine to the daily practice. Balzanelli MG, Distratis P, Lazzaro R, Pham VH, Del Prete R, Dipalma G, Inchingolo F, Aityan SK, Hoang LT, Palermo A, et al. Eur Rev Med Pharmacol Sci. 2023 Dec; 27(23):11653-11663.
- Comparative Analysis of Oxygen Saturation by Pulse Oximetry and Arterial Blood Gas in Hypoxemic Patients in a Tertiary Care Hospital. [Cureus. 2023] Comparative Analysis of Oxygen Saturation by Pulse Oximetry and Arterial Blood Gas in Hypoxemic Patients in a Tertiary Care Hospital. Abraham EA, Verma G, Arafat Y, Acharya S, Kumar S, Pantbalekundri N. Cureus. 2023 Jul; 15(7):e42447. Epub 2023 Jul 25.
- Prediction of arterial blood gas values from venous blood gas values in patients with acute respiratory failure receiving mechanical ventilation. [J Formos Med Assoc. 2003] Prediction of arterial blood gas values from venous blood gas values in patients with acute respiratory failure receiving mechanical ventilation. Chu YC, Chen CZ, Lee CH, Chen CW, Chang HY, Hsiue TR. J Formos Med Assoc. 2003 Aug; 102(8):539-43.
- Review Arterial Blood Gases. [Clinical Methods: The History,...] Review Arterial Blood Gases. Trulock EP III. Clinical Methods: The History, Physical, and Laboratory Examinations. 1990
- Review Comparing Central Venous Blood Gas to Arterial Blood Gas and Determining Its Utility in Critically Ill Patients: Narrative Review. [Anesth Analg. 2021] Review Comparing Central Venous Blood Gas to Arterial Blood Gas and Determining Its Utility in Critically Ill Patients: Narrative Review. Chong WH, Saha BK, Medarov BI. Anesth Analg. 2021 Aug 1; 133(2):374-378.
Recent Activity
- Arterial Blood Gas - StatPearls Arterial Blood Gas - StatPearls
Your browsing activity is empty.
Activity recording is turned off.
Turn recording back on
Connect with NLM
National Library of Medicine 8600 Rockville Pike Bethesda, MD 20894
Web Policies FOIA HHS Vulnerability Disclosure
Help Accessibility Careers

ABG Examples and Case Studies
- 📖 Geeky Medics OSCE Book
- ⚡ Geeky Medics Bundles
- ✨ 1300+ OSCE Stations
- ✅ OSCE Checklist PDF Booklet
- 🧠 UKMLA AKT Question Bank
- 💊 PSA Question Bank
- 💉 Clinical Skills App
- 🗂️ Flashcard Collections | OSCE , Medicine , Surgery , Anatomy
- 💬 SCA Cases for MRCGP
To be the first to know about our latest videos subscribe to our YouTube channel 🙌
Table of Contents
Suggest an improvement
- Hidden Post Title
- Hidden Post URL
- Hidden Post ID
- Type of issue * N/A Fix spelling/grammar issue Add or fix a link Add or fix an image Add more detail Improve the quality of the writing Fix a factual error
- Please provide as much detail as possible * You don't need to tell us which article this feedback relates to, as we automatically capture that information for you.
- Your Email (optional) This allows us to get in touch for more details if required.
- Which organ is responsible for pumping blood around the body? * Enter a five letter word in lowercase
- Phone This field is for validation purposes and should be left unchanged.
Test your arterial blood gas (ABG) interpretation skills with the following ABG case studies .
For each case, we encourage you to interpret the ABG systematically , commenting on oxygenation, pH, PaCO 2 , HCO 3 – , base excess and compensation.
For each blood gas case study, consider the most likely diagnosis and formulate a management plan .
For more information, see our guide to ABG interpretation .
Case study 1
A 21 year old woman presents with a five day history of vomiting and lethargy. She is confused and hypotensive.
An arterial blood gas is performed on room air .
Review the blood gas and document your interpretation below.
| 7.3 | 7.35 – 7.45 | |
| 13 kPa | 11-13 kPa (82.5 – 97.5 mmHg) | |
| 4.1 kPa | 4.7 – 6.0 kPa (35.2 – 45 mmHg) | |
| 13 mEq/L | 22 – 26 mEq/L | |
| -5 | -2 to +2 | |
| Na | 135 mmol/L | 135 – 146 mmol/L |
| K | 4.9 mmol/L | 3.5 – 5.3 mmol/L |
| Cl | 102 mmol/L | 98 – 106 mmol/L |
| Glucose | 27 mmol/L | 3.6 – 5.3 mmol/L (64.8 – 95.4 mg/dL) |
| Lactate | 2.6 mmol/L | 0.5 – 2.2 mmol/L |
Interpretation
| normal, ruling out hypoxia as the cause of confusion | |
| low, indicating an acidaemia | |
| low, the respiratory system isn’t contributing to the acidaemia | |
| low, suggesting a metabolic acidosis | |
| low, in keeping with the established metabolic acidosis | |
| the PaCO is low, suggesting partial respiratory compensation | |
| significantly raised glucose and raised lactate |
Explanation
Oxygenation.
- The PaO 2 is within normal limits and appropriate to the % inspired oxygen concentration (FiO 2 )
- FiO 2 in room air is 21%, and as a rule of thumb, the PaO 2 should be approximately 10 kPa less than the %FiO 2
Acid-base disturbance
Primary acid-base disturbance
- The patient has an acidaemia with a pH of 7.3 (7.35-7.45)
- Acidaemia can either be driven by a respiratory cause (high CO 2 ) or a metabolic cause (low HCO 3 )
- The bicarbonate is low, suggesting a metabolic acidosis
Compensation
- The PaCO 2 is low, suggesting respiratory compensation . The lungs are blowing off CO 2 to compensate for the acidosis. Blowing off CO 2 moves the carbonic acid equation to the left in order to remove excess H + .

- The anion gap can help differentiate between the different causes of metabolic acidosis
- Anion gap = Na + – (Cl – + HCO 3 – )
- A normal anion gap is 4 to 12 mmol/L
- In this case the anion gap is high ([135 – [102 +13] = 20 )
Causes of a high anion gap metabolic acidosis include:
- Diabetic ketoacidosis
- Lactic acidosis
- Aspirin overdose
- Renal failure
Other significant findings
- Raised lactate and significantly raised glucose
This patient has a high anion gap metabolic acidosis with partial respiratory compensation . The raised glucose makes diabetic ketoacidosis (DKA) the most likely diagnosis.
A blood ketone level is needed to confirm the diagnosis. Respiratory compensation is commonly seen in DKA, and the increased respiratory effort in these cases is known as Kussmaul breathing .
Management priorities in DKA are: fluid replacement (patients can be significantly dehydrated), starting a fixed rate insulin infusion , identifying and treating underlying causes and close monitoring of glucose and potassium levels.
Case study 2
A 24 year old asthmatic patient presents with a wheeze and shortness of breath.
| 7.49 | 7.35 – 7.45 | |
| 11 kPa | 11-13 kPa (82.5 – 97.5 mmHg) | |
| 4.1 kPa | 4.7 – 6.0 kPa (35.2 – 45 mmHg) | |
| 24 mEq/L | 22 – 26 mEq/L | |
| +1 | -2 to +2 | |
| Na | 137 mmol/L | 135 – 146 mmol/L |
| K | 5.1 mmol/L | 3.5 – 5.3 mmol/L |
| Cl | 99 mmol/L | 98 – 106 mmol/L |
| Glucose | 5.1 mmol/L | 3.6 – 5.3 mmol/L (64.8 – 95.4 mg/dL) |
| Lactate | 1.3 mmol/L | 0.5 – 2.2 mmol/L |
| normal | |
| alkalaemia (pH > 7.45) | |
| low ~ respiratory alkalosis | |
| normal | |
| normal | |
| no evidence of compensation | |
| no other significant abnormalities |
- FiO 2 in room air is 21% , and as a rule of thumb, the PaO 2 should be approximately 10 kPa less than the %FiO 2
- The patient has an alkalaemia with a pH of > 7.45
- Alkalaemia on a blood gas can either be driven by a respiratory cause (low CO 2 ) or a metabolic cause (high HCO 3 )
- The patient has a low CO 2, suggesting a respiratory alkalosis
Carbon dioxide diffuses rapidly between the capillaries and alveoli, making blood carbon dioxide levels very sensitive to respiratory rate (↑RR = ↓PCO 2 and ↓RR = ↑PCO 2 ).
Compensation
- The bicarbonate is within normal limits ~ there is no evidence of metabolic compensation for the respiratory alkalosis
- No other significant abnormalities
This patient is having an asthma attack , and her ABG demonstrates a respiratory alkalosis caused by a raised respiratory rate .
This is an expected finding during an asthma exacerbation. A normal PaCO 2 in a patient experiencing an asthma exacerbation is a life-threatening feature as it indicates respiratory fatigue.
Case study 3
A 57 year old man suffers an out of hospital cardiac arrest. Return of spontaneous circulation occurs, and he is being ventilated with a Bag-Valve-Mask (BVM).
An arterial blood gas is performed on 15 L/min O 2 .
| 6.9 | 7.35 – 7.45 | |
| 17 kPa | 11-13 kPa (82.5 – 97.5 mmHg) | |
| 9.2 kPa | 4.7 – 6.0 kPa (35.2 – 45 mmHg) | |
| 16 mEq/L | 22 – 26 mEq/L | |
| -12 | -2 to +2 | |
| Na | 136 mmol/L | 135 – 146 mmol/L |
| K | 7.9 mmol/L | 3.5 – 5.3 mmol/L |
| Cl | 101 mmol/L | 98 – 106 mmol/L |
| Glucose | 7.1 mmol/L | 3.6 – 5.3 mmol/L (64.8 – 95.4 mg/dL) |
| Lactate | 11 mmol/L | 0.5 – 2.2 mmol/L |
| impaired oxygenation relative to the FiO | |
| significant acidaemia | |
| significantly elevated CO (suggesting respiratory acidosis) | |
| decreased (suggesting metabolic acidosis) | |
| low, in keeping with metabolic acidosis | |
| no evidence of compensation | |
| severe hyperkalaemia, lactate significantly raised and glucose elevated |
- Oxygen levels are low , given the expected FiO 2
- As a rule of thumb, the PaO 2 should be approximately 10 kPa less than the percentage of inspired O 2 (%FiO 2 )
- The FiO 2 for a patient receiving 15 L/min O 2 via a BVM with a good seal can approach 100%
- The hypoxia here may be secondary to a primary hypoxic event leading to the cardiac arrest or secondary to poor ventilation with the BVM
- The PaCO 2 is also significantly elevated, indicating poor ventilation
Primary acid base disturbance
- There is a mixed respiratory and metabolic acidosis
- Acidosis can either be driven by a respiratory cause (high CO 2 ) or a metabolic cause (low HCO 3 )
- In this case, both the CO 2 is high, and the HCO 3 is low, suggesting a mixed acidosis
- There is no evidence of compensation as both the respiratory and metabolic systems are contributing to the acidosis
- Lactate is significantly raised, contributing to the metabolic acidosis
- It is common to see lactic acidosis following organ hypoperfusion during a cardiac arrest
- Glucose is mildly elevated, which may be a stress response
Potassium
- Severe hyperkalaemia (K + > 6.5 mmol/L)
- Hyperkalaemia can occur in cardiac arrest secondary to cell death and secondary to acidosis (which pushes K+ extracellularly in exchange for H + )
- Hyperkalaemia is also one of the reversible causes of cardiac arrest
This patient has a mixed respiratory and metabolic acidosis following a cardiac arrest.
It is imperative to identify and treat the potential underlying causes (think 4Hs and 4Ts ).
The patient has severe hyperkalaemia , which requires immediate treatment with IV calcium to stabilise the myocardium, followed by K + lowering measures such as an insulin-dextrose infusion.
They are also significantly hypoxic relative to the FiO 2 and require a definitive airway with optimised oxygenation and ventilation.
Case study 4
A 52 year old with severe COPD is reviewed in respiratory clinic.
An arterial blood gas is performed on room air.
| 7.35 | 7.35 – 7.45 | |
| 7.2 kPa | 11-13 kPa (82.5 – 97.5 mmHg) | |
| 7.5 kPa | 4.7 – 6.0 kPa (35.2 – 45 mmHg) | |
| 33 mEq/L | 22 – 26 mEq/L | |
| +6 | -2 to +2 | |
| Na | 140 mmol/L | 135 – 146 mmol/L |
| K | 4.2 mmol/L | 3.5 – 5.3 mmol/L |
| Cl | 102 mmol/L | 98 – 106 mmol/L |
| Glucose | 5.1 mmol/L | 3.6 – 5.3 mmol/L (64.8 – 95.4 mg/dL) |
| Lactate | 1.2 mmol/L | 0.5 – 2.2 mmol/L |
| low, significantly impaired oxygenation | |
| normal range (lower end of normal) | |
| high, type 2 respiratory failure (low O and high CO ) | |
| high, suggesting metabolic compensation | |
| high, due to excess bicarbonate | |
| high bicarb & BE suggesting metabolic compensation for chronic CO retention | |
| no other abnormalities |
- Type 2 respiratory failure ~ hypoxaemia (PaO 2 <8 kPa) with hypercapnia (PaCO 2 >6.0 kPa)
- pH is within normal limits (either suggesting no acid-base disturbance or a compensated acid-base abnormality)
- The PaCO 2 is high and the bicarbonate is high
- Theoretically, this could either be due to a respiratory acidosis with metabolic compensation or a metabolic alkalosis with a respiratory compensation
- The first clue is the clinical context: this is a patient with chronic COPD who is likely to be a retainer of carbon dioxide
- The second clue is the pH: the pH is tending towards acidosis, indicating the primary abnormality is a respiratory acidosis
Remember that overcompensation does not occur . Therefore, this could not be a primary metabolic alkalosis, as that would mean the respiratory system has overcompensated and pushed the blood pH back down to borderline acidaemia.
This is an ABG of a chronic CO 2 retainer showing chronic respiratory acidosis with a compensatory metabolic alkalosis .
Patients with chronic CO 2 retention can become desensitised to high CO 2 levels and rely instead on oxygen levels to guide the adequacy of ventilation. This is sometimes referred to as the hypoxic drive .
Giving patients too much O 2 in this setting can cause respiratory depression and further increase CO 2 retention. Therefore, it is essential that chronic CO2 retainers and those at risk of hypercapnic respiratory failure have their oxygen saturations titrated to between 88% and 92% .
Case study 5
A 72 year old woman presents to the emergency department with profuse vomiting. Examination reveals global abdominal tenderness and a CT abdomen has been requested.
A venous blood gas is performed on room air .
| 7.48 | 7.35 – 7.45 | |
| 7.8 kPa | 11-13 kPa (82.5 – 97.5 mmHg)* | |
| 6.7 kPa | 4.7 – 6.0 kPa (35.2 – 45 mmHg)* | |
| 33 mEq/L | 22 – 26 mEq/L | |
| +7 | -2 to +2 | |
| Na | 136 mmol/L | 135 – 146 mmol/L |
| K | 3.5 mmol/L | 3.5 – 5.3 mmol/L |
| Cl | 94 mmol/L | 98 – 106 mmol/L |
| Glucose | 4.0 mmol/L | 3.6 – 5.3 mmol/L (64.8 – 95.4 mg/dL) |
| Lactate | 1.1 mmol/L | 0.5 – 2.2 mmol/L |
*Note that reference ranges here are for arterial blood samples (ABG), as is standard for blood gas analysers. Key differences between arterial and venous blood gas samples are covered in our venous blood gas (VBG) analysis article.
| VBG cannot be used to assess oxygenation | |
| raised, indicating an alkalaemia | |
| high, suggesting respiratory system is not the cuase of the alkalaemia | |
| high, indicating this is a metabolic alkalosis | |
| high, in keeping with a metabolic alkalosis | |
| cannot accurately comment on the extent of hypercapnia as this is a VBG | |
| hypochloraemia |
- Venous oxygen tension (PvO 2 ) cannot be used to equate to arterial oxygen tension (PaO 2 ), thus a VBG cannot be used to assess oxygenation
- The patient is alkalaemic with a pH of 7.48
- Alkalaemia can either be driven by a respiratory cause (low CO 2 ) or a metabolic cause (high HCO 3 )
- In this case, there is a high HCO 3 suggesting a metabolic alkalosis
- This is a VBG therefore, we cannot comment accurately on respiratory compensation
- An elevated PCO 2 on an arterial blood gas would suggest respiratory compensation
- Hypochloraemia
This patient has a metabolic alkalosis with associated hypochloraemia . This is in keeping with loss of chloride-rich stomach contents. Remember that gastric juice is rich in hydrochloric acid (HCl), thus marked vomiting leads to a loss of both H + and Cl – ions.
A high degree of suspicion for significant underlying pathology is required in older people with abdominal pain. A CT scan has been ordered in this case to look for surgical causes such as small bowel obstruction .

Other pages
- Product Bundles 🎉
- Join the Team 🙌
- Institutional Licence 📚
- OSCE Station Creator Tool 🩺
- Create and Share Flashcards 🗂️
- OSCE Group Chat 💬
- Newsletter 📰
- Advertise With Us
Join the community
Your cart is empty
Have an account?
Log in to check out faster.
ABG Interpretation, part 8: Example problems 1-4
In this video, Cathy goes through four example ABG Interpretation problems and answers. You can download the questions and answers below to print out and follow along. We've also explained the steps for interpretation below.
Need help interpreting ABGs? Check out our Arterial Blood Gas Interpretation Flashcards for Nursing Students .
ABG practice question 1
A patient's arterial blood gas measurements read pH = 7.29, PaCO₂ = 47 mmHg, and HCO₃ = 24 mEq/L. How would you interpret this?
Steps for interpretation
- Check the pH to determine if we have acidosis or alkalosis. The normal range for pH is 7.35 - 7.45.
- The pH here is 7.29, which is out of range on the acidic side. Therefore, we have ACIDOSIS.
- Determine which system, metabolic or respiratory, is causing the acidosis. Check the PaCO₂, which represents the respiratory system, first. The normal range for PaCO₂ is 35 - 45 mmHg.
- The PaCO₂ here is 47 mmHg, which is out of range on the acidic side.
- Therefore, the respiratory system is causing the acidosis and we have RESPIRATORY ACIDOSIS.
- Check if there is compensation. Because it's respiratory acidosis, it's the metabolic system that would be compensating. The metabolic system is represented by HCO₃. So we'll check HCO₃ to check for compensation. The normal range for HCO₃ is 22 - 26 mEq/L.
- The HCO₃ here is 24 mEq/L, which is within normal range. There is no compensation.
- Therefore, we have UNCOMPENSATED RESPIRATORY ACIDOSIS
Lab Values & ABG Interpretation - Nursing Flashcards
4.915789473 / 5.0
(95) 95 total reviews
ABG practice question 2
A patient's arterial blood gas measurements read pH = 7.31, PaCO₂ = 49 mmHg, HCO₃ = 30 mEq/L. How would you interpret this?
- The pH here is 7.31, which is out of range on the acidic side. Therefore, we have ACIDOSIS.
- The PaCO₂ here is 49 mmHg, which is out of range on the acidic side.
- The HCO₃ here is 30 mEq/L, which is out of range on the basic side. This means there is metabolic compensation.
- To determine if the compensation is partial or full, we check the pH again. The pH was 7.31 which is outside the normal range, so the metabolic system has not succeeded in fully compensating.
- Therefore, we have PARTIALLY COMPENSATED RESPIRATORY ACIDOSIS.
ABG practice question 3
A patient's arterial blood gas measurements read pH = 7.35, PaCO₂ = 48 mmHg, HCO₃ = 29 mEq/L. How would you interpret this?
- The pH here is 7.35 , which is normal but on the acidic side. This might be acidosis.
- The PaCO₂ here is 48 mmHg , which is out of range on the acidic side.
- The HCO₃ here is 29 mEq/L , which is out of range on the basic side. This means there is metabolic compensation.
- To determine if the compensation is partial or full, we check the pH again. The pH was 7.35, which is within the normal range, so the metabolic system has succeeded in fully compensating.
- Therefore, we have FULLY COMPENSATED RESPIRATORY ACIDOSIS.
ABG practice question 4
A patient's arterial blood gas measurements read pH = 7.49, PaCO₂ = 33 mmHg, HCO₃ = 24 mEq/L. How would you interpret this?
- The pH here is 7.49 , which is out of range on the basic side. So we have ALKALOSIS.
- Determine which system, metabolic or respiratory, is causing the alkalosis. Check the PaCO₂, which represents the respiratory system, first. The normal range for PaCO₂ is 35 - 45 mmHg.
- The PaCO₂ here is 33 mmHg , which is out of range on the basic side.
- Therefore, the respiratory system is causing the alkalosis and we have RESPIRATORY ALKALOSIS .
- Check if there is compensation. Because it's respiratory alkalosis, it's the metabolic system that would be compensating. The metabolic system is represented by HCO₃. So we'll check HCO₃ to check for compensation. The normal range for HCO₃ is 22 - 26 mEq/L.
- The HCO₃ here is 24 mEq/L , which is within the normal range. There is no compensation.
- Therefore, we have UNCOMPENSATED RESPIRATORY ALKALOSIS.
Full Transcript: ABG Interpretation, part 8: Example problems 1-4
Starting in this video, we are going to start going over some ABG interpretation problems. We're going to go through a lot of problems and try to hit all the different variations you may get. So let's start with problem number 1. And again, you can download these problems from our website, leveluprn.com, and kind of follow along with me. There is also an answer key on the website. So if you want to later work through these problems independently and check your answers, you can do that.
So problem number one, pH is 7.29, PaCO₂ is 47, HCO₃ is 24. So step one is figuring out if we have acidosis or alkalosis. So let's look at the pH for that. So pH should be between 7.35 and 7.45. But it is out of range on the low side, which means we have acidosis. So that's step one, all done, acidosis. Now, second step, we need to figure out who's to blame for that acidosis. Is it the respiratory system, or is it the metabolic system? Right? So the respiratory system, we're going to be looking at PaCO₂; metabolic system, we're going to be looking at HCO₃. So when we look at PaCO₂, we see we have 47. The normal range for PaCO₂ is between 35 and 45. If we are too high out of that range on the high side, then we have acidosis. So in this case, we know that the respiratory system is to blame for the acidosis, okay? So we have respiratory acidosis. Now we need to see if the metabolic system is trying to compensate for that. Are they trying to fix the problem? So our normal HCO₃ level should be between 22 and 26. And here we have 24, so it's totally within normal range. So the metabolic system is not doing anything to fix the situation. We have just kind of normal HCO₃ level. So in this case, we have uncompensated respiratory acidosis. So the respiratory system is causing the acidosis, and the metabolic system isn't doing anything to fix the situation, so uncompensated respiratory acidosis. Hopefully, you can read that okay.
Alright. Let's do another problem. Here, with problem two, we have a pH of 7.31. Again, this is out of range on the low side, which means again we have acidosis. So that's step one. Now we're going to figure out, is it the respiratory system to blame, or is it the metabolic system to blame? We look at the PaCO₂ to evaluate whether the respiratory system is to blame. PaCO₂ should be between 35 and 45. When it is high, when it is out of range on the high side, we have acidosis. So we know again in this situation that we have respiratory acidosis. Now let's look and see if the metabolic system is trying to fix the situation. HCO₃ should be between 22 and 26. In this case, it's 30, so it's on the basic side. So it is trying to compensate for this acidosis. Respiratory system is acting up, causing this acidosis. The metabolic system is basic, so it's trying to neutralize the situation. But does it fully compensate for the situation? No, because here you can see the pH is 7.31. It's not within normal range. So in this case, we have partially compensated respiratory acidosis. Metabolic system is trying to compensate but hasn't fully compensated so, again, partially compensated respiratory acidosis. okay! So that's problem two. And we will pick it up with more problem sets.
Okay, problem three. We have a pH of 7.35, PaCO₂ of 48, and HCO₃ of 29. So let's first look at the pH, see if we have acidosis or alkalosis. You'll notice that 7.35 is within the normal range for pH, but it is on the acidic side. So we have a normal pH, but we'll want to note that it is on the acidic side, okay? So let's see what's going on with the respiratory system. PaCO₂ is 48, which is out of range, on the acidic side. So we have some respiratory acidosis going on. Let's see what the metabolic system is doing about the situation. So the metabolic system is HCO₃, it should be between 22 and 26, and it is high, right? It's 29, which is out of range on the basic side. So the metabolic system is fixing the situation, right? We have respiratory acidosis, the metabolic system is making it more basic, and it is fully compensating for this because our pH is within normal range. So again, respiratory system is acting up, causing respiratory acidosis. Metabolic system saves the day by becoming more basic, and it basically fixes the situation because we have a pH that's within the normal range, 7.35 to 7.45. So in this case, we have fully compensated respiratory acidosis. Alright. That's problem three.
Let's do problem four. pH is 7.49. PaCO₂ is 33. HCO₃ is 24. So let's first determine do we have acidosis or alkalosis? It's always our first step. So in this case, our pH should be between 7.35 and 7.45. It is out of range on the high side, which means we have alkalosis. Now let's see who is to blame for the alkalosis, right? So our pH is alkalosis, which is like another word for being basic. So let's see if the respiratory system is to blame or the metabolic system is to blame. PaCO₂, again, represents the respiratory system, should be between 35 and 45. It is 33, so it is out of range on the low side, which means we have alkalosis and we have respiratory alkalosis. So right off the bat, we know that the respiratory system is to blame for the alkalosis. Now we need to check and see if the metabolic system is trying to compensate at all. If it is trying to compensate, then we'll see that it is acidic, okay? HCO₃ should be between 22 and 26. Our value is 24, so it's within normal range. So it's not basic; it's not trying to compensate. This is normal. So for this problem, we have uncompensated respiratory alkalosis, okay? Respiratory system is acting up, causing the alkalosis, and the metabolic system is not saving the day. They're not even trying because we have a normal HCO₃ level. So in this case, we have uncompensated respiratory alkalosis. Alright. That's problem four. We'll pick it up with more next!
Sign up for the Pharmacology Challenge
The Five-a-Day Pharmacology Challenge - Cardiovascular Medications is a free community challenge, helping more nursing students master (and review) critical meds with just a few minutes a day.
BONUS: There's prizes. Lots of prizes.
Well simplified. Thank you
I’m still struggling whether an ABG is compensated, uncompensated, or partially compensated.
This is so helpful, no need to know which arrow is moving in the same direction or opposite direction. Thank you.
I struggle with whether an ABG is compensated, uncompensated, or partially compensated. The interpretations here really helped. Thank you!
Leave a comment
Please note, comments need to be approved before they are published.
Videos by Subject
- ABG Interpretation
- Clinical Nursing Skills
- Dosage Calculation
- EKG Interpretation
- Fundamentals
- Health Assessment
- Medical-Surgical
- Nutrition Essentials
- Pediatric Nursing
- Pharmacology
- Psychiatric Mental Health
Tips & More
- Ask a Nurse
- Nursing Tips
- Resources for Nursing Students
- Why Flashcards Work

Exam Information
Subscribe to our emails.
- Choosing a selection results in a full page refresh.
- Opens in a new window.
Please select your country / currency.
Free economy shipping on The Survival Kit OR The Comprehensive Collection !
Select "USD" to purchase digital products including Level Up RN Membership.

- Advanced Life Support
- Endocrinology
- Gastroenterology
- Infectious disease
- Intensive care
- Palliative Care
- Respiratory
- Rheumatology
- Haematology
- Endocrine surgery
- General surgery
- Neurosurgery
- Ophthalmology
- Plastic surgery
- Vascular surgery
- Abdo examination
- Cardio examination
- Neurolo examination
- Resp examination
- Rheum examination
- Vasc exacmination
- Other examinations
- Clinical Cases
- Communication skills
- Prescribing

Arterial Blood Gas (ABG) interpretation for medical students, OSCEs and MRCP

Arterial Blood Gas (ABG) interpretation for medical students, OSCEs and MRCP PACES
This section presents how to interpret arterial blood gases. It explains each component in turn followed by clinical examples to work through.
The most important points when assessing a patient are the history, examination and basic observations. Investigations such as arterial blood gases add to the information you have already gained to guide your management.
Before starting…
- Arterial blood gas analysis can be used to assess gas exchange and acid base status as well as to provide immediate information about electrolytes.
- It is also useful to have access to any previous gases. This is particularly important if your patient is known to have chronic respiratory disease with existing chronic ABG changes.
Normal values for arterial blood gas (ABG)
- Normal values are given below. Note that these may vary slightly between analysers. Be sure to know the normal ranges and units for the analyser you will be using.
- pH: 7.35 – 7.45
- pO2: 10 – 14kPa*
- pCO2: 4.5 – 6kPa*
- Base excess (BE): -2 – 2 mmol/l
- HCO3: 22 – 26 mmol/l
*1kPa = 7.5mmHg. p stands for the ‘partial pressure of…’
Click here for related pages: ABG examples and ABG exam questions
Components of the abg.
- pH is a logarithmic scale of the concentration of hydrogen ions in a solution. It is inversely proportional to the concentration of hydrogen ions.
- When a solution becomes more acidic the concentration of hydrogen ions increases and the pH falls.
- Normally the body’s pH is closely controlled at between 7.35 – 7.45. This is achieved through buffering and excretion of acids. Buffers include plasma proteins and bicarbonate (extracellular) and proteins, phosphate and haemoglobin (intracellularly).
- Hydrogen ions are excreted via the kidney and carbon dioxide is excreted via the lungs.
- Changes in ventilation are the primary way in which the concentration of H+ ions is regulated. Ventilation is controlled of the concentration of CO 2 in the blood.
- If the buffers and excretion mechanisms are overwhelmed and acid is continually produced, the he pH falls. This creates a metabolic acidosis.
- If the ability to excrete CO 2 is compromised this creates a respiratory acidosis.
- Note that a normal pH doesn’t rule out respiratory or metabolic pathology. This why you must always look at all the values other than pH as there may be a compensated or mixed disorder.
Partial pressure (PP)
- Partial pressure is a way of assessing the number of molecules of a particular gas in a mixture of gases. It is the amount of pressure a particular gas contributes to the total pressure. For example, we normally breathe air which at sea level has a pressure of 100kPa, oxygen contributes 21% of 100kPa, which corresponds to a partial pressure of 21kPa.
- When used in blood gases, Henry’s law is used to ascertain the partial pressures of gases in the blood. This law states that when a gas is dissolved in a liquid the partial pressure (i.e. concentration of gas) within the liquid is the same as in the gas in contact with the liquid. Therefore you can measure the partial pressure of gases in the blood.
- PaO 2 is the partial pressure of oxygen in arterial blood
- PaCO 2 is the partial pressure of carbon dioxide in arterial blood.
Base excess (BE)
- This is the amount of strong acid which would need to be added or subtracted from a substance in order to return the pH to normal (7.40).
- A value outside of the normal range (-2 to +2 mEq/L) suggests a metabolic cause for the acidosis or alkalosis.
- In terms of basic interpretation
- A base excess more than +2 mEq/L indicates a metabolic alkalosis.
- A base excess less than -2 mEq/L indicates a metabolic acidosis.
Bicarbonate (HCO 3 )
- Bicarbonate is produced by the kidneys and acts as a buffer to maintain a normal pH. The normal range for bicarbonate is 22 – 26mmol/l.
- If there are additional acids in the blood the level of bicarbonate will fall as ions are used to buffer these acids. If there is a chronic acidosis additional bicarbonate is produced by the kidneys to keep the pH in range.
- It is for this reason that a raised bicarbonate may be seen in chronic type 2 respiratory failure where the pH remains normal despite a raised CO 2 .
Electrolytes
- A venous or arterial blood gas is a good way to quickly check potassium and sodium values. This is particularly important in the immediate management of cardiac arrhythmias as it gives an immediate result.
- Lactate is produced as a by-product of anaerobic respiration. A raised lactate can be caused by any process which causes tissue to use anaerobic respiration. It is a good indicator of poor tissue perfusion.
Haemoglobin (Hb)
- Haemoglobin acts as a guide but is notoriously inaccurate in an ABG. Lab samples should be used to verify results.
- Don’t forget to check this. Glucose is especially pertinent in the management of the patient who has decreased consciousness or seizures. It is also important in patients with known or suspected diabetes.
- Glucose may also be raised in patients with severe sepsis or other metabolic stress.
Other components of the ABG
- These are rarely deranged and often overlooked. However, it is important to notice them if they are abnormal. This is especially true in the case of carbon monoxide as there may be other people at risk.
Carbon monoxide (CO)
- Normally CO is <10%. In city dwellers or smokers levels can be raised up to 10% but a level >10% indicates poisoning, commonly from poorly ventilated boilers or old heating systems.
- At levels of 10 -20% symptoms such as nausea, headache vomiting and dizziness will be predominant. At higher levels patients may experience arrhythmias, cardiac ischaemia, respiratory failure and seizures.
Methaemoglobin (metHb)
- MetHb is an oxidized form of haemoglobin. Levels of >2% are abnormal.
- Methaemoglobinaemia is a rare condition but again it is important not to miss. It may be caused by errors of metabolism or by exposure to toxins such as nitrates.
Compensation
pH is closely controlled in the human body and there are various mechanisms to maintain it at a constant value. It is important to note that the body will never overcompensate as the drivers for compensation cease as the pH returns to normal. In essence compensation for an acidosis will not cause an alkalosis or visa versa.
Respiratory Compensation
- If a metabolic acidosis develops the change is sensed by chemoreceptors centrally in the medulla oblongata and peripherally in the carotid bodies.
- The classic example of this is ‘Kussmaul breathing’ the deep sighing pattern of respiration seen in severe acidosis including diabetic ketoacidosis. Here you will see a low pH and a low pCO2 which would be described as a metabolic acidosis with partial respiratory compensation (partial as a normal pH has not been reached).
Metabolic Compensation
- In response to a respiratory acidosis, for example in CO2 retention secondary to COPD, the kidneys will start to retain more HCO3 in order to correct the pH.
- Here you would see a low normal pH with a high CO2 and high bicarbonate.
- This process takes place over days.
- It is important to ensure that the compensation that you see is appropriate, i.e. as you would expect. If not then you should start to think about mixed acid base disorders.
How to interpret an ABG
A systematic approach to ABG interpretation leads to easy interpretation. Here is one such system:
- Look at the patient! Review history and examination findings.
- What is the pO2 – how much oxygen was your patient on when the gas was taken?
- What is the pH? Is the patient acidaemic or alkalaemic.
- What is the pCO2?
- What is the HCO3 and base excess?
- Is the patient compensating?
- What are the other values? Ensure that you look at all other figures on the gas.
How to present an ABG
- State that this is an arterial blood gas sample (rather than venous).
- State the patients name and outline history/pertinent examination findings.
- State the time the sample was taken and how much oxygen the patient was on at the time.
- Present your findings: e.g. this showed type one respiratory failure with a p02 of 7
- Present any abnormal findings or important negatives from the rest of the values.
- A one line summary of your findings.
For example:
- “This is an arterial blood gas sample taken from Mrs Smith, a 70 year old lady who presented this morning with shortness of breath. She has a back ground of heart failure and diabetes and on auscultation of her chest she has bibasal crackles.
- This gas was taken at 10 a.m. today when Mrs Smith was on 15l per minute of oxygen via a non rebreathe mask.
- It showed type one respiratory failure with a p02 of 10.0 and a pCO2 of 4.1.
- There is no acid base disturbance although her glucose was noted to be 15.
- N.B. the pO2 of 10 whilst on 15l/min of oxygen is indicative of severe respiratory disease. This is why including all the information in the presentation is incredibly important as a pO2 of 10 on air would be far less worrying.
- In patients with chronic respiratory disease it is very useful to see an old ABG as this may give useful clues as to a patient’s normal respiratory status.
Respiratory failure
Respiratory failure can be split into Type one or Type 2 respiratory failure. These are differentiated by the pCO2.
Type 1 Respiratory failure (T1RF)
- Type one respiratory failure is defined as a PaO2 less than 8 and a PaCO2 which is low or normal.
- T1RF is caused by pathological processes which reduce the ability of the lungs to exchange oxygen, without changing the ability to excrete CO2.
- Examples of T1RF are pulmonary embolus, pneumonia, asthma and pulmonary oedema.
Type 2 respiratory failure (T2RF)
- This is defined as a PaO2 of less than 8 and a raised PaCO2.
- You can think of it as being caused by a problem with the lungs or by a problem with the mechanics or control of respiration.
| COPD | Chest wall trauma | Opiate overdose |
| Pulmonary oedema | Muscular dystrophies | Acute CNS disease |
| Pneumonia | Motor neurone disease | |
| Myasthenia Gravis |
For medical student exam, OSCE and MRCP PACES questions on ABGs click here
Arterial Blood Gas Analysis Made Easy with Tic-Tac-Toe Method

Interpretation of arterial blood gases (ABGs) is a crucial skill that a lot of student nurses and medical practitioners need to learn. In this guide, we’ll help you understand the concepts behind arterial blood gas and teach you the easiest and most fun way to interpret ABGs using the tic-tac-toe method.
Table of Contents
- What is arterial blood gas?
- PaCO2 (Partial Pressure of Carbon Dioxide)
- PaO2 (Partial Pressure of Oxygen)
SO2 (Oxygen Saturation)
- HCO3 (Bicarbonate)
BE (Base Excess)
Normal values, goals of arterial blood gas analysis, steps in abg analysis using the tic-tac-toe method, application and examples.
- How to draw Arterial Blood Gas?
Respiratory Acidosis
- Respiratory Alkalosis
Metabolic Acidosis
- Metabolic Alkalosis
Arterial Blood Gas Interpretation Quiz
References and sources, what is arterial blood gas.
An arterial blood gas is a laboratory test to monitor the patient’s acid-base balance . It is used to determine the extent of the compensation by the buffer system and includes the measurements of the acidity (pH), levels of oxygen, and carbon dioxide in arterial blood. Unlike other blood samples obtained through a vein, a blood sample from an arterial blood gas (ABG) is taken from an artery (commonly on radial or brachial artery).
What are the components of arterial blood gas?
There are six components of arterial blood gas (ABGs):
The pH is the concentration of hydrogen ions and determines the acidity or alkalinity of body fluids. A pH of 7.35 indicates acidosis and a pH greater than 7.45 indicates alkalosis. The normal ABG level for pH is 7.35 to 7.45 .
PaCO 2 (Partial Pressure of Carbon Dioxide)
PaCO 2 or partial pressure of carbon dioxide shows the adequacy of the gas exchange between the alveoli and the external environment (alveolar ventilation ). Carbon dioxide (CO2) cannot escape when there is damage in the alveoli, excess CO2 combines with water to form carbonic acid (H2CO3) causing an acidotic state. When there is hypoventilation in the alveolar level (for example, in COPD ), the PaCO 2 is elevated, and respiratory acidosis results. On the other hand, when there is alveolar hyperventilation (e.g., hyperventilation), the PaCO 2 is decreased causing respiratory alkalosis. For PaCO 2 , the normal range is 35 to 45 mmHg (respiratory determinant) .
PaO 2 (Partial Pressure of Oxygen)
PaO 2 or partial pressure of oxygen or PAO2 indicates the amount of oxygen available to bind with hemoglobin . The pH plays a role in the combining power of oxygen with hemoglobin: a low pH means there is less oxygen in the hemoglobin. For PaO 2 , the normal range is 75 to 100 mmHg
SO 2 or oxygen saturation , measured in percentage, is the amount of oxygen in the blood that combines with hemoglobin. It can be measured indirectly by calculating the PAO2 and pH Or measured directly by co-oximetry. Oxygen saturation , the normal range is 94–100%
HCO 3 (Bicarbonate)
HCO 3 or bicarbonate ion is an alkaline substance that comprises over half of the total buffer base in the blood. A deficit of bicarbonate and other bases indicates metabolic acidosis. Alternatively, when there is an increase in bicarbonates present, then metabolic alkalosis results.
BE . Base excess or BE value is routinely checked with HCO 3 value. A base excess of less than –2 is acidosis and greater than +2 is alkalosis. Base excess, the normal range is –2 to +2 mmol/L
To determine acid-base imbalance, you need to know and memorize these values to recognize what deviates from normal. The normal range for ABGs is used as a guide, and the determination of disorders is often based on blood pH. If the blood is basic, the HCO 3 level is considered because the kidneys regulate bicarbonate ion levels. If the blood is acidic, the PaCO 2 or partial pressure of carbon dioxide in arterial blood is assessed because the lungs regulate the majority of acid. The normal ABG values are the following:
- For pH, the normal range is 7.35 to 7.45
- For PaCO 2 , the normal range is 35 to 45 mmHg (respiratory determinant)
- For PaO 2 , the normal range is 75 to 100 mmHg
- For HCO 3 , the normal range is 22 to 26 mEq/L (metabolic determinant)
- Oxygen saturation , the normal range is 94–100%
- Base excess, the normal range is –2 to +2 mmol/L
Interpreting Arterial Blood Gas Imbalances
Interpreting arterial blood gases is used to detect respiratory acidosis or alkalosis, or metabolic acidosis or alkalosis during an acute illness. To determine the type of arterial blood gas the key components are checked. The best (and fun) way of interpreting arterial blood gas is by using the tic-tac-toe method below:
For the purpose of this guide, we have set three (3) goals that we need to accomplish when interpreting arterial blood gases. The goals are as follows:

- Based on the given ABG values, determine if values interpret ACIDOSIS or ALKALOSIS.
- Second, we need to determine if values define METABOLIC or RESPIRATORY.
- Lastly, we need to determine the compensation if it is: FULLY COMPENSATED, PARTIALLY COMPENSATED, or UNCOMPENSATED.
We need to keep these goals in mind as they’ll come up later in the steps for the ABG interpretation technique.
There are eight (8) steps simple steps you need to know if you want to interpret arterial blood gases (ABGs) results using the tic-tac-toe technique.
1. Memorize the normal values.
The first step is you need to familiarize yourself with the normal and abnormal ABG values when you review the lab results. They are easy to remember:
- For PaCO 2 , the normal range is 35 to 45
- For HCO 3 , the normal range is 22 to 26
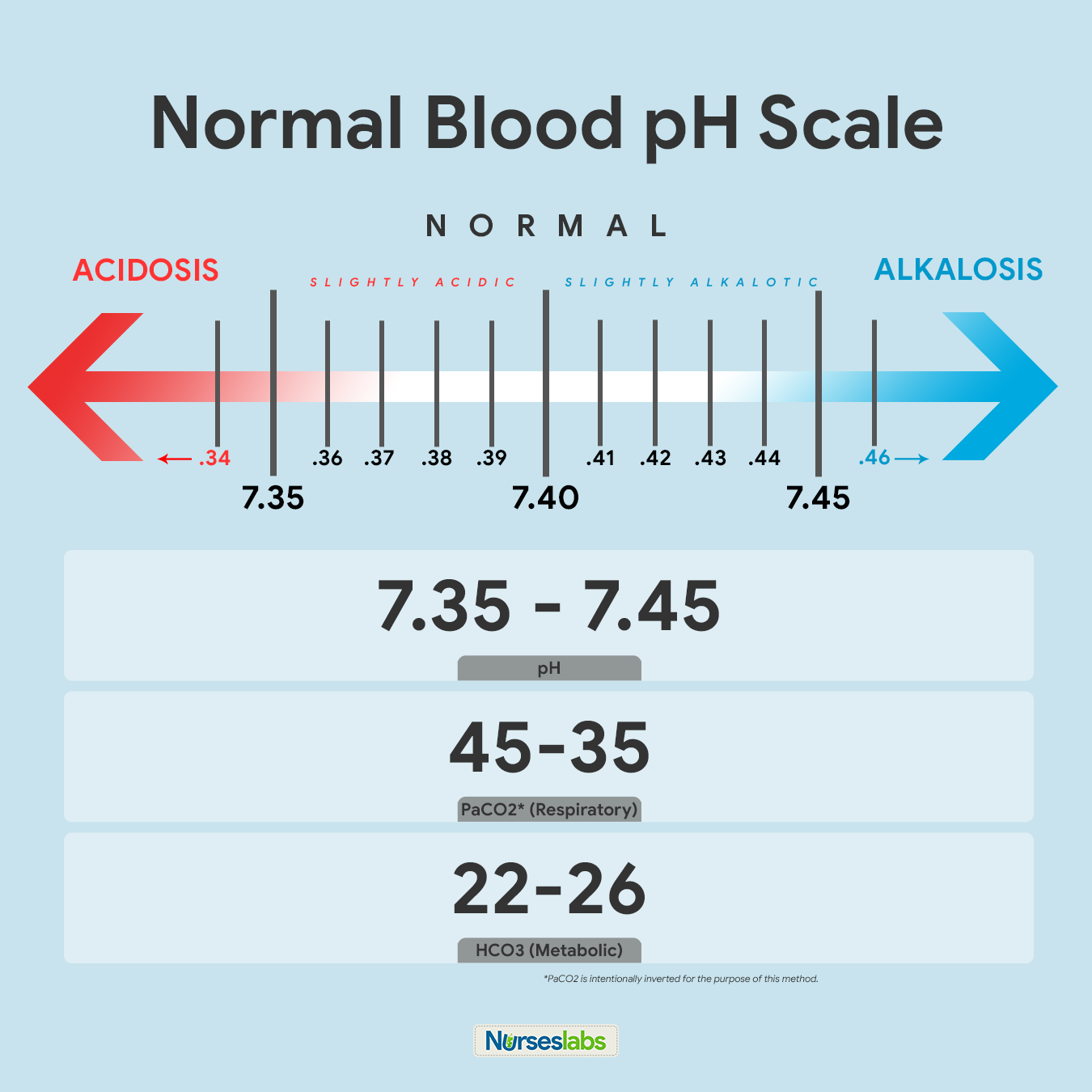
The recommended way of memorizing it is by drawing the diagram of normal values above. Write it down together with the arrows indicating ACIDOSIS or ALKALOSIS. Note that PaCO 2 is intentionally inverted for the purpose of the Tic-Tac-Toe method.
2. Create your tic-tac-toe grid.
Once you’ve memorized the normal values and the diagram, create a blank your tic-tac-toe grid and label the top row as ACIDOSIS, NORMAL, and ALKALOSIS. Based on their values, we need to determine in which column we’ll place pH, PaCO 2 , and HCO 3 in the grid.
3. Determine if pH is under NORMAL, ACIDOSIS, or ALKALOSIS.
The third step of this technique is to determine the acidity or alkalinity of the blood with the given value of the pH as our determining factor. Remember in step #1 that the normal pH range is from 7.35 to 7.45.
- If the blood pH is between 7.35 to 7.39, the interpretation is NORMAL but SLIGHTLY ACIDOSIS, place it under the NORMAL column.
- If the blood pH is between 7.41 to 7.45, interpretation is NORMAL but SLIGHTLY ALKALOSIS, place it under the NORMAL column.
- Any blood pH below 7.35 (7.34, 7.33, 7.32, and so on…) is ACIDOSIS, place it under the ACIDOSIS column.
- Any blood pH above 7.45 (7.46, 7.47, 7.48, and so on…) is ALKALOSIS, place it under the ALKALOSIS column.
Please use the diagram below to help you visualize whether the normal value is ACIDOSIS or ALKALOSIS.
Once you’ve determined whether the pH is under the ACIDOSIS or ALKALOSIS, plot it on your tic-tac-toe grid under the appropriate column.
4. Determine if PaCO 2 is under NORMAL, ACIDOSIS, or ALKALOSIS.
For this step, we need to interpret if the value of PaCO 2 is within the NORMAL range, ACIDIC, or BASIC and plot it on the grid under the appropriate column. Remember that the normal range for PaCO 2 is from 35 to 45:
- If PaCO 2 is below 35, place it under the ALKALOSIS column.
- If PaCO 2 is above 45, place it under the ACIDOSIS column.
- If PaCO 2 is within its normal range, place it under the NORMAL column.
5. Determine if HCO 3 is under NORMAL, ACIDOSIS, or ALKALOSIS.
Next, we need to interpret if the value of HCO 3 is within the NORMAL range, ACIDIC, or BASIC and plot it under the appropriate column in the tic-tac-toe grid. Remember that the normal range for HCO 3 is from 22 to 26:

- If HCO 3 is below 22, place it under the ACIDOSIS column.
- If HCO 3 is above 26, place it under the ALKALOSIS column.
- If HCO 3 is within its normal range, place it under the NORMAL column.
6. Solve for goal #1: ACIDOSIS or ALKALOSIS.
Now, we will start solving for our goals. Looking at the tic-tac-toe grid, determine whether in what column the pH is placed and interpret the results:
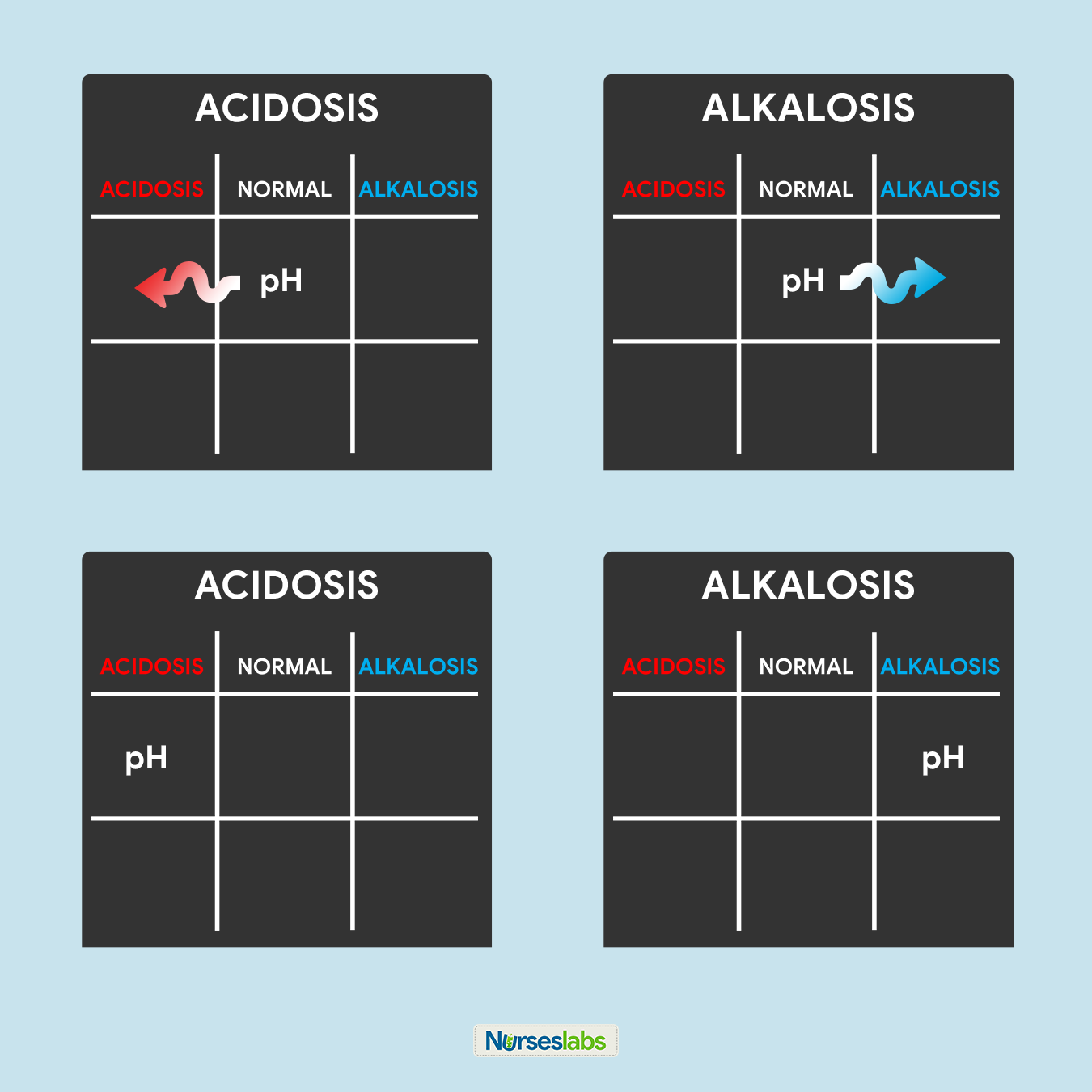
- If pH is under the ACIDOSIS column, it is ACIDOSIS.
- If pH is under the ALKALOSIS column, it is ALKALOSIS.
- If pH is under the NORMAL column, determine whether the value is leaning towards ACIDOSIS or ALKALOSIS and interpret accordingly.
In this step, we can accomplish goal #1 of determining ACIDOSIS or ALKALOSIS.
7. Solve for goal #2: METABOLIC or RESPIRATORY.
Looking back again on the tic-tac-toe grid, determine if pH is under the same column as PaCO 2 or HCO 3 so we can accomplish our goal #2 of determining if the ABG is RESPIRATORY or METABOLIC. Interpret the results as follows:

- If pH is under the same column as PaCO 2 , it is RESPIRATORY.
- If pH is under the same column as HCO 3 , it is METABOLIC.
- If pH is under the NORMAL column, determine whether the value is leaning towards ACIDOSIS or ALKALOSIS and interpret accordingly.
8. Solve for goal #3: COMPENSATION.
Lastly, we need to determine the compensation to accomplish our goal #3. Interpret the results as follows:
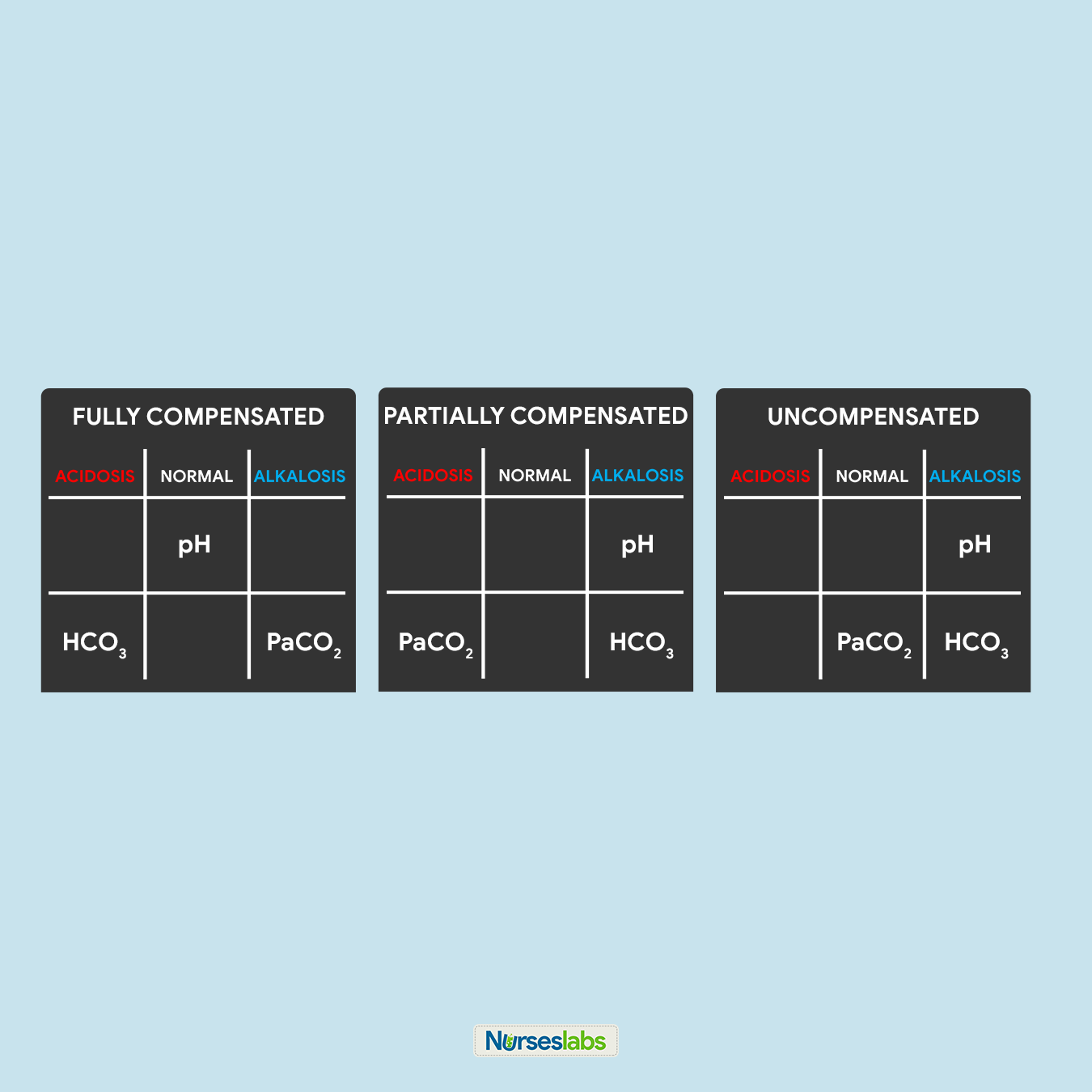
- It is FULLY COMPENSATED if pH is normal.
- It is PARTIALLY COMPENSATED if all three (3) values are abnormal.
- It is UNCOMPENSATED if PaCO 2 or HCO 3 is normal and the other is abnormal.
Let’s solve for the ABG interpretation with the examples below:
Practice Problem #1: pH=7.26 | PaCO 2 =32 | HCO 3 =18
- Remember the normal values.
- Make your tic-tac-toe grid.
- pH of 7.26 ABNORMAL and under ACIDOSIS, so we place pH under ACIDOSIS.
- PaCO 2 of 32 is ABNORMAL and under ALKALOSIS, so we place PaCO 2 under ALKALOSIS.
- HCO 3 of 18 is ABNORMAL and under ACIDOSIS, so we place HCO 3 under ACIDOSIS.
- pH is under ACIDOSIS, therefore solving for goal #1, we have ACIDOSIS.
- pH is on the same column as HCO 3 , therefore solving for goal #2, we have METABOLIC.
- All three values are ABNORMAL, therefore solving for goal #3, we have a PARTIALLY COMPENSATED ABG.
Metabolic Acidosis, Partially Compensated
Practice Problem #2: pH=7.44 | PaCO 2 =30 | HCO 3 =21
- pH of 7.44 is NORMAL but slightly leaning towards ALKALOSIS, so we place pH under the NORMAL column with an arrow pointing towards the ALKALOSIS column.
- PaCO 2 of 30 is ABNORMAL and ALKALOSIS, so we place PaCO 2 under the ALKALOSIS column.
- HCO 3 of 21 is ABNORMAL and ACIDOSIS, so we place HCO 3 under the ACIDOSIS column.
- pH of 7.44 is NORMAL but leaning towards ALKALOSIS, therefore solving for goal #1, we have ALKALOSIS.
- pH is NORMAL but is leaning towards ALKALOSIS, therefore under the same column as PaCO 2 . Solving for goal #2, we have RESPIRATORY.
- pH is NORMAL, therefore solving for goal #3, we have a FULLY COMPENSATED ABG.
Respiratory Alkalosis, Fully Compensated
Practice Problem #3: pH=7.1 | PaCO 2 =40 | HCO 3 =18
- pH of 7.1 is ABNORMAL and ACIDOSIS, therefore, we place pH under the ACIDOSIS column in the tic-tac-toe grid.
- PaCO 2 of 40 is NORMAL, therefore, place it under the NORMAL column.
- HCO 3 of 18 is ABNORMAL and ACIDOSIS, so we place HCO 3 under the ACIDOSIS column.
- pH of 7.1 is ACIDOSIS, therefore, solving for goal #1, we have ACIDOSIS.
- pH is under the same column as HCO 3 , therefore, solving for goal #2, we have determined that it is METABOLIC.
- pH is ABNORMAL so as HCO 3 , but PaCO3 is under the NORMAL column. Solving for goal #3, we can interpret it as UNCOMPENSATED.
Metabolic Acidosis, Uncompensated
How to draw Arterial Blood Gas?
Arterial blood is usually drawn via the brachial or radial artery.
- Inform the client about the procedure and that there is no food or fluid restriction imposed.
- Note if the client is taking anticoagulant therapy or aspirin as this may affect results.
- Note if the client is receiving oxygen therapy (flow rate, type of administration device), and the client’s current temperature.
- Using a heparinized needle and syringe, collect 1 to 5 mL of arterial blood. Common sites for drawing arterial blood are the radial and brachial artery.
- Put the syringe with arterial blood in an ice-water bag to minimize the metabolic activity of the sample.
- Deliver the blood sample immediately to the laboratory.
- Apply pressure to the puncture site for 5 minutes or longer.
Acid-Base Balance and Imbalances
Acid-base imbalances develop when a person’s normal homeostatic mechanisms are dysfunctional or overwhelmed. One type of acid-base imbalance is acidosis wherein the blood is relatively too acidic (low pH). The body produces two types of acid, therefore, there are two types of acidosis: respiratory acidosis and metabolic acidosis. On the contrary, alkalosis is a condition wherein the blood is relatively too basic (high pH), there are also two types of alkalosis: respiratory alkalosis and metabolic alkalosis.
When acid-base imbalances occur, the body activates its compensatory mechanisms (the lungs and kidneys) to help normalize the blood pH. The kidneys compensate for respiratory acid-base imbalances while the respiratory system compensates for metabolic acid-base imbalances. This does not correct the root cause of the problem, if the underlying condition is not corrected, these systems will fail.
Respiratory acidosis occurs when breathing is inadequate (alveolar hypoventilation) and the lungs are unable to excrete enough CO2 causing PaCO 2 or respiratory acid builds up. The extra CO2 combines with water to form carbonic acid, causing a state of acidosis — a common occurrence in emphysema . The kidneys activate its compensatory process (albeit slow, often 24 hours or more) by increasing the excretion of metabolic acids through urination , which increases blood bicarbonate.
Types of Respiratory Acidosis
There are two forms of respiratory acidosis: Acute and Chronic.
- Acute respiratory acidosis. This form of respiratory acidosis occurs immediately. Left untreated, symptoms will get progressively worse. It’s a medical emergency and can become life-threatening.
- Chronic respiratory acidosis. This form of respiratory acidosis develops over time. It doesn’t cause symptoms. Instead, the body adapts to the increased acidity. For example, the kidneys produce more bicarbonate to help maintain balance. Chronic respiratory acidosis may not cause symptoms. Developing another illness may cause chronic respiratory acidosis to worsen and become acute respiratory acidosis.
Risk Factors
Respiratory acidosis is typically caused by an underlying disease or condition. This is also called respiratory failure or ventilatory failure.
- Hypoventilation. A decrease in ventilation increases the concentration of carbon dioxide in the blood and decreases the blood’s pH ( brain trauma , coma, hypothyroidism : myxedema ).
- Chronic Obstructive Pulmonary Disease (COPD). In chronic respiratory acidosis in COPD patients, the body tries to compensate by retaining more bicarbonate to overcome acidosis.
- Respiratory Conditions. The lungs are not able to eliminate enough of the carbon dioxide produced by the body. Excess carbon dioxide causes the pH of the blood and other bodily fluids to decrease, making them too acidic. ( pneumothorax , pneumonia , status asthmaticus)
- Drug Intake. Overdose of an opiate or opioid, such as morphine , tramadol , heroin , fentanyl , or magnesium sulfate (MgSO4) can cause respiratory acidosis.
Signs and Symptoms
Signs and symptoms of respiratory acidosis are as follows:
- Altered level of consciousness. Respiratory acidosis may be the result of an altered level of consciousness caused by encephalopathy or cerebral edema .
- Confusion. Acute respiratory acidosis may also cause symptoms involving the brain, including confusion , stupor, drowsiness, and muscle jerks.
- Disorientation. Respiratory acidosis may result in disorientation , headache, or even focal neurologic signs.
- Coma. When the lungs can’t remove all of the carbon dioxide produced by the body through normal metabolism, the blood becomes acidified, leading to increasingly serious symptoms, from sleepiness to coma.
- Tremors. Manifest as shaking or jerking muscle movements.
- Asterixis. An inability to maintain the posture of part of the body.
Management of Respiratory Acidosis
Medical and nursing management of an arterial blood gas of respiratory acidosis includes the following:
- Medications. Bronchodilator medicines and corticosteroids may be used to reverse some types of airway obstruction , like those linked to asthma and COPD.
- Weight loss . In the case of obesity hypoventilation syndrome, significant weight loss may be necessary to reduce abnormal compression of the lungs.
- Provide mechanical ventilation through oxygen supplementation . Additional oxygen may be provided to alleviate the low oxygen level in the blood.
- Manage hyperkalemia through the use of Kayexalate. Acidosis causes potassium to move from cells to extracellular fluid ( plasma ) in exchange for hydrogen ions, and alkalosis causes the reverse movement of potassium and hydrogen ions. Kayexalate increases fecal potassium excretion through the binding of potassium in the lumen of the gastrointestinal tract.
- Maintain adequate hydration. Provide intravenous fluids and electrolytes as ordered.
Respiratory Alkalosis
Respiratory alkalosis can result from hyperventilation since the lungs excrete too much carbonic acid which increases pH. Since respiratory alkalosis occurs quickly, the kidneys do not have time to compensate. Neurological symptoms such as confusion , paresthesias, and cell membrane excitability occur when the blood pH, CSF, and ICF increases acutely.
Causes of hyperventilation include:
- Panic. Panic attacks and anxiety are the most common causes of hyperventilation.
- Hyperthermia. Fever may manifest as hyperventilation. The exact mechanism is not known but is thought to be due to carotid body or hypothalamic stimulation by the increased temperature.
- Brainstem damage. Central neurogenic hyperventilation (CNH) is the human body’s response to reduced carbon dioxide levels in the blood. This reduction in carbon dioxide is caused by the contraction of cranial arteries from damage caused by lesions in the brain stem .
- Metabolic acidosis. Hyperventilation occurs most often as a response to hypoxia, metabolic acidosis, increased metabolic demands, pain , or anxiety .
- Diabetic ketoacidosis (DKA). The only known compensatory response to metabolic acidosis in DKA is hyperventilation with consecutive respiratory alkalosis.
- Pregnancy. Progesterone levels are increased during pregnancy. Progesterone causes stimulation of the respiratory center, which can lead to respiratory alkalosis.
- Salicylate toxicity. Salicylate toxicity causes respiratory alkalosis and, by an independent mechanism, metabolic acidosis.
Hyperventilation is a sign that respiratory alkalosis is most likely to occur. However, low carbon dioxide levels in the blood also have a number of physical effects, including:
- Numbness. Increased neuromuscular irritability in which a person loses feeling in a particular part of their body.
- Tingling sensation. Prickling sensation that is usually felt in the hands, arms, legs, or feet, but can also occur in other parts of the body.
- Palpitations. Palpitations are the perceived abnormality of the heartbeat characterized by awareness of cardiac muscle contractions in the chest.
- Tetany. Tetany or tetanic seizure is a medical sign consisting of the involuntary contraction of muscles.
- Convulsions. A medical condition where body muscles contract and relax rapidly and repeatedly, resulting in uncontrolled actions of the body.
- Signs and symptoms of hypokalemia and hypocalcemia . Persistent respiratory alkalosis can induce secondary hypocalcemia and hypokalemia that may cause cardiac arrhythmias, conduction abnormalities, and various somatic symptoms such as paresthesia, hyperreflexia, convulsive disorders, muscle spasm, muscle twitching, positive Chvostek’s sign, and tetany.
Management of Respiratory Alkalosis
The treatment for respiratory alkalosis depends on the underlying cause. Treating the condition is a matter of rising carbon dioxide levels in the blood. The following strategies and tips are useful for respiratory alkalosis caused by over-breathing due to panic and anxiety.
- Breathe into a paper bag . Breathing through a paper bag fills it with carbon dioxide helping in inhaling exhaled air back into the lungs.
- Medications. Administering an opioid pain reliever or anti-anxiety medication to reduce hyperventilation.
- Relaxation techniques. Breathing exercises that help relax and breathe from the diaphragm and abdomen, rather than chest wall.
- Safety. Stay with the patient.
- Lavage. After massive aspirin ingestions, aggressive gut decontamination is advisable, including gastric lavage.
- Correction of hypokalemia and hypocalcemia .
- Oxygenation as indicated. Providing oxygen to help keep a person from hyperventilating.
Metabolic acidosis is when there is a decrease in bicarbonates and a buildup of lactic acid occurs. This happens in diarrhea , ketosis, and kidney disorders. It has three main root causes: increased acid production, loss of bicarbonate, and a reduced ability of the kidneys to excrete excess acids.
- Diabetic Ketoacidosis (DKA). DKA develops when substances called ketone bodies (which are acidic) build up during uncontrolled diabetes . DKA occurs mostly in Type 1 Diabetes Mellitus (DM).
- Chronic Renal Failure (CRF). This is due to reduced tubular bicarbonate reabsorption and insufficient renal bicarbonate production in relation to the number of acids synthesized by the body and ingested with food.
- Chronic Hypoxia. With chronic hypoxia, metabolic and hypercapnic acidosis develop along with considerable lactate formation and pH falling to below 6.8.
- Obesity. Obesity, especially in conjunction with insulin resistance, can increase metabolic acidosis and thus result in a reduction of urinary citrate excretion.
- Diarrhea . Loss of bicarbonate stores through diarrhea or renal tubular wasting leads to a metabolic acidosis state characterized by increased plasma chloride concentration and decreased plasma bicarbonate concentration.
- Dehydration . Electrolyte disturbances caused by prolonged vomiting or severe dehydration can cause metabolic acidosis.
- Aspirin Toxicity. Aspirin overdose causes the body to not produce ATP, leading to anaerobic metabolism with consequent raised lactate and ketone bodies. Acute aspirin or salicylates overdose or poisoning can cause initial respiratory alkalosis through metabolic acidosis ensues thereafter.
- Methanol Poisoning. Significant methanol ingestion leads to metabolic acidosis, which is manifested by a low serum bicarbonate level. The anion gap is increased secondary to high lactate and ketone levels. This is probably due to formic acid accumulation.
- Altered level of consciousness
- Disorientation
- Lack of appetite
Management of Metabolic Acidosis
Patients with arterial blood gas indicating metabolic acidosis are managed and treated by:
- Sodium bicarbonate. Indicated in the treatment of metabolic acidosis which may occur in severe renal disease, uncontrolled diabetes , circulatory insufficiency due to shock or severe dehydration , extracorporeal circulation of blood, cardiac arrest, and severe primary lactic acidosis.
- Treat the underlying condition.
- Hydration for diabetic ketoacidosis . The major treatment of this condition is the initial rehydration.
- Dialysis for chronic renal failure . The control of metabolic acidosis in hemodialysis is mainly focused on the supply of bicarbonate during the dialysis sessions.
- Use of diuretics .
- Initiate safety measures.
- Kayexalate. Acidosis causes potassium to move from cells to extracellular fluid (plasma) in exchange for hydrogen ions, and alkalosis causes the reverse movement of potassium and hydrogen ions. Kayexalate increases fecal potassium excretion through the binding of potassium in the lumen of the gastrointestinal tract.
Metabolic Alkalosis
Metabolic alkalosis occurs when bicarbonate ion concentration increases, causing an elevation in blood pH. This can occur in excessive vomiting , dehydration , or endocrine disorders.
- Vomiting . Vomiting causes metabolic alkalosis by the loss of gastric secretions, which are rich in hydrochloric acid (HCl). Whenever a hydrogen ion is excreted, a bicarbonate ion is gained in the extracellular space.
- Sodium bicarbonate overdose. Administration of sodium bicarbonate in amounts that exceed the capacity of the kidneys to excrete this excess bicarbonate may cause metabolic alkalosis.
- Hypokalemia. Due to a low extracellular potassium concentration, potassium shifts out of the cells. In order to maintain electrical neutrality, hydrogen shifts into the cells, raising blood pH.
- Nasogastric suction. Just like in vomiting , nasogastric (NG) suction also generates metabolic alkalosis by the loss of gastric secretions, which are rich in hydrochloric acid (HCl).
Metabolic alkalosis may not show any symptoms. People with this type of alkalosis more often complain of the underlying conditions that are causing it. These can include:
- Swelling in the lower legs (peripheral edema )
- Tingling sensation
Management of Metabolic Alkalosis
- Antiemetic. In the case of vomiting , administer antiemetics, if possible.
- Ammonium chloride. Ammonium chloride is a systemic and urinary acidifying agent that is converted to ammonia and hydrochloric acid through oxidation by the liver . Intravenous (IV) ammonium chloride is a treatment option for severe cases of metabolic alkalosis.
- Acetazolamide (Diamox). Acetazolamide also appears to be safe and effective in patients with metabolic alkalosis following treatment of respiratory acidosis from exacerbations of chronic obstructive pulmonary disease (COPD).
If you need to practice your new skills acquired here, check out our Arterial Blood Gas Interpretation for NCLEX (40 Questions)
The following sources are used as references for this guide. You may find them interesting for your additional reading:
- Barnette, L., & Kautz, D. D. (2013). Creative ways to teach arterial blood gas interpretation . Dimensions of Critical Care Nursing , 32 (2), 84-87.
- Samuel, R. (2018). A Graphical Tool for Arterial Blood Gas Interpretation using Standard Bicarbonate and Base Excess . Indian J Med Biochem , 22 (1), 85-89.
- Sood, P., Paul, G., & Puri, S. (2010). Interpretation of arterial blood gas . Indian journal of critical care medicine: peer-reviewed, official publication of Indian Society of Critical Care Medicine , 14 (2), 57.
- Williams, A. J. (1998). Assessing and interpreting arterial blood gases and acid-base balance . Bmj , 317 (7167), 1213-1216.
- Verma, A. K., & Roach, P. (2010). The interpretation of arterial blood gases . Aust Prescr , 33 (4), 124-129.
30 thoughts on “Arterial Blood Gas Analysis Made Easy with Tic-Tac-Toe Method”
Awesome page for starters .Great work.
Lovely and awesome notes. Simple and to the point.
I learned how to find if its Compensated, Uncompensated, or Partially Compensated.
Thanks,this material is helpful.
Loved the explanation
Thank you very much
Dear Nurse Labs, Thank you so much for putting in the effort in setting up this website and helping thousands of nursing students. I was in nursing school and I had high anxiety in my first semester I had to drop out because I was struggling a lot. I had huge exam anxieties and my temperature will be all over the place, I haven’t been in class for over 23yrs and then I wanted to become a nurse these past years and in class, I felt like I had to look up every single word in other to under a whole sentence. I was really struggling but I realized that I simply wasn’t ready or (well) prepared mentally. So, I finally decided to drop out to get myself (mentally) prepared, and will surely re-enroll again in 2022.
I’ve made it my goal to spend between 2-3hrs a day reading a lot of materials on your website ever since I discovered it through Google, and I feel like I’m super ready now, I’ve found your website about 2 months ago, I spent some time on the care plans tabs because in my class care plan was a tough subject for everyone. I want to read everything on your website and I believe that, even though I will not be able to memorize EVERYTHING, but I’m sure when I come across certain Med Terms, I will remember seeing them somewhere before and it won’t sound or look so strange to me.
I’ve also memorized about 34 suffixes and prefixes and I’m so grateful for the internet! I will continue to make use of your website and I hope to cover the entire website before Dec of 2022! Once again, Thank you very much to all the people on this website helping strangers like myself. Thank you!
Thank you for your kind words, Abb! Best of luck to you! :)
I hope you made it back to nursing school and graduated by now!!
Thanks I get V . Good information
Thanks you so much you guys are a life saver . God bless you so much.
Explanations in this ABG interpretation are easy to understand. It is very detailed. Honestly, I had a hard time before in the ABG interpretation most specifically on finding whether its fully compensated, partially compensated and uncompensated. But after reading the full text of this lecture, I can now identify which is fully, partial and uncompensated ABG. Thank you so much for sharing and generosity.
This was made so easy thank u
Thank you. You helped me a lot. I bow understand how to interpret blood gas
Wow, a whole nursing degree and I still never truly understood ABG interpretation until now. You made it so easy.
It was easiest method,, being easy to understand is best point for any article 👌
Thank you.. This means a lot for me
This presentation was really comprehensive and reader-friendly, I was searching for a document to make for easy teaching and this was it. Simply wonderful I love the straightforward explanations.
Hello NurseLabs, I have been in nursing school for 4 years now and I’m about to graduate. I NEVER understood ABGs and how to calculate them until now. I really wish they taught this method in class!! Thank you so much, I have been so anxious about this chapter since I used to always guess on these problems. I can confidently take the test this time around!! You made it so easy to understand.
Hi lovely Nurseslab team, I am very happy and you made it so easy to understand I am so confident It is very straightforward Thank you so much.
Hi Vaji, Super happy to hear you found the ABG tic tac toe method helpful and easy to get! It’s awesome when things just click, isn’t it? If you’ve got more topics you want to crack or any questions, just holler. Always here to keep things easy and fun!
i love how you explain the ABG interpretation, it just confuses me that we have different values in UK
Hi A. Gonzales, Thanks for the love on the ABG interpretation explanation! I’m really glad to hear it helped clear things up for you. It’s interesting how different regions like the UK can have varying normal values – it definitely adds an extra layer to consider when interpreting results.
Thank You all with helping us to be updated with current scenario.
Thank you so much Nurse Labs. Such a simple way to understand ABGs. Great interpretation
You’re absolutely welcome! I’m delighted to hear that our explanation of ABGs (Arterial Blood Gases) has been helpful for you and that you find it easy to understand.
Best explanation!
Thank you Nurselabs, the explanation of ABGs is very simple and easy to understand and has made me confident in solving ABGs interpretation, I am now more confident.
Thanks very much you have made things easier,be blessed.
Glad you liked it!
Leave a Comment Cancel reply

IMAGES
COMMENTS
Interpreting an arterial blood gas (ABG) is a crucial skill for physicians, nurses, respiratory therapists, and other health care personnel. ABG interpretation is especially important in critically ill patients. The following six-step process helps ensure a complete interpretation of every ABG. In addition, you will find tables that list ...
Step 1: Analyze the pH. The first step in analyzing ABGs is to look at the pH. Normal blood pH is 7.4, plus or minus 0.05, forming the range 7.35 to 7.45. If blood pH falls below 7.35 it is acidic. If blood pH rises above 7.45, it is alkalotic.
Below are some brief clinical scenarios with ABG results. Try to interpret each ABG and formulate a differential diagnosis before looking at the answer. Question 1. You are called to see a 54 year old lady on the ward. She is three days post-cholecystectomy and has been complaining of shortness of breath. Her ABG is as follows: pH: 7.49 (7.35-7.45)
go to YouTube, and type in "ABG tic-tac-toe" method.Wallace. 1 recommends using color to simplify ABG interpretation. In this approach, blue depicts. base, red is used for acid, and black signifies neutral. The use of color may not only help students and new nurses learn, but also aid exp. rienced.
ABG analysis. As part of the body's buffering system, the kidneys retain or excrete the alkalotic bicarbonate ion as needed. The HCO 3 - value can be used to determine if the source of an acid-base disturbance is respiratory or metabolic. An HCO 3 - level below 22 mEq/L indicates metabolic acidosis; above 26 mEq/L indicates metabolic alkalosis.
HCO3-: This is bicarbonate, a chemical buffer made in the kidneys to neutralize acids. It is the metabolic component of the ABG. Normal is 22-28 mEq/L. SaO2: This is a measure of the % of oxygen that is attached to hemoglobin in red blood cells. Normal is > 95% in healthy humans (avg is 98% for a healthy pt).
Abstract. Arterial blood gases (ABG) results reflect underlying pathology and interpretation of the results are often compounded by ongoing disease processes and clinical interventions. While ABG ...
J.M. (2008) Arterial blood gas a. alysis 1: understanding ABG reports. Nursing Times; 104: 18, 28-29. This is the first of a two-part unit on arterial blood gas (ABG) analysis, and focuses on background information and basic. interpretation of ABGs where no evident compensation is taking place. It discusses the various components on an AB.
The arterial blood gas (ABG) is one of the most powerful and frequently used tests in critical care and in the operating room. An ABG may be ordered to obtain information about the patient's acid/base status, arterial carbon dioxide tensions (PaCO 2) and arterial oxygen (PaO 2) tensions.Frequently, other information such as the calculated sodium bicarbonate, base deficit, hemoglobin, basic ...
Metabolic Acidosis. Metabolic acidosis is defined as a bicarbonate level of less than 22 mEq/L with a pH of less than 7.35. Metabolic acidosis is caused by either a deficit of base in the bloodstream or an excess of acids, other than CO2. Diarrhea and intestinal fistulas may cause decreased levels of base.
An ABG can be taken by sampling blood from an arterial line or performing an arterial punc-ture (if staff are trained and competent to do so). This article focuses on ABGs. A glossary of key terms is given in Box 1. Blood gases are taken using point-of-care devices when a result is needed quickly (namely, from sampling to analysis in min - utes).
Understanding and using blood gas analysis enables providers to interpret respiratory, circulatory, and metabolic disorders. A "blood gas analysis" can be performed on blood obtained from anywhere in the circulatory system (artery, vein, or capillary). An arterial blood gas (ABG) explicitly tests blood taken from an artery.
Explanation Oxygenation. The PaO 2 is within normal limits and appropriate to the % inspired oxygen concentration (FiO 2); FiO 2 in room air is 21%, and as a rule of thumb, the PaO 2 should be approximately 10 kPa less than the %FiO 2; Acid-base disturbance. Primary acid-base disturbance. The patient has an acidaemia with a pH of 7.3 (7.35-7.45); Acidaemia can either be driven by a respiratory ...
I'm still struggling whether an ABG is compensated, uncompensated, or partially compensated. Hetty Naphata May 13, 2024. This is so helpful, no need to know which arrow is moving in the same direction or opposite direction. Thank you. JEM February 2, 2024. I struggle with whether an ABG is compensated, uncompensated, or partially compensated.
Arterial PO 2 (PaO 2) zNormal: 80 - 100 mm Hg breathing room air at sea level in healthy young adults (103- 0.5 x age) zPaO 2 affected by - FIO 2 PEEP Lung function - Age Ventilation Altitude PAO 2 = FIO 2(PB-PH 20) - PaCO 2 x 1.2 PAO 2 = FIO2(700) - PaCO 2 x 1.2 Always interpret PaO
ABG Examples (ABG exam questions for medical students and PACES) OSCE and PACES-style clinical ABG examples and questions. ABG Exam Questions. OSCE and PACES-style test on ABG background and physiology. ABG Procedure. How to take an arterial blood gas (ABG) Venous blood gas (VBG) interpretation. How to interpret a VBG and its comparison to an ABG
Reassess all acutely ill patients regularly, and consider repeat arterial blood gas analysis Errors in blood gas analysis are dependent more on the clinician than on the analyser Table 1|Report of arterial blood gases for the hypothetical patient described Value (reference range) pH 7.25 (7.35-7.45) Partial pressure of oxygen (PaO 2) (kPa) 8.9 ...
Arterial blood gas analysis can be used to assess gas exchange and acid base status as well as to provide immediate information about electrolytes. ... (ABG) Normal values are given below. Note that these may vary slightly between analysers. Be sure to know the normal ranges and units for the analyser you will be using. pH: 7.35 - 7.45; pO2 ...
This document provides an overview of arterial blood gas (ABG) analysis using an ABG analyzer. It discusses what an ABG test measures, its clinical significance, the principles and parts of an ABG analyzer, quality control processes, and interpretation of ABG results. The document is a student assignment that analyzes the topic of ABG analysis in 12 pages with an index and bibliography.
They are easy to remember: For pH, the normal range is 7.35 to 7.45. For PaCO 2, the normal range is 35 to 45. For HCO 3, the normal range is 22 to 26. Normal Blood pH Scale Diagram for the Tic-Tac-Toe Method for ABG Analysis. The recommended way of memorizing it is by drawing the diagram of normal values above.
ASSIGNMENT ON ABG ANALYSIS 1 INTRODUCTION. Arterial blood gases are an important routine investigation to monitor the acid-base imbalance of the patient. They may help to make diagnosis, indicate the severity of condition and help to assess treatment. Blood for ABG analysis can be obtained by arterial puncture usually from radial and femoral ...